
Gluttony and Sloth—Behaviors
Driven by Hormones

Marie is a sixteen-year-old girl with a brain tumor of the hypothalamus (the area at the base of the brain that regulates the hormones of the body). When she was ten, cranial radiation was required to kill the tumor. Since then, she has gained 30 pounds per year; she weighed 220 pounds when I first saw her. Her insulin levels spiked to incredible heights every time she ate. She had a form of intractable weight gain due to brain damage called hypothalamic obesity. She wouldn’t do any activity at home, couldn’t study in school, and was severely depressed. As part of a research study, I started her on a drug called octreotide, which lowered her insulin release. Within one week Marie’s mother called me to say, “Dr. Lustig, something’s happening. Before, we would go to Taco Bell where she would eat five tacos and an encharito and still be hungry. Now we go, she has two tacos and she’s full. And she’s starting to help me around the house.” After beginning the medication, Marie commented to me, “This is the first time my head hasn’t been in the clouds since the tumor.” Within a year, she was off antidepressants and had lost 48 pounds.
Who’s at fault here? Is this a case of free will? And what happened to cause Marie’s reversal? If obesity is truly a result of too much energy intake (gluttony) and too little energy burned (sloth), then my last sixteen years taking care of obese children has been a complete and utter waste. Because it’s become painfully evident, after years of motivating, pleading, and arguing, that I can’t change children’s behavior. And I certainly can’t change their parents’ behavior. It was this insight from Marie, and other children like her, that exposed the inherent problems in our current thinking. Biochemistry and hormones drive our behavior.
The idea that biochemistry comes first is not a new one, but it is one that physicians, scientists, and the public should embrace. Think about the following: You see a patient who drinks ten gallons of water a day and urinates ten gallons of water a day (highly abnormal). What is wrong with him? Could he have a behavioral disorder and be a psychogenic water-drinker? Could be. Much more likely he has diabetes insipidus, a defect in a water-retaining hormone at the level of the kidney. You see a twenty-five-year-old who falls asleep in his soup. Was he up partying all night? Perhaps. But he may have narcolepsy, which is a defect in the hormone that stimulates arousal (orexins) in the midbrain. The biochemistry drives the behavior. Schizophrenia for one hundred years was a mental health disorder. Now we know that it’s a defect in dopamine neurotransmission and that no amount of psychotherapy is going to help until you treat the biochemical defect. Thus, we routinely infer “biochemical” defects in many “behavioral” disturbances.
Introducing Energy Processing and Storage
To appreciate how hormones control eating behavior, first we have to look at what happens to the food we eat. In response to various brain signals (hunger, reward, stress) we ingest various calorie-laden foodstuffs (combinations of fat, protein, carbohydrate, and fiber, with some micronutrients thrown in for good measure) to build muscle and bone for growth and/or to burn for energy. These calories arrive at the stomach, a muscular bag in the abdomen about the size of a baseball glove, which releases hydrochloric acid, to begin to digest the food into smaller components. The food makes its way into the next part of the digestive tract, called the small intestine. There, a bunch of enzymes (proteins) digest the food into even smaller components, such that dietary fats are digested into fatty acids, dietary protein is sliced into amino acids, and carbohydrate is cleaved into simple sugars (mostly glucose, with varying amounts of the sweet molecule fructose). But we can’t digest dietary fiber, so it remains intact. The fiber speeds the rate of transit of the food through the small intestine (see chapter 12), while limiting the rate of absorption of the other nutrients.
Once absorbed in the small intestine, the amino acids and simple sugars travel via the portal vein to the liver for immediate processing. The fatty acids are transported to the liver by a different route (the lymphatic system). The liver has first dibs on the processing of each of these three classes of nutrient. Whatever the liver can’t take up appears in the general circulation. Rising levels of glucose or amino acids or fatty acids reach the pancreas, where the beta-cells release the hormone insulin.
Insulin, in common parlance, is known as the diabetes hormone. Diabetics inject insulin to lower their blood glucose. But where does the glucose go? To the fat. Insulin’s actual job is to be your energy storage hormone. When you eat something (usually containing some form of carbohydrate), your blood glucose rises, signaling the pancreas to release insulin commensurate with the rise in blood glucose. (This is the theory behind the concept of glycemic index, which is discussed in chapter 17.) Insulin then tops off the liver’s energy reserve by making liver starch (called glycogen), and shunts any amino acids from the blood into muscle cells. Excess fatty acids, or blood lipids, are cleared into fat cells for storage for a “rainy day,” where they get turned into greasy triglycerides (such as the fat surrounding your steak). There is no energy storage without insulin—it is the key that unlocks the door to the fat cell to let energy enter and subsequently be stored as fat. Insulin makes fat—the more insulin, the more fat. And there it sits…and sits…as long as there is insulin around. When the insulin levels drop, the process goes in reverse: the triglycerides get broken down, causing the fat cells to shrink—when it happens, that’s weight loss!—and the fatty acids reenter the bloodstream and travel back to the liver, where they are burned by the liver or other organs. In this way, by cycling our insulin up and down, we burn what we need, and store the rest.
For the past sixty years we’ve known that the brain, especially the one cubic centimeter at the base of the brain called the hypothalamus, controls this process of energy balance. It’s about the size of a thumbnail, and it is “ground zero” for the control of almost all the hormonal systems in the body.
Imagine the organization of a taxicab company. At the bottom are the taxicab drivers, getting their orders from a central dispatcher by radio and shuttling passengers all over town. The target organs—the thyroid, the adrenal, the testicles, the ovaries—are like the cabbies. They receive their orders from the central dispatcher, or, in this case, the pituitary or “master gland,” which acts as the main control system. The hormones released are similar to the taxi’s computerized system, signaling to the pituitary to tell it how things are going out in the field. Like the central dispatcher who directs the cabs based on their location, the pituitary will then adjust its message.
However, there is another layer of control: the chief executive officer, or CEO, who decides on hiring and firing, contracts, upgrades, and mergers and acquisitions. The company can’t turn a profit without the cabbies, be efficient without the dispatcher, or be sustainable long term without a CEO. Furthermore, the CEO can alter the direction of the company based on the profitability of its cabdrivers. The CEO is akin to the hypothalamus. It sends blood-borne hormonal signals to tell the pituitary what to do. It then makes large-scale decisions based on the function of the peripheral glands, which send it information via the bloodstream. And it integrates information from other areas of the brain to alter the long-term hormonal milieu. Marie’s hypothalamus was damaged beyond repair, which caused it to be ineffective in controlling her hormones and, therefore, her behavior.
The Ventromedial Hypothalamus (VMH) and Energy Balance
The hierarchy of energy balance is even more complicated. A subarea of this thumbnail is called the ventromedial hypothalamus (VMH), which serves the executive function of controlling energy storage versus expenditure. Because energy balance is so important to survival, there are redundant systems in case one goes amiss to ensure that the organism doesn’t die. It’s clear that energy balance is the most complex function we humans perform. It’s likewise apparent that energy storage, or the creation of fat cells, is the default strategy. Bottom line, we humans won’t give up our hard-earned energy without a fight.
There are afferent (incoming) and efferent (outgoing) systems that control energy balance1 (see figure 4.1). The VMH receives acute meal-to-meal information from the GI (gastro-intestinal) tract on both hunger and satiety (not shown in the figure). Either one can turn the feeling of hunger on or off by itself. But that’s not all. In addition, the VMH receives more long-term information on one’s fat stores and nutrient metabolism: in other words, whether your body needs to consume more calories for longer-term survival. This information is conveyed via the hormones leptin and insulin to the hypothalamus, where it is decoded and either stimulates or suppresses appetite, and adjusts energy expenditure accordingly.
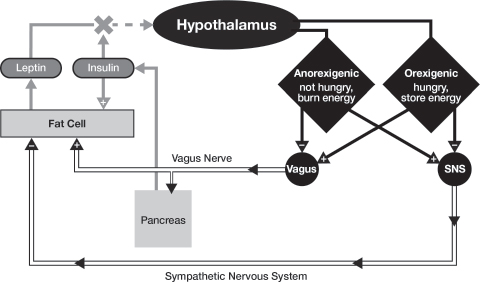
Fig. 4.1. How the Brain and Hormones Work Together (or Don’t) to Regulate Energy Balance. The hypothalamus receives hormonal information from the fat cells (leptin). This information is processed into one of two signals: (a) anorexigenesis (I’m not hungry and I can burn energy) or (b) orexigenesis (I’m hungry and I want to store energy). Anorexigenesis turns on the sympathetic nervous system (responsible for muscle activity and fat loss), and turns off the vagus nerve (responsible for appetite and fat gain); while orexigenesis does the opposite. However, high insulin blocks the leptin signal, mimicking “brain starvation” and driving orexigenesis, so that we feel hungry even when we have eaten.
From there, the hypothalamus sends signals from the brain to the body via two components of the autonomic nervous system. The autonomic nervous system is that portion of your body that controls your heart rate, blood pressure, and energy metabolism without your conscious effort. It is composed of two parts: the sympathetic nervous system (responsible for the fight-or-flight response) and the parasympathetic nervous system (responsible for “vegetative” functions such as food absorption and energy storage). The vagus nerve is one of the key components of the parasympathetic nervous system. There is a delicate balance and feedback loop between the sympathetic and parasympathetic systems. When that balance changes, that’s when problems ensue.
The vagus nerve is fascinating. It connects the brain to all the digestive organs in the abdomen: the liver, the intestine, the pancreas, and also to the fat cells. It performs many different functions but with one ultimate goal: to store energy. The vagus is your energy storage nerve. The vagus has two parts: the afferent part (organs to brain), and the efferent part (brain to organs). The afferent vagus communicates the sensation of hunger between the stomach and brain, and also communicates information on energy processing during a meal between the liver and brain. The VMH interprets all these afferent signals, which leads to one of two physiologic states: anorexigenesis (I don’t need any more food, I can burn energy as needed, and I feel good) or orexigenesis (I don’t have enough food, I don’t want to burn any energy, and I will feel lousy until I get some more).
The anorexigenesis signal turns on the sympathetic nervous system (SNS), which promotes energy expenditure by telling the adipose (fat) tissue and the muscles to burn energy, thereby resulting in weight loss and a sense of well-being. Anorexigenesis also turns off the vagus nerve and, in so doing, reduces appetite. Conversely, orexigenesis stimulates the vagus nerve to promote energy storage by increasing appetite. It accomplishes this by sending multiple signals through the vagus nerve: to the gastrointestinal tract to digest and absorb the food; to the adipose tissue to store more energy (make more fat); and to the pancreas to increase the amount of insulin released (promoting more energy storage into adipose tissue).
Leptin and the Elusive “Holy Grail” of Obesity
When the hormone leptin (from the Greek Leptos, for “thin”) was discovered in 1994, for the first time, scientists thought that obesity might have a biochemical basis. Leptin has been a veritable godsend to scientists who study obesity. It provided the starting point to understanding the biochemistry of the brain pathways that control food intake and the impetus for scientists and the National Institutes of Health (NIH) to believe that there was a simple way out of this mess, one that could be easily treated with medicine and science. The U.S. government began, and continues today, to shovel money at obesity research, hoping for a treatment that works. Conversely, leptin has been the biggest disappointment to those who suffer from obesity. And woe to the pharmaceutical industry, which hoped to harness its potential for a cure and generate megabucks in the process. The pharmaceutical company Amgen was so enamored of leptin’s blockbuster marketing potential that it offered $30 million for the exclusive marketing rights to the hormone, even before a human experiment had been performed. Amgen has since become so disillusioned that it has farmed leptin out to another company, Amylin Pharmaceuticals, to see if it will have better luck.
Leptin is a protein made and released by fat cells. It circulates in the bloodstream, goes to the hypothalamus, and signals the hypothalamus that you’ve got enough energy stored up in your fat.2 The discovery of leptin closed the loop, providing a servomechanism (like your home’s thermostat) in which the body’s fat cells told the hypothalamus whether the animal was in energy surplus (obesity) or dearth (starvation). Obese animals and humans deficient in leptin respond immediately to leptin treatment with remarkable losses of fat and also with increased activity.3 Leptin replacement corrected both behaviors, the gluttony and the sloth. The thought was, if you’re obese, then your leptin doesn’t work—you must be deficient and you just need more. Problem solved, right? Unfortunately, for the obese population, this simple-minded explanation was just that.
Defective Leptin Signaling: Brain Starvation
The VMH is constantly looking for the leptin signal. In the short-term, hormonal inputs can govern the size or the quality of this meal or that, but long term it’s all about leptin. Leptin tells the VMH that you have enough energy on board to burn the excess, feel good, reduce your long-term food intake, and remain weight stable. When your leptin signal works, you’re in energy balance, burning energy at a normal rate and feeling good.4 Every human has a “personal leptin threshold” above which the brain interprets a state of energy sufficiency. Thus, the leptin-replete state is characterized by appropriate appetite, normal physical activity, and feelings of well-being. Woe to the 97-pound weakling who can’t bulk up and gain weight; his leptin threshold is set too low, and his leptin is telling his brain to burn off any excess.
But what if leptin doesn’t work or the threshold is set too high? When the VMH can’t see the leptin signal, the brain interprets this as “starvation” and will direct the rest of the body to do whatever it can to increase its energy stores. The VMH relays messages to the sympathetic nervous system (SNS) to conserve energy and reduce activity. Energy expenditure is reduced by 20 percent, a great reason to feel like a sloth.5 Furthermore, the VMH wants the body to increase energy storage. It will increase the firing of the vagus nerve in order to amplify insulin release from the pancreas and shunt more energy into fat cells, with the ultimate goal of making more leptin. The vagus makes you hungry in order that you store more energy (gluttony). Simply put, defective leptin signaling in the VMH is what brain starvation is all about. This phenomenon occurs in two ways:
Leptin deficiency. Dr. Jeff Friedman of Rockefeller University is credited with cloning the leptin gene from leptin-deficient mice,6 which are the rodent equivalents of a 400-pound couch potato. While normal weight at birth, these mice immediately eat like there’s no tomorrow and just sit there—the only time they ever get off their behinds is if you put food on the other side of the cage; then they’ll waddle over to it, devour it, and sit there instead. These mice are deficient in leptin due to a genetic mutation. Their behaviors of gluttony and sloth are genetically determined. Their brain can’t see their fat and in turn thinks the body is starving.
Friedman’s lab also showed that giving these mice back the leptin they were missing by daily injection reduced their food intake and increased their physical activity back to normal. They lost the weight. Not only that, but all the physiological problems associated with their obesity—the diabetes, the lipid problems, and early death from heart disease—all disappeared. This made leptin look for all intents and purposes like the “holy grail” of obesity. If leptin deficiency was the cause of this pandemic, we could simply replace it, and all the unfortunate souls afflicted could be saved.
Thus far, fourteen children with mutations of the leptin gene have been identified in the entire world. These children cannot make leptin no matter how big their fat cells are, and their brains are in constant starvation mode. Amazingly, with a shot of leptin every day, they lose weight rapidly, and it’s all fat (no muscle). They stop their ravenous behavior, start moving, and their puberty goes into gear.7 For these patients, leptin is hormone-replacement therapy; while not a cure, it’s the next best thing.
Leptin resistance. This is the key to the obesity epidemic. With a few rare exceptions, the other 1.5 billion overweight or obese people on the planet suffer from this. Deciphering leptin resistance is the “holy grail” of obesity. These people have plenty of leptin, and each one’s blood leptin level correlates with his or her amount of body fat. This suggests that obese people are not leptin deficient but rather leptin resistant.8 Their hypothalami can’t see their leptin, so their brains think they’re starving, and will therefore try to increase energy storage (gluttony) and conserve energy usage (sloth).
In 1999, Steven Heymsfield, then at Columbia University, gave daily injections of leptin at varying doses to obese adults for six months. All these people had high leptin levels to start. The degree of weight loss, even with the highest dosage of leptin, was underwhelming.9 Clearly these obese people were leptin resistant. They couldn’t respond to their own leptin, and no amount of extra leptin was going to make a difference. Heymsfield’s study was the end of the promise of leptin as a stand-alone therapy for obesity and the end of Amgen’s interest.
Hypothalamic Obesity: Behavior or Biochemistry?
This is where I enter the story. In 1995, I arrived in Memphis to start work at St. Jude Children’s Research Hospital as a pediatric neuroendocrinologist. My training is in taking care of kids with brain tumors, and St. Jude had a large population of survivors, Many of these children develop hormonal deficiencies because of damage to the hypothalamus—due to the tumor itself, the neurosurgery to remove it, or the radiation and chemotherapy they receive to try to kill it. The good news is that we endocrinologists can treat these children by replacing most of the hormones that are missing—we can affect their growth, energy metabolism, and cognitive status; induce puberty when the children are age appropriate; and improve their overall health.
However, a relatively small number of children like Marie (and adults) who survive their brain tumors become massively obese after their tumor therapy is complete. Their hypothalamus is damaged, and their weight skyrockets. Their appetites aren’t that different from those of other obese children, but their energy expenditure is markedly decreased. (Marie didn’t move.) Those affected sit on the couch, watch TV, eat, poop, sleep, and generally lose interest in the world around them. As one parent stated, “It’s double jeopardy. To think you might lose your kid to a cancer, and survive it, but then to lose your kid to a complication instead.” Patients with this form of obesity, called hypothalamic obesity, can’t lose weight. Even if these kids eat only 500 calories a day, they gain weight.10 The neurons in the hypothalamus, which sense the leptin signal, are all dead. The “servo-mechanism” for energy balance had been short-circuited. This is leptin resistance at its worst—an anatomic leptin resistance. Rodent studies dating back to the early 1950s show that when you damage the VMH, the animal will become massively obese, and not even food restriction will reverse that. The VMH-lesioned rats ate more than they needed and burned less than they should have. Unlike the leptin-deficient mice, no amount of leptin would fix the problem. These animals had anatomic leptin resistance.11 The leptin had no place to act.
The obese children I saw at St. Jude were similar to these VMH-lesioned rats. There was no fixing them because there was no way to regrow those neurons. Those kids were stuck forever in bodies that just kept storing energy instead of burning it,12 with brains that constantly thought the bodies were starving. They would forever get fatter on fewer calories, never feel good, and would lose interest in everything around them. If this isn’t hell on earth for parent and child, I don’t know what is.
Worst yet, there was no treatment. Diet and exercise is notoriously ineffective in these children. Weight loss drugs also didn’t work. In 1995, I was faced with a clinic full of patients with hypothalamic obesity following their brain tumor therapy. How to help them? I couldn’t give them leptin, because the block at the hypothalamus would not allow leptin to work. If any therapy were to be successful, it would have to work downstream of the leptin neuron, somewhere between the brain and the fat cell.
Insulin: The “Leptinator”
Normally, the amount of insulin released in response to a meal is yoked to the blood sugar rise. But there are a few things that force the pancreas to make extra insulin, the vagus nerve being chief among them. When the brain can’t see the leptin signal, as in children such as Marie, it interprets starvation. The vagus nerve goes into overdrive to store more energy, and kick-starts the pancreas to make extra insulin—even more than the glucose rise would predict. This excess insulin release drives nonstop energy storage and nonstop weight gain.
As it happens, there is a drug available that can lower insulin secretion as a side-effect. It is called octreotide (Sandostatin, made by Novartis Pharmaceuticals) and is what we used to treat Marie. It is normally used to reduce pituitary growth hormone secretion in patients who have tumors of the pituitary gland, a disease called acromegaly. But it also happens to reduce pancreatic insulin secretion. It doesn’t wipe it out completely—that would cause diabetes—but it does reduce the rapid early release of insulin in response to a meal or a glucose tolerance test. But it’s expensive, requires injections, has side-effects, and with regard to obesity, it is for experimental studies only.
We have treated many children with hypothalamic obesity with octreotide.13 When we were successful in reducing their insulin release, the patients lost weight and started to feel better. Parents were calling me up within the first few weeks, saying, “I’ve got my kid back!” Most amazingly, the children had started to be active. When we got the insulin down, Marie and patients like her improved physically, mentally, and socially.
These studies highlight a crucial concept of obesity. Each of us is really two compartments: lean body mass (heart, liver, kidneys, brain, and muscles), which burns energy; and fat, which stores energy. Every molecule of energy consumed has a choice: to which compartment does the energy go? Is the energy burned or stored? Your consumption of energy is never high enough to overwhelm both compartments at the same time; no one can eat that much. This means that there is an issue of energy flux to the two compartments. What factor determines which compartment gets the energy?
Your insulin does. The more insulin there is, the more energy goes to fat. Normally your fat makes more leptin, which would feedback on your hypothalamus and decrease your insulin by reducing appetite and limiting your energy intake. In this way, the “servo-mechanism” between leptin, the brain, your pancreas, your insulin, and your fat cells maintains normal energy balance. But…if your hypothalamus can’t see your leptin (in this case, because those neurons are dead from a brain tumor), then your brain thinks it’s starving. It will reduce your activity to conserve energy, and increase your appetite to store more energy. When leptin doesn’t work, the biochemistry comes first and the behaviors of gluttony and sloth are secondary.
This is all well and good for Marie and the few unfortunate souls with hypothalamic obesity. They have a brain tumor. They have a legitimate excuse for being fat, and at least there is now a rational, if painful and expensive, approach to treatment. For them, the biochemistry dictates the behavior. However, the overwhelming majority of obese people do not have a goombah sitting in the middle of their heads wreaking havoc on their energy balance pathway. What does this phenomenon have to do with the obesity pandemic? As you will see, everything.
Back in 1998, after three years of my working at St. Jude, the response of these patients was quite a revelation. My colleagues at the University of Tennessee and I wondered, “Is it possible that an adult population without brain tumors might manifest the same problem? Did they also have increased vagal tone driving excess insulin secretion and causing their obesity? If we gave them octreotide to suppress their insulin, might they lose weight, feel better, and start exercising?” We didn’t know what these patients looked like. So we did a pilot study in forty-four morbidly obese adults recruited from off the street. We treated all of them with octreotide for six months, courtesy of Novartis Pharmaceuticals. No dieting, no exercise, just the drug. We told them, “If the drug works, it will work by itself.”
We’ve done this experiment twice, first as a pilot and then as a placebo-controlled trial. The majority of patients did not respond to the drug. But in about 20 percent of the adults, there was big-time weight loss. The thing that predicted their success was their insulin status. The lucky responders released insulin rapidly and in high amounts at baseline, just like the brain tumor kids,14 and their quality of life improved with the drug.
There is one final lesson to glean from these studies. All these obese adult subjects had high leptin levels. They were leptin resistant; if their leptin worked right, they wouldn’t have been obese. If leptin falls, the brain should interpret this as starvation and reduce the patient’s resting energy expenditure accordingly. But these patients’ resting energy expenditures went up! And their improvement in energy expenditure correlated with the suppression of their insulin levels, the same as with the brain tumor kids. When we were successful in getting their insulin down, their leptin resistance improved.15 This suggests that insulin can block leptin signaling in the brain, and therefore insulin acts as a “leptin antagonist.”16
Many scientists have now shown that insulin actions in the VMH block leptin signaling.17 A reduction in insulin concentrations results in a decline in leptin. Insulin and leptin are independent hormones that bind to separate receptors in the VMH. They have their own separate pathways of action, but they share the same signaling cascade. When insulin levels at the VMH are chronically high, leptin cannot signal the hypothalamus.
Deconstructing Darwin
Whenever paradoxical events occur in biology, one has to look for an evolutionary explanation. Why should insulin block leptin signaling? What’s the advantage for insulin, the hormone that tells the body to store energy, to block leptin, the hormone that tells your brain to burn energy? Leptin is a necessary signal to the VMH for the initiation of high-energy processes, such as puberty and pregnancy. If leptin always worked right, then nobody could gain weight. Think of the 97-pound weakling at the beach. The crucial weight gain during puberty and pregnancy would be compromised, and our reproductive capacity would be shot. Twice in our lives we need to stop leptin from working, or we can’t gain the weight, and the species dies out. Since insulin drives energy storage, it makes sense that it should do double-duty, and also be the central blocker of leptin—one hormone, two coordinated actions. Indeed, both puberty and pregnancy are hyperinsulinemic states. When adulthood or the postpartum state is reached, the insulin levels fall, weight stabilizes or is lost, and leptin levels return toward baseline.18 However, in maladaptive conditions, when insulin is high all the time and leptin signaling is impaired, the energy gets stored yet the brain sees starvation, and obesity worsens.
When you examine the symptoms of obese and starved individuals, they are very similar. On first thought this sounds ludicrous, but it actually makes sense. Both claim fatigue, malaise, and depression. The reason for this in both groups is the inability to adequately respond to the leptin signal—in starvation because of the inadequacy of leptin, and in obesity because of the resistance to leptin. Furthermore, leptin concentrations drop precipitously during periods of short-term fasting (within twelve hours), declining faster than body fat stores. You haven’t lost any weight in that time, but your fat cells are already telling your brain you’re starving, driving your food intake back up. By the time you’re one day into any weight-loss regimen you’re already leptin deficient on top of being leptin resistant, meaning, you really can’t see the signal. Trying not to eat for a day to fit into that little black dress? Oops. This actually drives gluttony and sloth to return your weight to its baseline level. In a nutshell, this is the recidivism of obesity. If your brain thinks there’s no leptin (due to either leptin deficiency or leptin resistance) you’re pretty miserable. Your sympathetic nervous system goes into conservation mode, driving down your energy expenditure, physical activity, and quality of life. Your vagus nerve then goes into overdrive, driving up your appetite, your insulin, and your energy storage.
The Alternate Interpretation of the First Law
No matter the mechanism, insulin blocks leptin signaling both in rodents and in humans. In the body, insulin causes energy storage in fat cells. In the brain, insulin causes leptin resistance and “brain starvation.” Insulin delivers a one-two punch to drive gluttony and sloth, weight gain, and obesity the world over. Insulin is the bad guy in this story.
This idea turns obesity on its head. The standard thinking in obesity is: “If you eat it, you had better burn it, or you’re going to store it”—in which case the weight gain is secondary to the two behaviors of increased energy intake (gluttony) and decreased energy expenditure (sloth). What these data are telling us is that it is the other way around. Storing energy is a biochemical process not under the patient’s control. Burning energy is synonymous with quality of life. Things that make you burn energy faster—such as exercise, ephedrine (off the market now), and caffeine (for about two hours)—make you feel good. Conditions that make you burn energy slower—starvation and hypothyroidism, for example—make you feel lousy. So, the first law needs to be reinterpreted: “If you are going to store it, and you expect to burn it, then you will have to eat it.”19 In this interpretation, the biochemical process is primary, the weight gain is secondary, and the behaviors are a result of the biochemistry.
Obesity is a biochemical alteration in the brain promoting leptin resistance with resultant weight gain and secondary changes in behavior to maintain energy balance. The apparent character defects of gluttony and sloth are not the cause of the problem; they are the result of the problem. The biochemistry drives the behavior, not vice versa. The linchpin in this biochemical alteration is the hormone insulin. The majority of humans, regardless of weight, release double the insulin today that we did thirty years ago for the same amount of glucose. Now we’re left with the $147 billion (the annual financial cost of obesity) question: If insulin is the bad guy and we’re all hyperinsulinemic as never before in the history of humankind, where did the excess insulin come from? And how do we reverse it?
The plot thickens.