1951
Alpha-Helix and Beta-Sheet
Robert Corey (1897–1971), William Astbury (1898–1961), Linus Pauling (1901–1994), Herman Branson (1914–1995)
Techniques like X-ray crystallography and NMR (nuclear magnetic resonance) have shown that although every protein is different, there are structural motifs and themes that appear over and over. The two most important are the alpha-helix and beta-sheet.
The alpha-helix looks a bit like what you would get if you held out a spool of ribbon and let some of it drop down. It’s a spiral staircase of amino acids, gradually corkscrewing around and forming favorable hydrogen bonds. Some amino acids are much more helix-prone than others, and the parts of a protein that curl up like this can be predicted pretty well by just their amino acid sequence. American chemist Linus Pauling had the idea for it in 1948 while in bed with a cold—he drew a chain of amino acids out on a sheet of paper and tried curling it around to figure out how the hydrogen bonds that held the molecule together could be formed. He then gave American physicist and chemist Herman Branson the task of determining which helical structures were possible.
The beta-sheet, proposed by English molecular biologist William Astbury and refined by Pauling and American biochemist Robert Corey in 1951, is a different animal: a tightly hydrogen-bonded network of zigzagging amino acids that are, roughly, lying on top of each other. In fact, sometimes beta-sheet structures are so well matched that the protein becomes an insoluble aggregate, as happens with the amyloid protein in Alzheimer’s disease. For this reason, there has surely been evolutionary pressure away from too much beta-sheet formation, but it’s still a very important motif, giving local order to many classes of protein.
These structures are fundamental to the shapes of all proteins, and thus to all life. A typical structure might have a few alpha-helices bundling together at angles to each other (like a bunch of baguettes standing on end in a tall basket) and connected by loops with the rest of the chain forming beta-sheet regions off to one side. Sometimes protein structures are drawn in a ribbon-type diagram just to emphasize the twists and turns of the backbone.
SEE ALSO Amino Acids (1806), Spider Silk (1907), X-Ray Crystallography (1912), Hydrogen Bonding (1920), NMR (1961)
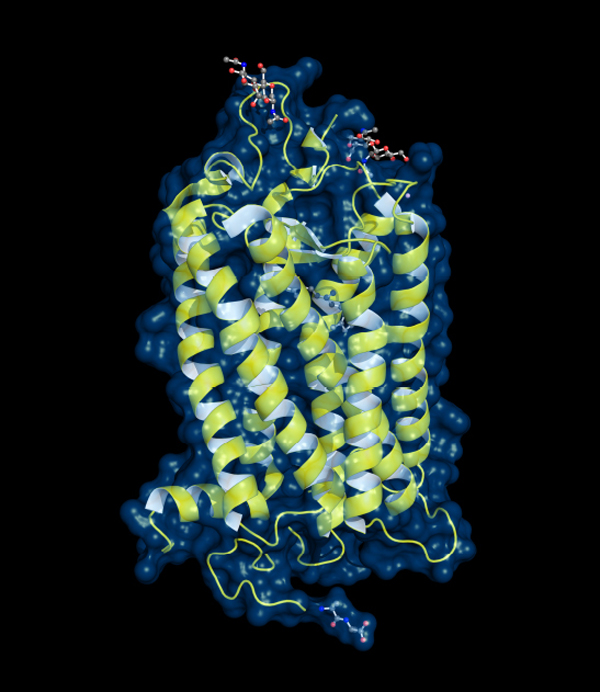
Rhodopsin, the light-sensitive pigment in the retina, sits in the cell membrane as a bundle of alpha-helix units. Such alpha-helix interactions are a key part of protein structure.