1961
NMR
John Dombrowski Roberts (b. 1918), Martin Everett Packard (b. 1921), Rex Edward Richards (b. 1922), James T. Arnold (b. 1923), James N. Shoolery (b. 1925)
NMR (nuclear magnetic resonance) is one of the most powerful analytical techniques in chemistry. You can learn more about an unknown compound in ten minutes on a modern NMR machine than a chemist used to be able to learn in a year. The physics behind it are complex, but essentially, an NMR spectrum shows every different sort of hydrogen atom in a compound (depending on what sort of group they’re attached to). This is extremely useful for determining compound structures, since all organic molecules have hydrogen atoms attached to them in different positions. Each of these hydrogen peaks is split into multiple lines depending on how many other hydrogens are attached nearby, and the distance between those lines is related to the angles between the atoms. But NMR isn’t just for hydrogen. Fluorine atoms work very well, as does carbon-13 (the minor stable isotope of carbon), and many other nuclei. More complicated NMR experiments display all the proton-carbon connections simultaneously, which can quickly solve the structures of complex organic molecules.
NMR came from the cutting-edge physics of the 1930s, and its applications to chemistry weren’t obvious. Many physicists didn’t think there were any, but American biochemist Linus Pauling’s advice at the time (to British chemist Sir Rex Edwards Richards, a 1950s NMR pioneer) was to ignore them. Still, building reliable NMR instruments, with their large, finicky electromagnets, took real perseverance. American physicist Martin Everett Packard first recorded the NMR spectrum of an organic molecule, and James T. Arnold built what was, in 1956, the world’s best magnet to study more of them. The year 1961 marked American chemist James N. Shoolery’s Varian A60, the first commercial NMR that seemed built to get results. American chemist John Dombrowski Roberts showed his fellow chemists how useful it could be for analysis of compound structures, and the field has never looked back.
SEE ALSO Natural Products (c. 60 CE), Helium (1868), Liquid Nitrogen (1883), Nonclassical Ion Controversy (1949), Alpha-Helix and Beta-Sheet (1951), Protein Crystallography (1965), Fullerenes (1985)
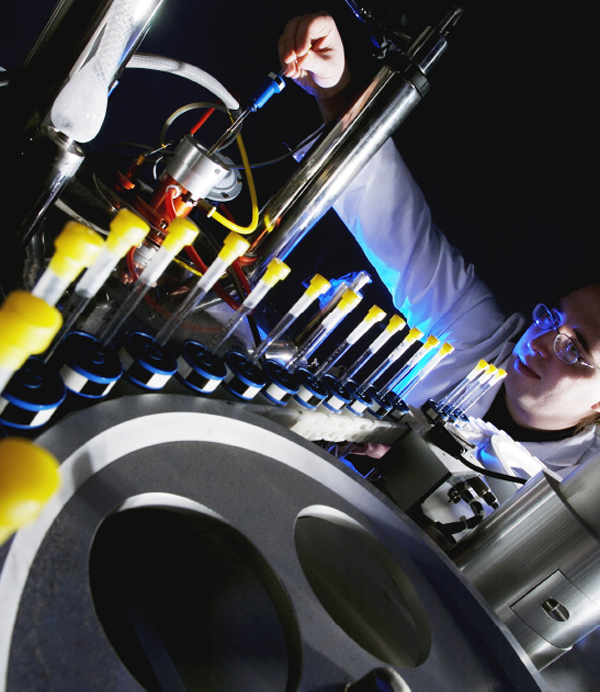
A chemist loads a sample into a modern NMR machine. The samples are contained in thin glass tubes, mounted in plastic “spinners.” These are taken down into the center of the magnet by compressed air, spinning rapidly in order to give a sharper signal.