

If you have had a brain or body scan for functional magnetic resonance imaging (fMRI), you know that it is done with your body inserted into a large circular magnet. This technology was developed in the 1990s. Like the use of 2-deoxyglucose (2DG), fMRI is based on local changes in blood flow within the brain that are related to the energy demands of locally active nerve cells. The magnets for humans must have wide internal chambers to receive the human body. Initially they were limited to modest magnetic strengths of 1 to 2 Tesla (the unit for measuring magnetic strength), although recently stronger magnets up to 4 Tesla and above have been introduced. The resolution of the recorded pattern is limited to roughly 0.00006 to 0.00012 cubic inch (about 1 to 2 mm3), about as wide as the end of thick pencil lead, which enables one to see different regions within the brain but not different layers within a region.
A New Approach: Smell Patterns Using fMRI
As fMRI was being developed for humans, the basic research to understand the method was being carried out in parallel in small animals such as rodents. The magnets for rodents have small, narrow chambers only a few inches across and go to higher magnet strengths, currently up to 11 Tesla and beyond, giving a higher resolution down to approximately 100 μm (0.1 mm). This is about the size of an olfactory glomerulus. In addition to the higher resolution, fMRI offers the opportunity to test different smells, use briefer stimuli, and record faster responses.
When I realized the technology could be applied to activity patterns in smell, it was time to act. As it happened, one of the world centers for developing fMRI in animal experiments was at Yale, just two floors down from my laboratory. Charles Greer, my colleague from our 2-deoxyglucose (2DG) work, and I sat down with the director, Robert Shulman, and his colleagues Douglas Rothman and Fahmeed Hyder to outline our proposal, emphasizing how great it would be if we could image the glomerular layer and even identify single active glomeruli. They immediately got excited and showed us an fMRI study they had just finished on a part of the rat brain that contains a representation of the whiskers, in which each of the 24 whiskers on a rat’s snout has its own group of cells, called a barrel, in the cortex. They showed us experiments in which they were able to tweak a single whisker and show activity in its corresponding barrel. It didn’t take long for us all to be persuaded that the olfactory experiments should work.
Our experiments started with a 4.7 Tesla magnet. Greer and I worked with the imaging group to devise an “olfactometer” (a smell-delivery apparatus) for stimulating a rat lying anesthetized within the narrow chamber of the magnet. The first images exceeded our hopes, showing strong fMRI activity patterns apparently within the glomerular layer just as in the 2DG case. Our colleagues told us that these were the strongest fMRI signals that they had seen in any part of the brain. We could immediately explain that this was likely due to the fact that each glomerulus is the site of convergence of thousands of incoming olfactory nerve fibers, focusing all the responses of those cells on their corresponding glomerulus.
The patterns demonstrated by fMRI in the rat olfactory bulb were consistent with the patterns obtained with 2DG: different odors give different patterns; the patterns have medial and lateral domains; the patterns are similar in the two olfactory bulbs; and the patterns increase in extent with increasing odor concentration. We could now apply this “high-resolution fMRI” method to a key question: Could the smell activity patterns reflect the different responses of olfactory receptors to chemically related odor molecules in the same animal?
Different Receptor Responses Encoded in Different Odor Patterns
To answer this question, we shifted our study to the mouse. The mouse was rapidly becoming the animal of choice for applying gene engineering because of its rapid reproduction cycle. It offered the promise of labeling different olfactory receptor cell populations and relating them to the smell patterns.
The mouse is much smaller than the rat and therefore poses an extreme challenge to the high-resolution fMRI approach. In contrast to an adult rat, which may weigh about 12 ounces (300 grams) or more, a mouse may be only one-tenth the size, around 1 ounce (30 grams). The mouse olfactory bulb is correspondingly much smaller. However, by then our imaging colleagues had moved up to a 7.6 Tesla magnet. By technical wizardry they were able to image down to about 100 μm2 (0.0001 mm2), which is near the size of a mouse glomerulus.
Fuqiang Xu, a postdoctoral fellow in the laboratory, took responsibility for carrying out these demanding experiments, using the same series of aldehyde molecules—containing a backbone of four to eight carbon atoms—used in the studies of the I7 receptors described in chapter 5. The animals were exposed to odors, with the magnet humming away, and the images were acquired and stored. As with the 2DG experiments, the functional images were overlaid on the anatomical images, and the glomerular activity patterns were reconstructed.
Summary of the Smell Image Evidence
The take-home message is illustrated in figure 8.1. On the left, the smell molecule activates the receptor within a binding pocket. The diagram shows the sheet of receptor cells in the nose. As explained in chapter 7, each cell sends its impulse response through its fiber (axon) to a glomerulus in the olfactory bulb. All the cells containing the same receptor molecule send their fibers usually to a pair of glomeruli on the medial and lateral sides of the olfactory bulb. This means that whenever a particular receptor is activated, the responses of all the cells are focused on these modules. Cells more or less strongly activated cause more or less strong activation of their corresponding glomeruli, resulting in a pattern such as that shown in the figure, as seen in this case from the medial surface.
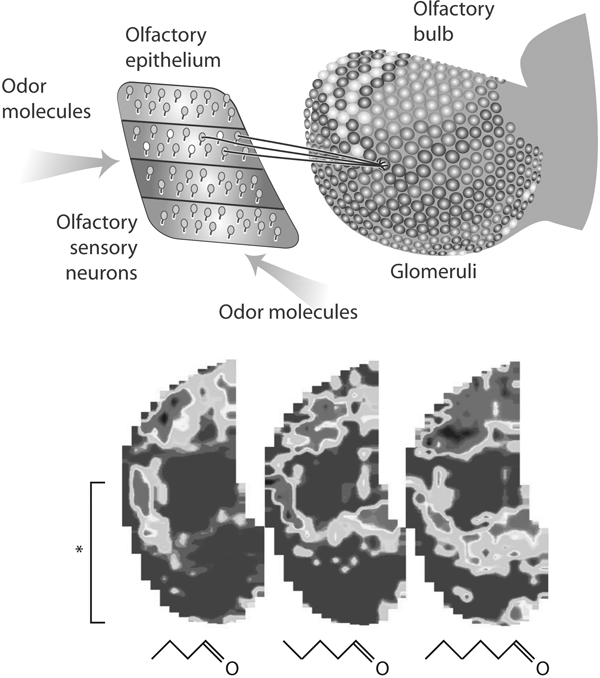
FIGURE 8.1 How the smell image is initially formed
Above, the olfactory receptor cells lie in zones in the olfactory epithelium. Representative cells are shown sending their axons to converge on a glomerulus in the olfactory bulb. The different shades of gray indicate different levels of activity in the glomerular layer. Below, flattened maps of patterns of activity in the glomerular layer produced by stimulation with three different odor molecules differing only by a single carbon atom. The bracket indicates the extent of the glomerular pattern on the medial side of the olfactory bulb shown above.
(Adapted from G. M. Shepherd, Smell images and the flavour system in the human brain, Nature 444 [2006]: 316–321)
In the lower part of figure 8.1 are three images of the activity patterns in the glomerular sheet constructed from imaging of the functional MRI responses to three closely related aldehyde smell molecules. You can relate the lower part of the image in the left panel (see asterisk and bracket) to the image on the medial side of the olfactory glomerular layer shown in the view of the olfactory bulb, in the upper part of the image. These molecules vary only in their number of carbon atoms, from four to six.
These spatial patterns can be regarded as representing the information carried in the odor molecules. By the analogy with the visual system, where a pattern on the retina is called a visual image, we can call a pattern on the olfactory bulb an odor image or smell image. These results indicate some of the key features of odor images: they extend over much of the olfactory bulb, and they are overlapping but different. As with 2DG patterns, they become more extensive with increasing concentration.
Behavioral Tests
Although these patterns, even while overlapping, are different, can a mouse actually distinguish behaviorally between these small differences? To test for this I was joined by Matthias Laska, then from Munich, Germany. Laska is a leading psychophysicist in the study of smell in monkeys and other species. He and a Yale undergraduate student, Dipa Joshi, carried out experiments in a behavioral olfactometer, consisting of a plastic box and two small openings for delivering two odors. Animals were trained to test the odor at each port and to indicate when they were different, for which they received a small reward. Most of the olfactometer consisted of a complicated array of glass and Teflon tubes and valves to ensure that the odors were pure and that they were delivered in brief pulses.
The results showed that mice are extremely good at distinguishing between these smell molecules. This applies to two odors that are different by only one carbon atom, as well as those that differ by two or more carbon atoms. This discrimination is much finer than shown by the immune system, in which an epitope, as mentioned in chapter 4, consists of up to a dozen or more amino acids within a protein chain on the antigen. By contrast, the ability to discriminate a single carbon atom appears to put the olfactory system in a class by itself.
Many Methods, Many Patterns
Any given method in biology has advantages and limitations. The methods using 2DG and fMRI display smell activity patterns that extend throughout the olfactory bulb, but they use persistent odor stimulation instead of short whiffs, and they can’t quite image a single glomerulus. As indicated in chapter 7, investigators have developed many other methods. Some of these involve microscopic viewing of the activation of individual glomeruli. This depends on labeling cells with fluorescent dyes that are sensitive to electrical changes in the cells, or observing minute changes in the microcirculation—so-called intrinsic imaging. Other methods use electrophysiological recordings of nerve cell activity. The results have extended the general conclusions from 2DG and fMRI to more local detailed levels. They show that when applied to the same series of aldehydes, the principles of overlapping but different patterns applies at the level of individual glomeruli.
A disadvantage of these microscopic methods is that one is usually limited to observing only the dorsal part of the olfactory bulb, which may account for only 10 to 15 percent of the surface. It is a bit like looking at a face and seeing only an ear or an eyebrow.
To overcome these limitations, Kensaku Mori and his colleagues in Tokyo in a study reported in 2010 have opened the view to the lateral surface of the olfactory bulb as well. This has permitted much more extensive exploration of the activity patterns. They have been able to plot the responses to a wide range of odors. In general, odors that are related chemically to each other activate glomeruli near each other. There is a tendency toward clustering of glomeruli responding to related types of odors.
With so many patterns from so many methods, it becomes essential to be able to compare them. The field is just beginning to do this. The Sense-Lab database is designed to aid in this effort. As indicated at the end of chapter 7, one can explore the thousands of different receptors, the different odor molecules that interact with them, and the odor maps that are produced by their interaction.
Odor Images, Pattern Recognition, and Faces
All these studies have supported and extended the initial findings obtained with the 2DG method. The patterns have been found in several vertebrate species, including fish, salamanders, mice, rats, rabbits, and monkeys. Odor patterns have also been found in the corresponding parts of the olfactory brains of invertebrates, including honeybees, fruit flies, and the tobacco hornworm. This shows that not only are the glomeruli a constant feature of the architecture of the smell pathway across the animal kingdom, but that activity patterns are a constant feature of their function. Together, these results provide direct experimental proof of the hypothesis that smells are encoded at least in part as spatial activity patterns, and they give the first insight into the mechanism: the neural basis of odor encoding involves differential activation of the olfactory glomeruli. When we followed Edgar Adrian’s advice and “looked to the glomeruli,” we were not disappointed.
This new evidence has stimulated new ways of thinking about the neural basis of smell. For the first time one can say that, just as the non-spatial modality of auditory frequency is represented by a frequency map in the cochlea, which is retained all the way up to the cerebral cortex, so is the nonspatial modality of smell represented in activity patterns, starting in the olfactory bulb. This is what is meant by “the uses of neural space by a nonspatial modality.”
Smell Images and Faces
The fact that smell molecules are represented as spatial patterns supports the hypothesis of an analogy with the visual system. When we have a visual perception, we are sensing a spatial pattern of activity that we call a visual image. Likewise, I wish to hypothesize, when we sense an odor we are sensing a spatial pattern of activity that we can also call an “odor image” or a “smell image.”
An advantage of thinking in this way is that it encourages drawing on the vast amount of work that has been done, and is being done currently, on pattern recognition in the visual pathway. Because the odor images are irregular patterns of activity, they seem less like the geometrical shapes of many visual objects in our surroundings and more like irregular shapes such as plants and animals, and particularly human faces.
Humans are in fact very good at recognizing faces. The classical illustration of this is that in a room full of grandmothers, you can readily identify your own grandmother. Yet if you are asked to describe your grandmother’s face to someone else, it is very difficult; we lack the vocabulary and an appropriate coordinate system to specify how this pattern recognition is carried out. But we do it unerringly. How?
Recognition of faces is an important subject, not only for designing machines to perform pattern recognition, but also for law enforcement agencies trying to identify criminal’s faces from witnesses’ descriptions. The process of face recognition has been characterized by Terry Landau in his book About Faces: The Evolution of the Human Face:
What you see and recognize in [the face of] another person is ultimately determined by the unique pattern created by the whole picture the face presents. That is identity. It’s not the details of the features. It’s not the spacing between features. Rather, it is the interrelationship among all of these things taken together [that is, the gestalt] that enables us to recognize the identity of a criminal or the face of a friend. You can’t take faces apart analytically. Most of the time you can’t even tell what you are looking at when you look at a face because verbal processes have little to do with visual recognition. Identity is encoded on the face and remembered as a whole. Though elusive and hard to define, it is experienced on many different levels, not the least of which is that identity enables us to carry out an important social skill: recognizing one another as individuals.
It seems reasonable to hypothesize that recognition of smell patterns in the olfactory bulb involves similar principles. We see a pattern as a whole, we learn to identify it with its “smell object,” and we learn to discriminate it from patterns for other smell objects that may be very similar but carry a different behavioral meaning.
To pursue the analogy a step further, the quotation from Landau applies only to recognizing a face that one has seen only once, in which one needs the entire gestalt to remember it (barring some noticeable single feature). This is quite different from recognizing a familiar face one has seen many times, in which case catching only a fleeting glimpse of part of the face, or of the face in poor light, can be sufficient; animals are very good at recognizing these minimal cues. An example is an increasing pixelation of photographs of Abraham Lincoln or the Mona Lisa. We have no trouble identifying the picture even though the resolution is poor, because we have trained our pattern recognition abilities on it so that we can match it with our stored memory despite the indistinct image. The adaptive value of this ability is obvious; identifying prey or a predator in the indistinct shadows of dusk is critical to survival, as is the ability to identify the face of a friend or enemy in shadows. Because most animals live by their sense of smell, an analogous ability to identify an odor image would be critical to survival.
In the case of the visual system, it is easy to grasp the concept of a visual image in the retina, because this image is maintained through several steps of processing from the retina to the higher centers in the cortex. By contrast, in the olfactory system, we are not conscious of the fact that our olfactory bulbs have constructed an image of an odor. We may speculate that this is because that image does not represent the real odor world, but is only the brain’s way of representing the odor world. What the brain cares about is how that image is processed to provide the basis for our perception of different smells and different smell objects.
It is therefore to higher stages of processing in the brain that one must look for understanding how the images are used as a basis for smell perception.