
CHAPTER 4
EPIGENETICS AND MENTAL HEALTH
Introduction
The new science of epigenetics46-47 has revolutionized our understanding of the brain and is leading to exciting breakthroughs in treatment of mental illnesses. Epigenetics is a rapidly growing field that investigates alterations in gene expression that do not involve changes in DNA sequence. Until recently, a person’s genetic characteristics were thought to be cast in concrete at the moment of conception, with a unique DNA sequence arising from a somewhat random collection of genetic factors from parents and ancestors. We now know this is only partially true and that the chemical environment in the womb can determine which genes are expressed and which are silenced in the various tissues and organs. In addition, environmental factors can alter genetic expression throughout life.
Many mental disorders result from environmental factors that cause genes to behave (or express themselves) improperly.48 For example, nutrient imbalances or toxic exposures can alter gene expression rates and may be the root cause of numerous psychiatric disorders. It’s not a coincidence that methylation is a dominant factor in epigenetics, and methylation abnormalities are common in mental illnesses. Recent advances in the science of epigenetics provide a roadmap for nutrient therapies that have potential for overcoming mental disorders and eventual elimination of the need for psychiatric medications.
Epigenetics 101
All cells in the human body contain an identical copy of DNA with the potential for producing more than 20,000 proteins. However, the proteins needed in liver cells are different from those of skin cells, pancreas cells, and so on. Epigenetics provides the blueprint that specifies the combination of proteins to be manufactured in each tissue.49 Without epigenetics, we could be an amorphous blob of identical cells instead of an organism with arms, eyeballs, teeth, and other diverse parts. Certain nutrients have a powerful role in determining which genes are expressed or silenced in different tissues, and proper balance between these nutrient factors is essential for good mental health.
DNA consists of billions of proteins that form a double-helix ribbon that would be about six feet in length if stretched out. This DNA is amazingly packed into a tiny ball that is about one-hundredth of a millimeter in diameter and neatly fits inside the nucleus of every cell. This fragile double helix is wrapped around tiny globs of protein called histones in a configuration known as beads on a string. The acidic DNA strand gently adheres to millions of histones which are slightly alkaline. The histone-DNA beads are called nucleosomes,50 and an array of nucleosomes is termed chromatin. Figure 4-1 is a schematic of a nucleosome. A histone consists of eight linear proteins clumped together like a ball of yarn, with several protein tails protruding from the array. The DNA ribbon wraps around each of the millions of histones slightly less than two times (146 base pairs). For years, scientists believed histones provided a support framework for the fragile DNA but did not have a role in gene expression. Researchers recently established that genes can be turned on or off, depending on which chemicals react with the histone tails. Abnormal histone modifications are common in mental illness,51-52 and nutrient therapy can assist in normalizing histone chemistry.
The two dominant epigenetic processes are DNA methylation53 and histone modification54 as shown in Fig. 4-2. DNA methylation involves addition of methyl groups to some of the cytosine molecules along the double helix. In most cases, methylation in the vicinity of a gene tends to silence expression of that gene. This process is a crucial part of human development that helps determine which proteins are produced in different tissues and organs. DNA methylation also prevents expression of viruses and other junk genes that have been implicated in disease conditions.
Figure 4-1. Schematic of a Nucleosome

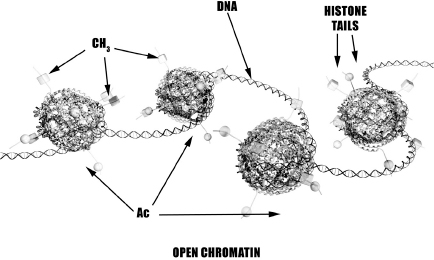
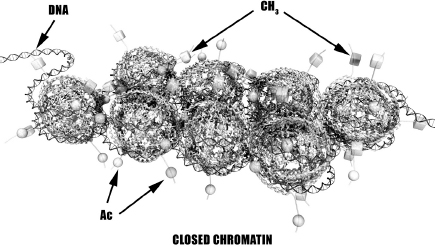
In many cases, genetic expression depends on competition between acetyl and methyl groups at histone tails. If tails are predominantly acetylated, genetic expression (protein production) is promoted. In contrast, highly methylated histones generally result in silencing of genetic expression. Many scientists believe that the ability to express genes is determined by the degree of compactness of chromatin as illustrated in Figures 4-3 and 4-4. This theory recognizes that acetyl groups lower the pH of alkaline histones, thus reducing the electrostatic attraction to DNA and allowing the array to open up and promote genetic expression. Methyl groups produce the opposite effect by increasing compactness of chromatin and silencing genetic expression.
Figure 4-2. The Two Main Components of the Epigenetic Code
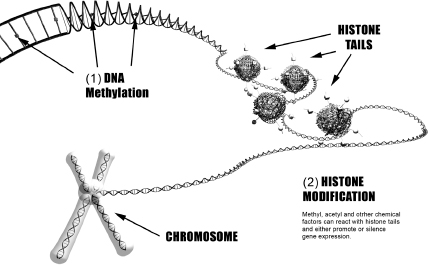
After conception, all of the methyl, acetyl, and other regulating chemicals from the parents’ DNA are removed from the DNA of the fetus and a new set of chemicals attached during early fetal development. These chemicals are called bookmarks (or marks) since they regulate the expression of every gene and can remain in place through a lifetime of cell divisions. Deviant marks that develop in utero can result in a variety of diseases and developmental disorders. Most deviant marks associated with mental illnesses are believed to involve inappropriate placement of methyl and acetyl groups on DNA or histone tails.
Figure 4-3. Low Methylation Promotes Gene Expression
Histone epigenetics is proving to be extraordinarily complex, and it may take another century to completely describe the processes involved. A total of 69 different histone proteins have been identified, each with different chemical characteristics. Acetyl and methyl levels dominate the expression/silencing of many genes, but other chemical factors such as phosphate, biotin, ubiquitin, and citrullin can react with histones and influence gene expression.55 In addition, special enzymes called transcription factors are recruited by specific combinations of histone proteins and reactants and interact with the local DNA to influence cell expression. There may be more than 2,000 transcription factors, indicating there are a great multitude of different histone reactions that can occur. A complex histone code is under development.
Figure 4-4. High Methylation Inhibits Gene Expression
Transcription factors
Gene expression requires separation (or uncoiling) of DNA from histones to allow certain large molecules to access areas near that gene. The process of gene transcription (copying of DNA by RNA polymerase into RNA) requires the presence of one or more transcription factors56 (complex proteins such as zinc fingers). Transcription factors are proteins that bind to specific areas of DNA and regulate the access of RNA polymerase (the enzyme that performs the transcription of genetic information from DNA to RNA). A defining feature of transcription factors is that they contain one or more DNA-binding domains, which attach to specific sequences of DNA adjacent to the genes that they regulate.
There are more than 2,500 proteins in the human genome that have DNA-binding domains, and most are assumed to function as transcription factors. Some transcription factors promote gene expression, while others are inhibitory. The enormous diversity of these proteins may be essential to development of in utero tissue differentiation. They have several mechanisms, including regulation of acetyl and methyl levels at CpG islands (genomic regions that contain a high frequency of cytosine-phosphate-guanine sites) and histones. It is very likely that many nutrients have an impact on transcription factors, but these relationships are poorly understood at present.
The Methyl-Acetyl Competition
Methyl groups are delivered to histones by SAMe and acetyl groups by acetyl coenzyme A. Both of these chemical factors are in high concentration throughout the entire body. SAMe is a natural protein produced in the liver from dietary methionine. SAMe provides methyl groups for dozens of important biochemical reactions in the body and is conserved by a process called the methylation cycle or one-carbon cycle.57 Acetyl coenzyme A is formed from the metabolism (breakdown) of protein, fats, and carbohydrates and delivers high-energy acetyl groups to the mitochondria for processing in the citric acid cycle. Both acetyl and methyl are essential to life.
Attachment or removal of methyl and acetyl at histone tails is dominated by enzymes called methylases, acetylases, demethylases, and deacetylases—NOT by the amounts of methyl and acetyl present. There is considerable research aimed at developing drugs that can regulate the relative amounts of these enzymes. However, certain nutrients have a powerful effect on these enzymes and epigenetic nutrient therapies may be equally effective. A good example58 is niacinamide (vitamin B-3) that reduces the activity of sirtuin, an important deacetylase enzyme. In another example,59 folic acid impacts methyl levels at histones. It’s interesting to note that folates increase methyl levels in tissues and the bloodstream, but they reduce methylation at certain histones that regulate gene expression. It’s clear that many nutrients have a powerful impact on gene expression and that epigenetic nutrient therapies have great promise.
Neurotransmitter Transporter Proteins
Neurotransmitter transporter proteins, also known as transporters (see Chapter 1), are transmembrane proteins that quickly remove neurotransmitter molecules from a synapse for reuse in future cell firings. Gene expression of serotonin, dopamine, and norepinephrine transporters is dominated by a competition between methyl and acetyl groups at the histone tails. If acetylation dominates, the production of transporters is increased and neurotransmitter activity is reduced. If methylation wins the battle, gene expression of transporters is inhibited, resulting in higher neurotransmitter activity. In essence, nutrients that promote histone methylation are natural serotonin reuptake inhibitors.
Placing acetyl groups on histones is enabled by enzymes called histone acetyltransferases (HATs).60 Acetyl groups can be readily removed by chemicals called histone deacetylases (HDACs). Several HAT and HDAC enzymes have been identified. Enzymes called histone methyltransferases (HMTs) promote the transfer of one to three methyl groups from SAMe to specific amino acid locations along the linear histone proteins. For many years, histone methylation was thought to be a permanent modification. Very recently, two families of histone demethylating enzymes were discovered. Nutrients or drugs that promote or inhibit expression of these enzymes can have a major impact on neurotransmitter reuptake and synaptic activity.
Epigenetics and Brain Functioning
Healthy mental functioning requires a proper level of synaptic activity at receptors for serotonin, dopamine, norepinephrine, and other neurotransmitters. Proper synaptic activity depends on:
 The amount of neurotransmitter produced in brain cells
The amount of neurotransmitter produced in brain cells
 The amount of synaptic neurotransmitters lost by diffusion or reaction with chemicals
The amount of synaptic neurotransmitters lost by diffusion or reaction with chemicals
 The availability of transporters that return synaptic neurotransmitters back into the original brain cell (reuptake)
The availability of transporters that return synaptic neurotransmitters back into the original brain cell (reuptake)
Decades of pharmaceutical research have shown that synaptic activity is dominated by the concentration of transporters. SSRI antidepressants,61 such as Prozac and Zoloft, inactivate transporters to increase serotonin levels within synapses. SNRI medications, such as Effexor, use the same mechanism to increase activity of both serotonin and norepinephrine. In contrast, benzodiazapine medications, such as Xanax and Valium, increase GABA activity by directly binding to GABA receptors, thus reducing the impact of excessive norepinephrine activity. All three classes of these medications can provide impressive benefits in combating depression, anxiety, etc. but are very addictive and often cause troubling side effects such as fatigue, loss of libido, weight gain, and headaches, among others.
Epigenetics research has identified several nutrient factors that have a powerful impact on transporters at neurotransmitter synapses, including methionine, SAMe, folic acid, niacinanide, and zinc. These are the same nutrients pioneered by Hoffer, Pfeiffer, and the author that have produced thousands of reports of recovery from schizophrenia, depression, anxiety, ADHD, and behavioral disorders. It is highly unlikely that this is a coincidence. In addition, epigenetics research has identified several other nutrients with potential for improving brain functioning. Since nutrient therapy involves normalization of brain chemistry, this approach has the great advantage of minimal adverse side effects.
Two types of epigenetic disorders
Epigenetic disorders can result from either (a) fetal programming errors or (b) deviant gene bookmarks that develop later in life. In both cases, environmental insults are believed responsible for the deviant marks that can persist throughout the remainder of life. Fetal programming errors can result in developmental disorders that are evident from birth. However, deviant fetal programming may also produce predisposition for disorders that appear after birth such as cancer, heart disease, and regressive autism. Epigenetic disorders that appear before age three may result in brain structure abnormalities that are irreversible. In contrast, deviant epigenetic marks that cause brain chemistry imbalances are likely to be reversible. Future epigenetic therapies may eventually result in an enduring cure for anxiety, depression, schizophrenia, and other mental disorders.
Two types of epigenetic therapy
In theory, abnormal gene expression can be corrected either temporarily or permanently. Except for recent cancer research, all epigenetic treatments have been the temporary type in which gene expression rates are modified without changes in the marks. These treatments cause one of two outcomes: (1) uncoiling DNA from histones to enhance gene expression rates or (2) tighter compaction of DNA and histones to reduce expression rates. However, the resulting benefits in mental function can disappear if the treatments are stopped. Future therapies to permanently correct deviant marks could achieve a cure for several mental illnesses within a decade or two. The development of advanced epigenetic treatments should be a high national priority.
Epigenetics and Nutrient Therapy
Methionine and SAMe
Carl Pfeiffer developed a methylation therapy28 for high-histamine schizophrenic patients that brought thousands of reports of improvement. Pfeiffer believed that histamine imbalances in the brain caused psychosis, and he prescribed methionine as a therapy to lower histamine levels in these patients. In the early 1990s, I discovered that histamine was relatively unimportant and that methyl status was a dominant factor in psychosis. Undermethylated persons were prone to depression that usually could be lessened by SSRIs. Overmethylated persons were prone to high-anxiety depression that usually worsened after SSRIs. Epigenetics has provided a clear insight into the importance of methylation therapy in mental health.62-63 Methionine and SAMe increase histone methylation, which can inhibit gene expression of serotonin transport proteins. The result is increased serotonin in synapses and higher serotonin activity. In effect, methionine and SAMe are natural serotonin reuptake inhibitors.
Folic acid
Abram Hoffer pioneered the use of folic acid therapy for schizophrenia in the 1950s. About 10 years later, Pfeiffer found that most paranoid schizophrenics responded very well to folic acid supplements but that delusional schizoaffective patients deteriorated if given folic acid. My experience with thousands of schizophrenic patients confirmed Pfeiffer’s folic acid observations. However, I became puzzled by the fact that folic acid is an effective methylating agent, but it has a negative effect on undermethylated schizophrenics and depressives. Overmethylated patients clearly thrive on folic acid, whereas methyl-deficient patients exhibit intolerance. In 1994, I reported this surprising finding at the Society for Neuroscience annual meeting42 and concluded that the methyl/folate ratio has a special significance in mental health. Recent epigenetic research has finally provided a convincing explanation for this phenomenon. Several studies64-66 have shown that reducing dietary folates can increase methylation at histone tails and DNA sites. Vanderbilt University biochemists have reported that folates enhance histone demethylation.61 A National Institutes of Health (NIH) study reported that activation of a folic acid receptor gene can increase histone acetylation.67 In effect, folic acid increases genetic expression of transporters, causing reduced activity of dopamine and serotonin. Folate and methyl produce opposite effects on neurotransmission. Folic acid is a serotonin reuptake enhancer, whereas methionine and SAMe are serotonin reuptake inhibitors. Giving folic acid to an undermethylated depression patient results in improved methyl levels (SAMe) throughout the body and brain but reduced methyl levels at key histones and CpG islands that regulate neurotransmitter activity.
Folic acid supplements can either increase or decrease methylation at histone tails and CpG islands, depending on the portion of the DNA strand that is involved. With respect to mental health, folic acid supplements generally must be avoided for undermethylated patients and emphasized for overmethylated patients.
Vitamin B-3 (niacin)
For many years, Abram Hoffer championed the use of niacin (vitamin B-3) in the treatment of schizophrenia. Later, Carl Pfeiffer reported that niacin was highly effective for patients with low blood histamine levels but less beneficial for others. In the early 1990s, I observed that niacinamide, the active form of niacin in the body, could be substituted for niacin in treatment of high-dopamine patients. After years of confusion and uncertainty, epigenetics has provided a convincing explanation for the efficacy of niacin. Niacinamide inhibits sirtuins, a class of proteins that effectively remove acetyl groups from histones and promote methylation.58 By this mechanism, increased intake of vitamin B-3 (niacin or niacinamide) results in higher gene expression of transporters and reduced dopamine activity. This is especially useful for paranoid schizophrenics who have excessive dopamine activity.
Other nutrients
In addition to the powerful impacts of methyl, acetyl, folate, and niacin on brain chemistry, several other nutrients are known to influence the epigenetics of neurotransmission.68 For example, biotin and phosphates can covalently bind to histones and impact gene expression. Zinc enhances the gene expression of metallothionein, an important antioxidant protein. Many other nutrients affect the production and functioning of enzymes and cofactors that govern epigenetic processes. Pantothenic acid, tryptophan, choline, and dimethylaminoethanol (DMAE) enhance acetylation of histones. In addition, various nutrients have an important impact on DNA promoter regions that regulate gene expression rates.
Identification of Epigenetic Disorders
In Chapter 3, I alluded to my work over the past 35 years, wherein I collected a database of more than three million blood, urine, and tissue chemistries from 30,000 patients diagnosed with a variety of mental disorders. Methylation status was assessed for each of these patients based on lab chemistries and medical history. I discovered that the incidence of a methylation disorder approaches 100% in certain psychiatric conditions. Since methyl status is a dominant factor in epigenetics, there is a strong possibility that these conditions are epigenetic in nature. This belief is supported by the fact that these disorders are heritable (run in families) but violate classical laws of Mendelian genetics with less than 100% concordance for identical twins.
An environmental insult that triggers an epigenetic illness would likely involve altered expression of several genes. As a result, epigenetic mental disorders would be expected to involve a consistent syndrome of symptoms and behavioral traits. For example, autism usually involves poor immune function; altered brain structure; digestive abnormalities; odd, repetitive movements; etc., all of which form a distinctive syndrome that is typical of an epigenetic disorder. The following disorders meet my criteria for an epigenetic disorder: abnormal methylation, non-Mendelian heritability, and a syndrome of distinctive symptoms and traits. However, the most convincing indicator of an epigenetic condition is a sudden onset followed by a permanent change in functioning. Since deviant marks survive many cell divisions, the condition doesn’t just go away. Future epigenetic therapies aimed at correcting aberrant gene expression have great potential for benefitting these patients.
Autism: In 1999, Bernard Rimland of the Autism Research Institute learned that I had amassed the world’s largest collection of chemistry data for autism spectrum children and asked me to identify any consistent abnormalities in this population. My data confirmed previous reports of disordered metal metabolism, B-6 deficiency, and elevated toxic metals, but the data also produced the surprising finding that more than 95% of autistics exhibited undermethylation. This has led to convincing research by S. Jill James,69 Richard Deth,70 and others, indicating that undermethylation is a distinctive feature of autism spectrum disorders. Both the autistic regression event and the persistence of autism symptoms are also consistent with a gene expression disorder. As described in Chapter 7, there is growing evidence that autism is epigenetic in nature.71-72
Schizoaffective disorder: This condition73 is a mixed thought, mood, and perceptual disorder consisting of delusional thinking, moods ranging from depression to mania, and perceptual hallucinations and illusions. In most cases, delusional thinking is the dominant symptom. Many of these patients also exhibit obsessive-compulsive tendencies, internal anxiety, and catatonic tendencies. Typically, this is an adult-onset condition featuring a mental breakdown after a history of high achievement. A review of the chemistry database for 500 persons with this diagnosis revealed that virtually all had evidence of undermethylation. I believe this devastating disorder is epigenetic in nature.
Paranoid schizophrenia: This severe mental disorder73 usually involves auditory hallucinations and paranoia along with a multitude of other troubling symptoms. Review of the database for more than 1,000 persons with this diagnosis revealed that more than 85% exhibited overmethylation. This condition is often misdiagnosed, and a careful study of 250 patients with classic symptoms indicated overmethylation in 94% of the patients. With more accurate diagnosis, the incidence of this chemical imbalance could approach 100%. A typical feature of this illness is a mental breakdown after age 15. It seems likely that classic paranoid schizophrenia is epigenetic in nature.
Obsessive-compulsive disorder (OCD): My chemistry database contains 92 individuals diagnosed with severe OCD.73 Many reported that the condition appeared quite suddenly and has been a chronic problem since that time. All but five exhibited severe undermethylation. This strongly suggests that OCD is epigenetic in origin.
Antisocial personality disorder (ASPD): My behavior database includes chemistry information for more than 800 convicted felons and thousands of very violent children. Examination of data for more than 400 persons diagnosed with ASPD73 indicated a high incidence of zinc deficiency, pyrrole disorder, toxic metal overload, and glucose dyscontrol. However, more than 96% also exhibited undermethylation, suggesting this condition may be epigenetic in origin and involve aberrant brain development and altered neurotransmitter activity. Future treatments that modify methyl and acetyl levels at histones and DNA CpG sites may represent an effective way to reduce crime and violence.
Anorexia: Examination of chemistry information for 145 persons diagnosed with anorexia73 revealed that all but five were undermethylated. Nutrient therapy to enhance methylation together with counseling has produced many reports of recovery. This is another strong candidate for epigenetics research.
Paraphilias:73 My chemistry database includes a few dozen persons with abnormal sexual behaviors including pedophiles, sexual sadists, masochists, and peeping toms. All of these patients were male and most were in trouble with the law. They complained of overwhelming intrusive thoughts that interfered with daily life. Only two said they had been sexually abused as children. In most cases, they first became aware of their condition between the ages of 14-16. More than 90% were undermethylated, suggesting that paraphilias may be epigenetic in origin. I believe that the term obsessive-compulsive perversion or OCP is more appropriate than paraphilia. It’s well known that child molesters rarely reform regardless of medications, counseling interventions, or the threat of imprisonment. Perhaps future epigenetic therapies may rid the world of this devastating and criminal disorder.
Transgenerational Epigenetic Inheritance (TEI)
Animal experiments have clearly shown that certain epigenetic errors can be transmitted to future generations, without changing DNA sequence.74 There is growing evidence that this mechanism also occurs in humans. This means that the harmful effects of a toxic exposure can be passed on to one’s children and grandchildren. I’m reminded of a quotation from the Bible: “The sins of the father are visited upon the son.” Imprinting of abnormal methylation of the genome is believed to be one of the major TEI mechanisms. We are all familiar with physical birth defects that can result from toxic exposures during pregnancy. TEI defects could also cause abnormal brain development, chemical imbalances, weakened immune function, and an inborn predisposition for a mental illness. In addition, TEI may have contributed to the mysterious recent epidemics in ADHD, autism, breast cancer, and other conditions that have a strong heritable component.
Nature, Nurture, and Epigenetics
For centuries, scientists have debated whether mental illness results from inborn or environmental factors. At long last, these arguments are fading away as most experts now agree that both factors are highly important. Schizophrenia and many other mental illnesses involve inherited predispositions but violate classic laws of Mendelian genetics, thereby indicating a strong influence of environment. The new science of epigenetics has shown that gene expression can go awry due to toxic chemicals, emotional trauma, chronic personal failures, oxidative stress, medication side effects, nuclear radiation, and abnormal nutrient levels. The good news is that deviant gene marks may be normalized by future epigenetic therapies. Present epigenetics research is focused on the development of drugs that can alter gene expression, but epigenetic nutrient therapies may be equally effective.
The initial book chapters have summarized the basic facts and overarching principles needed to understand underlying chemical imbalances in the brain associated with mental illnesses as well as other conditions encompassing cognitive deficits. We have shown, in a general sense, how nutrients can often accomplish what drugs hope to achieve but in a more natural manner that is less prone to adverse side effects. The remaining chapters will focus on schizophrenia, depression, autism spectrum disorders, behavioral disorders, and Alzheimer’s disease. Some information that we’ve included in the preceding chapters is, by necessity, included, and we hope this serves to reinforce key points to the reader.