 Copper overload
Copper overloadCHAPTER 3
THE DECISIVE ROLE OF NUTRIENTS IN MENTAL HEALTH
The Brain – A Chemical Factory
Considering the complexity of brain chemistry and the multiplicity of gene processes that could go wrong, it is surprising that more humans do not have serious mental problems. Effective brain function requires a proper concentration of neurotransmitters in specific areas of the brain. These complex chemicals are continuously produced by enzymatic reactions within special brain cells. For example most of our brain’s serotonin is synthesized in the raphe nuclei along the brainstem and transported by axons to areas throughout the brain. In another example, dopamine is produced in several areas of the brain, including the substantia nigra and the ventral tegmental area.
As mentioned in Chapter 1, the primary raw materials for neurotransmitter synthesis in the brain are nutrients: amino acids, vitamins, minerals, and other natural biochemicals. The levels of these ingredients are well-regulated for most persons, but gene abnormalities can result in a nutrient deficiency or overload. The net result can be an abnormal neurotransmitter concentration and a serious mental illness. Psychiatric medications can be effective in coping with neurotransmitter imbalances. However, as we have emphasized, advanced nutrient therapy to promote normalization of neurotransmitter levels in the brain can also be effective, with the advantage of reduced adverse side effects.
Database Studies, Early Nutrient Therapies, and Beyond: Examples from Schizophrenia
Canadian psychiatrist Abram Hoffer25 was an early pioneer in nutrient therapy for schizophrenic patients. In 1951, Hoffer and his colleague Humphrey Osmond began experimenting with high doses of niacin and reported major reductions in auditory hallucinations and other schizophrenia symptoms. They conducted six double-blind, placebo-controlled experiments that yielded impressive evidence of improvement in the niacin group. Best results were reported for young schizophrenics, with lesser efficacy for chronic patients. After many years of research, Hoffer recommended a protocol involving the combined use of niacin, folic acid, vitamin B-12, vitamin C, essential oils, and special diets for schizophrenic patients.
Dr. Hoffer theorized that schizophrenia resulted from excessive levels of adrenaline and adenochrome (breakdown products of dopamine and norepinephrine), and his therapy was aimed at normalizing brain levels of these chemicals. However, new epigenetics research indicates that folates and niacin can powerfully reduce dopamine activity by enhancing acetylation of histones (see Chapter 4). This mechanism may be responsible for the thousands of reports of benefits from Hoffer’s protocols. In collaboration with Dr. Carl Pfeiffer, Hoffer’s group also discovered a condition termed pyroluria or the mauve factor that is the dominant imbalance in about 20% of schizophrenics. They identified pyroluria as an inborn disorder resulting in severe deficiencies of zinc and vitamin B-6. Hoffer’s therapy approach became known throughout the world and was used by hundreds of doctors over the next 20 years. In 1973, an American Psychiatric Association (APA) task force reviewed a large number of published, controlled studies and concluded that Hoffer’s claims of efficacy were not justified.26 This finding was very controversial and continues to be debated today. Hoffer’s nutrient therapy has never been accepted by mainstream medicine, but doctors throughout the world continue to use his methods. This modality is still championed by the International Society of Orthomolecular Medicine and the Journal of Orthomolecular Medicine.
The possible role of pyrroles in mental health was identified more than 50 years ago when researchers noticed that urine samples of certain psychotic subjects developed a reddish-purple or mauve color. When these individuals were found to possess similar traits and symptoms, the mauve factor became a subject of active research. Hoffer and Pfeiffer reported that more than 20% of schizophrenics exhibited mauve urine. In collaboration, their research groups isolated the mauve chemical and found it to be a pyrrole. For many years, this chemical was misidentified as kryptopyrrole and the medical condition termed pyroluria. The actual source of the mauve coloration is the complex chemical hydroxyhemopyrrolin-2-one (HPL). The syndrome of elevated pyrroles is now termed pyrrole disorder or mauve. In 2006, McGinnis and colleagues published an extensive review of pyrrole chemistry and its role in mental health.27
Hoffer’s early work inspired a prominent American doctor, Carl Pfeiffer, MD, PhD, to study the role of nutrients in mental illness. While working at a research hospital in the late 1950s, he discovered abnormal blood chemistry in a catatonic patient who had been nearly motionless for months. Pfeiffer treated the man with an intravenous cocktail of amino acids, vitamins, and minerals, and within seven days, the man experienced a near-complete recovery. The hospital instituted a study to determine the reason for his sudden ability to walk, talk, and behave normally but concluded that Pfeiffer’s nutrient therapy had no role in his improvement. The patient voluntarily stopped Pfeiffer’s nutrients and within two weeks returned to his catatonic state. Pfeiffer then cycled the man in and out of his catatonia several times, but his medical director refused to believe that nutrients were responsible. This was the beginning of Pfeiffer’s difficulties with mainstream medicine, and it launched him on a new career researching the use of nutrient therapies for mental illness. Pfeiffer eventually concentrated his efforts on schizophrenia and founded the Princeton Brain Bio Center in Skillman, New Jersey.
Pfeiffer evaluated more than 20,000 schizophrenics and developed the world’s largest chemistry database for this condition. His greatest contribution was the discovery of individual schizophrenia biochemical types, each with distinctive symptoms and blood/urine chemistries.28-29 This finding was in harmony with the widespread belief that schizophrenia is an umbrella term that includes several different mental disorders. Pfeiffer identified body chemistries and symptoms and recommended individualized nutrient therapies for each biotype. He reported that 90% of schizophrenics fit into one of the three major biochemical types that he called histapenia, histadelia, and pyroluria, with an additional 4% suffering from wheat gluten allergy. He also identified several low-incidence disorders that could cause schizophrenia symptoms, including porphyria, homocysteinuria, hypothyroidism, and polydipsia.
Pfeiffer believed that histamine deficiency (histapenia) and copper overload were responsible for classic paranoid schizophrenia that usually involved auditory hallucinations. He treated this condition with folic acid, vitamin B-12, niacin, zinc, and augmenting nutrients. In contrast, Pfeiffer’s histadelia (histamine overload) biotype typically involved delusions or catatonic behaviors that he treated with methionine, calcium, and sometimes with antihistamines. Another 20% of schizophrenia patients fit into Pfeiffer’s pyroluria biotype that was often characterized by both auditory hallucinations and delusions. Pfeiffer treated the pyrolurics with strong doses of vitamin B-6 and zinc.
Histamine is a neurotransmitter, and Pfeiffer believed that abnormal histamine levels were the underlying cause of most cases of schizophrenia. His treatments concentrated on normalizing histamine levels in the brain. In the early 1990s, I amassed evidence that methyl and folate levels had a far greater impact on mental health than histamine and concluded that histamine was not a decisive factor in schizophrenia. For example, hundreds of patients achieved impressive recoveries while their abnormal histamine levels remained unchanged. It appears that Pfeiffer developed effective nutrient therapies but proposed the wrong theory to explain their efficacy. Today, blood histamine is used as a marker for methylation status but not for mental status. Histamine and methyl groups are present in measurable levels throughout the body and an inverse relationship exists between them. Eventually, Pfeiffer’s terms histadelia and histapenia were replaced by undermethylation and overmethylation, with the understanding that serotonin, dopamine, and norepinephrine activities are powerfully influenced by methyl status. However, this approach did not explain why a powerful methylating treatment (folic acid and vitamin B-12) caused dramatic worsening in undermethylated schizophrenics. This problem was resolved in 2009 with the discovery that methyl and folate have opposite epigenetic impacts on neurotransmitter reuptake at synapses.
The activities of serotonin, dopamine, and norepinephrine neurotransmitters in the brain are dominated by transporters present in the membranes of presynaptic brain cells. You may recall from our earlier discussion that the transporters enable the return of neurotransmitters to cells after they are sprayed into a synapse. This process is called reuptake, and most modern psychiatric drugs are aimed at altering transporter function. The genetic expression of transporters is inhibited by methylation and enhanced by acetylation, processes which are described in Chapter 4. Acetylation is the process of adding an acetyl group (CH3CO) to a molecule. The relative amounts of methyl and acetyl attached to DNA and histone tails impact the synaptic concentration of reuptake proteins and the activity of dopamine, serotonin, and norepinephrine. By different mechanisms, folates and niacin promote dominance of acetylation at DNA and histones (see Chapter 4). Methionine and SAMe produce the opposite effect by promoting methylation of DNA and histones. The net result is that activities of serotonin, dopamine, and other neurotransmitters are strongly influenced by the methyl/folate ratio. After 25 years of searching, we finally have a convincing explanation for the apparent effectiveness of folate, niacin, and methylation therapies developed by Abram Hoffer and Carl Pfeiffer.
Dr. Pfeiffer28-29 and I30 studied more than 30,000 patients with a diagnosis of mental illness and an additional 15,000 with a behavioral disorder (BD), ADHD, or autism. These evaluations have generated millions of laboratory analyses measuring the concentrations of biochemical factors in blood, urine, and tissues. Comparison with known normal levels for healthy persons reveals a very high incidence of chemical imbalances in these clinical populations. Considering the vast amounts of data, it is extremely unlikely that this is a coincidence.
Thousands of Pfeiffer’s schizophrenic patients reported improvement, but the absence of published controlled studies measuring treatment effectiveness of Pfeiffer’s nutrient protocols prevented acceptance of his protocols by mainstream medicine. Prior to his death in 1988, Pfeiffer was nominated for a Nobel Prize (which he didn’t win) and was widely regarded as the world’s leading expert in biochemical therapy. Pfeiffer’s nonprofit Princeton Brain Bio Center experienced financial problems in the 1990s and no longer exists.
The work of Carl Pfeiffer has been continued by several doctors and clinics in the two decades since his death. Dr. Pfeiffer trained numerous physicians in his protocols, and these therapies are still used by complementary medicine practitioners throughout the world.
I had the privilege of collaborating with Pfeiffer in the evaluation of 500 patients diagnosed with behavioral disorders or mental illness over a 12-year period. After a few years of prodding by Dr. Pfeiffer, I founded the nonprofit Pfeiffer Treatment Center in Illinois, which treated more than 25,000 patients, including thousands diagnosed with schizophrenia. In the early years, the Pfeiffer Treatment Center treatment methods duplicated the lab testing, medical history, diagnosis, and treatment protocols of the Princeton Brain Bio Center. In subsequent years, we introduced improvements as brain science progressed and the impact of key nutrients on neurotransmitter activity became clearer. In 2008, I left the Pfeiffer Treatment Center to develop an international physician training program. Sadly, the Pfeiffer Treatment Center experienced financial problems and closed its doors in July 2011. Fortunately, there are many talented physicians throughout the world who are now quite expert in these treatment protocols.
Coping with Biochemical Imbalances
Longitudinal studies over the past 30 years indicate that a person’s biochemical tendencies persist throughout life, suggesting that these tendencies are genetic or epigenetic in origin. In many cases, symptoms of a particular imbalance have been clearly evident since age two, for example, as in the case of cruelty to childhood pets. The impact on a person’s life depends on the severity of the chemical imbalance and on exposure to environmental factors. For example, a child with a mild tendency for aggressive behavior may develop normally if there is a good diet, an absence of serious traumatic events, and a nurturing family. However, a child born with the severe chemistry observed in serial killers (see Chapter 8) is likely to become a criminal unless brain chemistry is corrected. Counseling and a good environment may be effective in mild-to-moderate behavioral disorders, but a severe chemical imbalance cannot be loved away, and treatment must focus on correcting brain chemistry. Similarly, a mild genetic tendency for depression may be overcome by factors such as a good environment, exercise, and counseling, whereas a severe tendency may require aggressive biochemical intervention. All of these patients are good candidates for individualized nutrient therapy.
The Repeat Offenders
For several years I was perplexed by the repeated presence of certain biochemical imbalances in completely different mental disorders. For example, copper overload is present in most cases of hyperactivity, learning disability, postpartum depression, autism, and paranoid schizophrenia. In another example, undermethylation is often present in antisocial personality disorder, clinical depression, anorexia, obsessive-compulsive disorder, and schizoaffective disorder. The primary repeat offenders are the following:
 Copper overload
Copper overload
 Vitamin B-6 deficiency
Vitamin B-6 deficiency
 Zinc deficiency
Zinc deficiency
 Methyl/folate imbalances
Methyl/folate imbalances
 Oxidative stress overload
Oxidative stress overload
 Amino acid imbalances
Amino acid imbalances
Eventually, I realized these factors had something in common—a direct role in the synthesis or functioning of a major neurotransmitter. It seems most unlikely that this is a coincidence. The situation is complicated since a mental illness may involve more than one imbalance. For example, antisocial personality disorder associated with criminality usually involves the combination of zinc deficiency, oxidative overload, undermethylation, and elevated toxic metals. Paranoid schizophrenia usually involves overmethylation, folate deficiency, and elevated blood copper. Because of genetic variations, a particular chemical imbalance may have a variety of outcomes for different persons. Each of these offending chemical imbalances can be effectively treated without the use of psychiatric medications.
Remediating the Repeat Offenders
Copper overload
In the early 1800s, W. Meissner31 identified the presence of copper in all forms of life. We now know that copper plays an important role in the synthesis of neurotransmitters,32 respiration, immune function, energy metabolism, and growth. In most persons, blood copper levels are kept in a narrow range through the action of metallothionein (MT), ceruloplasmin, and other proteins. Unfortunately, many persons have a genetic inability to regulate copper levels and a serious copper overload can result.
Copper is a cofactor in the synthesis of norepinephrine, a neurotransmitter associated with several mental illnesses.32 Norepinephrine is formed in the brain by the addition of a hydroxyl group to a dopamine molecule as shown in Figure 3-1. This reaction occurs in dopamine storage vesicles and is enabled by the enzyme dopamine β-hydroxylase (DBH) together with doubly-charged copper ions (Cu++), vitamin C, and O2 cofactors. DBH is a complex molecule containing 576 amino acids that binds to several copper ions. Vitamin C protects the DBH enzyme from oxidative reactions and supplies electrons for the reaction. The O2 cofactor provides the oxygen atom for creation of the hydroxyl group.
Figure 3-1. Synthesis of Norepinephrine
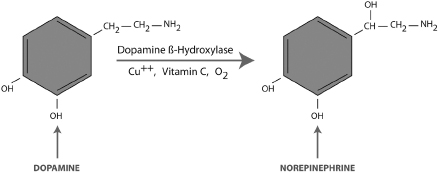
Copper overloads tend to lower dopamine levels and increase norepinephrine in the brain. Imbalances in these important neurotransmitters have been associated with paranoid schizophrenia, bipolar disorder, postpartum depression, ADHD, autism, and violent behavior. In two separate animal studies, a diet that lowered blood copper levels by 75% had a massive effect on norepinephrine and dopamine levels in the brain.33-34
Most persons with elevated blood copper also exhibit depressed zinc and excessive oxidative stress. In healthy persons, copper levels are regulated by MT and other proteins that bind to excess copper and carry it out of the body. However, MT activity can be significantly reduced by either zinc deficiency or elevated oxidative stress. Many persons diagnosed with mental illness have an inborn tendency for elevated copper levels, and this predisposes them to psychiatric disorders. Nutrient therapy to normalize copper levels can be effective in balancing dopamine and norepinephrine levels for these persons. This treatment approach is inexpensive and relatively free of side effects when administered properly.
Vitamin B-6 deficiency
Vitamin B-6 concentrations in the brain are about 100 times higher than levels in blood, and this nutrient has important roles in mental functioning. Severe deficiency of vitamin B-635-36 has been associated with irritability, depression, poor short-term memory, and psychosis. This is not surprising since it is required for efficient synthesis of serotonin,37 dopamine,38 and GABA,39 three critically important neurotransmitters. There are three different chemical forms of B-6, the most common being pyridoxine hydrochloride, which converts to pyridoxal-5-phosphate (PLP, which is also known as P5P), the activated form of B-6 in the body and brain.
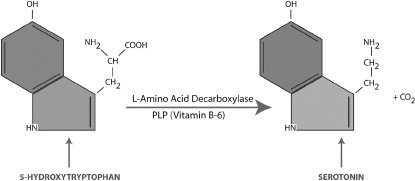
PLP is a strong aldehyde that has the ability to remove carboxyl groups (COOH) from molecules. An important example is the conversion of 5-HTP to serotonin (5-HT). Figure 3-2 shows the final step in the synthesis of serotonin that involves PLP as a coenzyme. In this reaction, PLP links to the enzyme aromatic L-amino-acid decarboxylase (AADC) and enables the removal of the OH- group from 5-HTP. Persons with a genetic or acquired B-6 deficiency tend to produce insufficient amounts of brain serotonin and are prone to clinical depression, OCD, and other mental problems. SSRI antidepressant medications, as an example, can increase serotonin activity in these persons, but nutrient therapy to normalize brain levels of vitamin B-6 and PLP may achieve the same result.
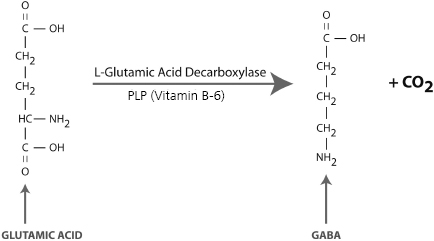
Vitamin B-6 in the form of PLP is also required for the synthesis of dopamine and GABA in the brain (Figures 3-3 and 3-4). A genetic or acquired deficiency of B-6 can result in abnormally low levels of these important neurotransmitters and a myriad of problems, including ADHD, depression, anxiety, and sleep disorders. A severe deficiency of B-6 can contribute to depressed levels of these neurotransmitters, resulting in mental disorders that are commonly treated with psychiatric medications such as amphetamines, SSRIs, or benzodiazapines (e.g., Xanax). Vitamin therapy to normalize B-6 levels can provide treatment benefits and, in some cases, may eliminate the need for medication altogether.
Figure 3-2. Synthesis of Serotonin
In addition to the production of neurotransmitters, vitamin B-6 is involved in more than 80 biochemical reactions in the body. Vitamin B-6 deficiency can result in a wide variety of physical symptoms that are often vague and difficult to diagnose. These may include nervousness, insomnia, muscle weakness, and difficulty walking. The gold standard test for B-6 status is the transaminase stimulation blood test.40 However, most B-6 deficient persons exhibit elevated pyrroles that can be detected by an inexpensive urine test.27 Since B-6 deficiency is often caused by genetics, very high dosages may be necessary to normalize levels in the bloodstream and brain.
Figure 3-3. Synthesis of Dopamine
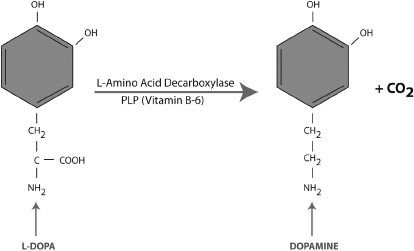
Figure 3-4. Synthesis of GABA
Overdoses of B-6 can cause neuropathy with loss of sensation in areas of the skin. However, this side effect is temporary and can be reversed by reducing B-6 intake. Another common symptom of B-6 overload is the onset of extremely troubling dreams. Persons who have a tendency for B-6 deficiency may tolerate very high dosages, but persons with B-6 sufficiency may react adversely to modest doses. In the mid-1980s, Dr. Carl Pfeiffer and I discovered that many slender malabsorbers diagnosed with schizophrenia failed to respond to the standard form of B-6 (pyridoxine hydrochloride) but improved significantly after receiving supplements of PLP. We later found that other patients responded better to the standard form of B-6. By the late 1980s, we began using a combination of standard B-6 together with PLP that appeared to benefit nearly all types of B-6 deficient patients, and this practice is still in use today.
Zinc deficiency
Zinc is a trace metal essential to all forms of life.41 Most healthy human beings receive all the zinc they need from their diet. Absorption is usually very efficient, with about 38% of zinc in foods passing into the bloodstream. This dietary zinc is transported to the liver by albumin, transferrin, and L-histidine proteins in the portal bloodstream. Once in the liver, most of the zinc is converted to zinc metallothionein that acts as a chaperone carrying zinc to cells throughout the body. Zinc is quite nontoxic when bound to a protein, and cases of zinc overload and poisoning are extremely rare.
Zinc deficiency is by far the most frequently observed chemical imbalance in mental health populations. More than 90% of persons diagnosed with depression, behavioral disorders, ADHD, autism, and schizophrenia exhibit depleted plasma zinc levels, ranging from low-normal to severe deficiency. One explanation for this curious fact is that most mental disorders involve oxidative stresses (described in Appendix B) that deplete zinc stores in the body. In addition, zinc has a special role in activation and inhibition of NMDA receptors that are essential to good mental health.
Zinc deficiency has been associated with delayed growth, temper control problems, poor immune function, depression, poor wound healing, epilepsy, anxiety, neurodegenerative disorders, hormone imbalances, and learning problems. Zinc is a component of more than 200 enzymes and is present in RNA polymerase, zinc fingers, and other special proteins that have key roles in cell division and genetic expression. Zinc has many important roles in brain function, which include the following:
 Zinc metallothionein is a key component of the blood-brain barrier that prevents harmful chemicals from entering the brain.
Zinc metallothionein is a key component of the blood-brain barrier that prevents harmful chemicals from entering the brain.
 Zinc proteins in the brain combat oxidative free radicals that could destroy brain cells, harm the myelin sheath, and alter neurotransmitter levels.
Zinc proteins in the brain combat oxidative free radicals that could destroy brain cells, harm the myelin sheath, and alter neurotransmitter levels.
 Zinc is required for the efficient conversion of dietary B-6 into PLP, which is needed for efficient synthesis of serotonin, dopamine, GABA, and other neurotransmitters.
Zinc is required for the efficient conversion of dietary B-6 into PLP, which is needed for efficient synthesis of serotonin, dopamine, GABA, and other neurotransmitters.
 Zinc deficiency can cause copper overloads that can alter brain levels of dopamine and norepinephrine.
Zinc deficiency can cause copper overloads that can alter brain levels of dopamine and norepinephrine.
 Zinc deficiency results in altered brain levels of GABA.
Zinc deficiency results in altered brain levels of GABA.
 Zinc is a neurotransmitter that is stored in vesicles and ejected into synapses.
Zinc is a neurotransmitter that is stored in vesicles and ejected into synapses.
 Zinc has a special role in the activation and inhibition of NMDA receptors.
Zinc has a special role in the activation and inhibition of NMDA receptors.
Genetic or acquired zinc deficiency can usually be corrected within two months using nutrient therapy. This treatment must be done gradually for persons exhibiting serious overloads of toxic metals or copper in order to prevent temporary blood elevation of toxins as they depart the body. Increasing blood zinc levels results in higher production of MT and other zinc-bearing proteins that drive toxins out of the body. Special caution must be taken for persons with a cadmium overload since rapid removal can damage kidney tubules.
Many neuroscientists regard zinc as unimportant in mental illness for the following reasons:
 Most persons receive sufficient zinc from their diet.
Most persons receive sufficient zinc from their diet.
 Homeostatic processes regulate blood zinc levels in most humans.
Homeostatic processes regulate blood zinc levels in most humans.
 Zinc is not directly involved in rate-controlling steps in the synthesis of most neurotransmitters.
Zinc is not directly involved in rate-controlling steps in the synthesis of most neurotransmitters.
However, millions of persons are born with a genetic tendency for severe zinc depletion that can disrupt brain chemistry and mental functioning. In working with thousands of violent children, we learned that most families report significant improvement once zinc levels are normalized. I believe lab testing for plasma zinc should be mandatory for all patients diagnosed with a behavioral disorder, ADHD, autism, or a mental illness.
Methyl/folate imbalances
In the 1960s, Abram Hoffer and colleagues proposed an adenochrome theory to explain auditory hallucinations. In the 1970s, Carl Pfeiffer published a theory that abnormal concentrations of brain histamine were responsible for paranoid schizophrenia and delusional disorders. In each case, subsequent research has failed to support the proposed theory. Medical history provides many examples of therapies that were highly effective many years before the correct scientific explanation was known.
My clinical experience with thousands of patients revealed the powerful impact of methylation status on several mental disorders.42 I learned that low-serotonin depressives thrive on methylating agents such as methionine or SAMe but are intolerant to folates. In contrast, patients with excessive activity of dopamine and norepinephrine (for example, classic paranoid schizophrenia) thrive on folic acid and react adversely to SAMe and methionine. For years I was perplexed by the well-known fact that folic acid supplements increase methylation. The question has been “Why are undermethylated mentally ill patients intolerant to folates?” I spent many fruitless years investigating the impact of methyl and folates on neurotransmitter synthesis, including genetic expression of enzymes needed for neurotransmitter production. Eventually, I learned that the solution to this mystery lies in epigenetics and the factors that promote or inhibit gene expression of transporters at the synapse.
Mainstream psychiatry learned decades ago that drug treatments aimed at normalizing levels of brain chemicals such as serotonin, dopamine, etc. were relatively slow and ineffective. In contrast, medications that impact neurotransmitter activities at synapses were much more potent.43 In early studies of depression, considerable attention was given to inhibiting proteins that destroy (metabolize) serotonin in the synapse. Monoamine oxidase is an enzyme that breaks down serotonin, and MAOI medications have been prescribed for depression since the 1970s. However, brain scientists eventually learned that the dominant factor at the synapse is the action of transporters, the special proteins that sweep neurotransmitters away from the synapse and return them back into the original brain cell. This reuptake process, previously introduced in Chapter 1, is the dominant factor at serotonin, dopamine, and norepinephrine synapses. This has been the basis for SSRI antidepressants, which bind to transporters and inactivate them.
There is little doubt that antidepressants can alleviate clinical depression in low-serotonin individuals. However, certain nutrient factors also can powerfully impact serotonin activity, and individualized nutrient therapy has the potential for normalizing serotonin activity and eliminating depression without significant side effects.
Abnormalities in methylation and folate chemistry are common in schizophrenia, bipolar disorder, depression, anxiety, and certain behavioral disorders.44 Nearly all low-serotonin depressives tend to be deficient in methionine and SAMe and react adversely to folic acid. In another example, high-dopamine schizophrenics respond nicely to folate therapy and deteriorate if treated with SSRIs or non-folate methylating supplements. This curious phenomenon results from the epigenetic impact of methyl and folates on the production of transporters that control synaptic activity. As described in Chapter 4, folate deficiency results in reduced production of transporters and elevated synaptic activity. Undermethylation has the opposite effect, resulting in excessive gene expression of transporters and reduced synaptic activity.
With respect to mental health, the nutrient SAMe is a natural reuptake inhibitor for serotonin, dopamine, and norepinephrine. Folic acid is a natural reuptake enhancer that can combat excessive dopamine activity. The individual levels of methyl and folate in the brain are not as important as the methyl/folate ratio. Genetic or acquired imbalances in methyl and folate may be responsible for more than 50% of all mental illness. Psychiatric drugs may produce benefits for these persons, but nutrient therapy to balance methyl and folate levels provides a more scientific and direct approach that can avoid unpleasant side effects.
Nutrient therapy to normalize methyl and folate levels has resulted in thousands of reports of great improvement in patients afflicted by psychosis, depression, anxiety, and other illnesses. However, anecdotal case histories are poorly regarded by medical and scientific experts, and double-blind, placebo-controlled studies to measure treatment effectiveness will be necessary before this treatment approach can be embraced by mainstream psychiatry.
Oxidative stress
The role of oxidative stress in schizophrenia was first noticed when researchers in the 1960s observed elevated pyrrole levels in these patients. Pyrrole is a natural organic chemical containing a five-membered ring with the formula C4H4NH.45 The term pyrrole is also used for several organic compounds containing a pyrrole ring. Pyrroles are involved in the synthesis of heme, the primary constituent of hemoglobin. Except for a role in the production of biochemicals, pyrroles are of minor importance and are efficiently excreted in urine. They have an affinity for binding with PLP and zinc, resulting in these valuable nutrients being transported out of the body together with the pyrroles. This process is a normal phenomenon for all human beings. However, some persons have a genetic (or acquired) tendency for very elevated levels of pyrroles, which can result in a deficiency of both PLP and zinc.
The alteration of neurotransmitter levels and mental functioning resulting from deficiency of either B-6 or zinc was described earlier in this chapter. Since pyrrole disorders involve simultaneous deficiency of both nutrients, behavioral and physical symptoms are generally more severe in this population. Classic symptoms of pyrrole disorder include high anxiety, frequent mood swings, poor short-term memory, reading disorder, morning nausea, absence of dream recall, and frequent anger and rages.
My colleagues and I have evaluated more than 40,000 urine pyrrole results for persons with a mental illness. Table 3-1 presents the incidence of elevated urine pyrroles (greater than 20 mcg/dl) in different clinical populations. As seen in the table, the incidence of pyrrole disorders is much higher in psychiatric disorders than in the general population. Most mental disorders involve oxidative stress, and elevated pyrroles may be secondary to a number of other biochemical conditions. However, psychiatric symptoms often recede or disappear after B-6 and zinc therapy and normalization of pyrrole levels. A genetic pyrrole disorder can result in low serotonin and GABA levels, and SSRI antidepressants and anti-anxiety medications often provide significant benefits. However, as is the case with other examples we’ve cited, nutrient therapy to normalize B-6 and zinc levels may provide similar benefits without unpleasant side effects.
Persons born with pyrrole disorder may have a lifetime tendency for deficiencies of B-6, zinc, and for high oxidative stress. A complication is that any source of oxidative stress can elevate urinary pyrrole levels. Many persons have elevated pyrroles resulting from factors such as physical accidents, illnesses, infections, emotional trauma, and toxic metals. Oxidative overloads from any source can cause psychosis in sensitive individuals by lowering glutamate neurotransmitter activity at NMDA receptors in the brain. Oxidative stresses deplete levels of glutathione (GSH) needed for efficient NMDA function. In schizophrenia, the term oxidative stress overload is more general and descriptive than the descriptors pyroluria, mauve factor, and pyrrole disorder. Biochemical markers for this condition include elevated urine pyrroles, low plasma zinc, depressed serum glutathione, elevated non-ceruloplasmin serum copper, and many others.
Table 3-1.
Incidence of Pyrrole Overload in Clinical Populations
| ADHD | 18 % |
| Behavioral Disorder | 28 % |
| Autism | 35 % |
| Depression | 24 % |
| Bipolar Disorder | 35 % |
| Schizophrenia | 30 % |
| Post-Traumatic Stress | 12 % |
| Alzheimer’s Disease | 14 % |
| Healthy Controls | 8 % |
Amino acid disorders
A number of amino acids have important roles in brain chemistry.44 Most human beings receive ample amounts of these amino acids from dietary protein and have proper concentrations in the brain. However, there are genetic errors that can alter the amounts of these chemicals in the brain and adversely impact mental functioning. Fortunately, this is a relatively rare occurrence. The roles of amino acids in brain chemistry are summarized below:
 Tryptophan is the initial starting point (substrate) for the synthesis of serotonin. For many years, tryptophan supplements have been used in an attempt to increase serotonin levels in the brain.
Tryptophan is the initial starting point (substrate) for the synthesis of serotonin. For many years, tryptophan supplements have been used in an attempt to increase serotonin levels in the brain.
 Dopamine and norepinephrine are synthesized from either phenylalanine or tyrosine, and supplements of these amino acids may assist in elevating levels of these neurotransmitters.
Dopamine and norepinephrine are synthesized from either phenylalanine or tyrosine, and supplements of these amino acids may assist in elevating levels of these neurotransmitters.
 Glutamine is an amino acid that is a substrate for glutamic acid and the neurotransmitter GABA.
Glutamine is an amino acid that is a substrate for glutamic acid and the neurotransmitter GABA.
 GABA is both an amino acid and a neurotransmitter, and depressed levels have been associated with anxiety, depression, and psychosis.
GABA is both an amino acid and a neurotransmitter, and depressed levels have been associated with anxiety, depression, and psychosis.
 Aspartate is the starting point for the synthesis of the neurotransmitter aspartic acid.
Aspartate is the starting point for the synthesis of the neurotransmitter aspartic acid.
 L-histidine is a precursor of the neurotransmitter histamine.
L-histidine is a precursor of the neurotransmitter histamine.
 Methionine is the precursor for SAMe that has a strong influence on genetic expression of several enzymes required for neurotransmitter synthesis and reuptake.
Methionine is the precursor for SAMe that has a strong influence on genetic expression of several enzymes required for neurotransmitter synthesis and reuptake.
Fatty acid imbalance
There are more than 300 different fats in the human body, and the brain has a very high fat content (about 65%). Unsaturated fatty acids are especially important because they provide fluidity to cell membranes and assist in communication between brain cells. At brain synapses where the action is, four fats make up more than 90% of the lipid content: docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), arachidonic acid (AA), and dihomo-gamma-linolenic acid (DGLA). Of these, DHA (an omega-3 fatty acid) is found in the highest concentration and appears to have the greatest impact on brain function. DHA deficiency has been associated with depression, ADHD, schizophrenia, bipolar disorder, and dementia. Second in importance may be EPA, another omega-3 fatty acid. DHA and EPA are essential fatty acids (EFAs) that have become very popular nutritional supplements. Seafood and fish oils are excellent omega-3 dietary sources. AA and DGLA are omega-6 EFAs and are at excessive levels in most persons who consume a junk food diet. The ideal proportion of EFAs in the diet is considered by nutritionists to be between 3 and 6 grams of omega-6 for each gram of omega-3. Unfortunately, a typical American diet contains an excessive amount of omega-6 and other unhealthy fats.
Because of genetic variations, there is biochemical individuality with respect to the ideal dietary intake of specific fatty acids, and indiscriminant use of omega-3 supplements could worsen symptoms for certain patients. For example, most persons with severe pyrrole disorder have sufficient levels of omega-3 oils but are very deficient in AA. In my experience, these patients thrive on primrose oil, which is rich in omega-6; however, they may deteriorate if they supplement with omega-3 alone. In contrast, most depressives and schizophrenics are deficient in DHA and EPA and thrive on omega-3 supplements.
Most mental disorders involve elevated oxidative stress that can be quite lethal to EFAs. Fortunately, phosphatidyls are fatty acids that provide a safe haven for DHA, EPA, AA, and DGLA in the presence of oxidative free radicals. The four major phosphatidyls have choline, serine, inositol, or ethanolamine attached to the end of the molecule. Several phosphotidyls are available commercially and can assist in combating certain mental illnesses.
Glucose dysregulation
Our database indicates a significant number of patients exhibit chronic low blood glucose levels. This problem doesn’t appear to be the cause of behavioral disorders or mental illnesses, but it is instead an aggravating factor that can trigger striking symptoms. Typical symptoms include drowsiness after meals, irritability, craving for sweets, trembling, anxiety, and intermittent poor concentration and focus. Treatment includes chromium, manganese, and other glucose-stabilizing nutrients, but the primary focus of treatment is on diet. These patients benefit from six or more small meals daily with emphasis on complex carbohydrates and protein. In essence, they cannot tolerate large meals or quick sugars. Complex carbohydrates provide the necessary glucose in a slow, gradual manner and may be thought of as timed-release sugar.
Toxic overload
My database includes many patients who exhibit elevated levels of toxic metals, pesticides, or other organic chemicals. Overloads of lead, mercury, and cadmium are especially common. Persons with depressed levels of zinc, glutathione, selenium, or metallothionein are especially sensitive to toxic metals. A high percentage of overmethylated mental patients exhibit severe sensitivities to pesticides and toxic industrial chemicals. Effective treatment of a toxic overload requires a three-part approach:
 Avoidance of additional exposures
Avoidance of additional exposures
 Biochemical treatment to hasten the exit of the toxins from the body
Biochemical treatment to hasten the exit of the toxins from the body
 Correction of underlying chemical imbalances to minimize future vulnerability to toxins
Correction of underlying chemical imbalances to minimize future vulnerability to toxins
Malabsorption
Although only 10% of mental illness cases involve serious malabsorption, more than 90% of autistics exhibit this problem. There are three primary classes of absorption problems:
 Stomach problems, including excessive or insufficient levels of hydrochloric acid
Stomach problems, including excessive or insufficient levels of hydrochloric acid
 Incomplete digestion in the small intestine
Incomplete digestion in the small intestine
 Problems at the brush-border of the intestine where most nutrients are absorbed into the portal blood stream
Problems at the brush-border of the intestine where most nutrients are absorbed into the portal blood stream
The consequences can include nutrient deficiencies, inflammation in the intestinal tract, Candida, and many other gastrointestinal (GI) disorders. Incomplete breakdown of protein and fats can cause physical problems and also adversely affect brain function. Elevated oxidative stress can destroy digestive enzymes needed for processing protein and is a frequent cause of malabsorption. A high percentage of malabsorbing patients have a compromised and, thus, ineffective intestinal barrier, allowing toxic metals and other undesirable substances to enter the body and access the brain. Treatment depends on the type of malabsorption present and may involve the adjustment of stomach acid levels, administration of digestive enzymes that survive stomach acid, administration of antioxidants, and use of special diets.
Other Nutrient Imbalances
Many other nutrients play a significant role in mental functioning. Selenium, vitamin C, vitamin E, and other natural antioxidants combat inflammation and free radicals in the brain and indirectly increase glutamate activity at NMDA receptors. Vitamin D deficiency has been associated with depression, schizophrenia, ADHD, and other mental disorders. Vitamin D levels increase during exposure to sunlight, and it’s not a surprise that northern Scandinavia, with its relatively low amount of sunlight, has an extremely high incidence of schizophrenia.
The Interface with Psychiatric Medication
Most schizophrenic patients are taking psychiatric medication at the time of the first appointment and express a desire to discontinue the drugs. However, the medication usually has produced definite benefits, and we urge continued compliance during the initial stages of biochemical therapy. If clear progress is achieved after several months of both therapies together, then we suggest the family return to their psychiatrist for a cautious trial of reduced medication dosages. The goal is not to eliminate psychiatric medication but to identify the dosage needed for maximum benefits.
Our internal outcome studies indicate that more than 70% of behavior, ADHD, and depression patients report they are at their best with zero medication after six months of biochemical therapy. The remaining 30% state that some medication support is needed to prevent a partial return of symptoms. In nearly all cases, medication dosages can safely be reduced with lessened side effects. The situation is very different for patients diagnosed with schizophrenia or bipolar disorder, with only 5% able to completely discontinue psychiatric medication after successful biochemical therapy. Many of these patients report elimination of psychosis and a return to independent living after a combination of nutrient therapy and greatly reduced medication levels. Reduction of side effects often increases a patient’s willingness to take medication and eliminates the dire consequences that can result from sudden noncompliance. In general, psychiatric medications appear to be much more effective after nutrient imbalances have been corrected. The two therapeutic approaches clearly are in harmony with each other.
For the past several decades, treatment of mental illness has focused on imbalanced brain chemistry and the use of drugs to alter the activity of serotonin, dopamine, and other neurotransmitters. Back in 1970, drugs were the only method known to have a powerful impact on molecular processes in the brain. This approach proved to be highly successful and has benefited millions of persons diagnosed with depression, schizophrenia, ADHD and other mental disorders. However, science has made great advances in the understanding of complex brain processes, and we are approaching an era in which psychiatric medications may no longer be necessary. It is time to move aggressively in the development of epigenetic therapies and other advanced techniques that have the potential to normalize brain function without drugs or significant adverse side effects.
Nutrient Therapy Response Times
Nutrient therapy can have a powerful impact on mental functioning, but several weeks or months are usually needed to achieve the full effect. In contrast, most psychiatric medications can affect symptoms within a few hours or days. For example, SSRI antidepressants quickly bind to transporters, resulting in a rapid increase in serotonin activity at the synapse. Methylation therapy for depression using SAMe or methionine reduces genetic expression of transporters, resulting in a slow and gradual increase in serotonin activity over a period of several months. In another example, nutrient therapy to eliminate copper overloads in the blood usually requires about 60 days.
The chemical imbalance with the fastest response is pyrrole disorder, with significant progress often reported during the first week. This rapid response results from the ability to normalize vitamin B-6 levels in a short time. Zinc deficiency usually can be corrected within 60 days. Treatment of overmethylation usually entails no improvement the first two weeks, with gradual progress over the next four to eight weeks. Undermethylation is the slowest chemical imbalance to resolve, with three to nine months often required for the full effect. Patients should be informed of the expected treatment time frame associated with biochemical treatments so they won’t become discouraged by lack of immediate progress.
The Value of Counseling
I’ve encountered thousands of depressed and psychotic patients who have received major benefits from counseling. Psychodynamic therapies not only provide insight, coping mechanisms, and self-image repair, but also can have an enduring impact on gene expression. There is evidence that effective counseling can also promote development of new synapses and neuronal minicolumns, thereby permanently improving the microstructure of the brain. Correcting brain chemistry often is not enough, and counseling can enhance the benefits patients can enjoy. For example, behavior-disordered teens may have a negative self-image and poor habits that cannot be corrected by chemistry alone. Many anorexic patients have reported nice improvement from nutrient therapies but needed effective counseling to achieve complete recovery. Nutrient therapy and counseling are natural partners.
Chemical Classification of Mental Illnesses
Over the past 20 years, psychiatry has achieved remarkable progress in the understanding of complex brain processes. However, development of improved treatments for depression, schizophrenia, and other illnesses has been hindered by the failure to separate these disorders into meaningful phenotypes. Depression is an umbrella term used to describe a collection of disorders that have very different brain chemistries, symptoms, and traits. The same thing is true for the terms schizophrenia, bipolar disorder, and ADHD. For example, some depressives have low serotonin activity, but others exhibit elevated serotonin activity. SSRIs, the serotonin-enhancing medications, have shown efficacy for mixed groups of depressives because more than 50% of the depressives have low activity of this neurotransmitter. However, more than 30% of depressives are not low-serotonin patients, and for them an SSRI will likely be either neutral or harmful. The most common phenotype of schizophrenia involves elevated dopamine activity, and most current antipsychotic medications are aimed at lowering activity of this neurotransmitter. Unfortunately, this is the wrong approach for schizophrenics with different brain chemistry. Most efficacy experiments for depression and schizophrenia continue to involve a mixture of disorders with different brain chemistry imbalances. This blurs the data and weakens the scientific findings. Some psychiatry experts have recently urged researchers to develop objective tests for separating these illnesses into meaningful phenotypes.
This book presents biochemical classifications for depression and schizophrenia based on (a) more than one million blood and urine chemical assays, and (b) identification of distinctive symptoms and traits for each chemical type. To the extent possible with present knowledge, I have indicated the primary neurotransmitter imbalances for each of the depression and schizophrenia phenotypes in the following chapters. This technology can help psychiatrists identify promising psychiatric medications for different patients through inexpensive blood/urine testing. This information also provides a roadmap for the development of nutrient therapies for these disorders.