As we work with cannabis to address specific conditions in the body, keep in mind that research on the efficacy of cannabis in humans is in its infancy, and scientific research on whole-plant extracts of cannabis is sparse. In this chapter, summaries are offered on research with more attention and merit given to human trials with cannabis, especially the long-term longitudinal studies that have been carried out for hundreds of years by practitioners. We will look at animal or cell culture studies, or studies utilizing artificial isolated constituents, only for clues to where cannabis could be potentially used rather than evidence of its benefit or lack thereof.
There are very few random, controlled trials on the efficacy of whole-plant extracts. There is, however, a wealth of research on isolated constituents of cannabis and pharmaceuticals and the biochemical pathways they interact with. We can glean some potential therapeutic uses for cannabis from this research. For example, we apply information about the effects of serotonin in the body and how serotonin reuptake inhibitors work when using high-CBD cultivars because CBD and CBDA bind to serotonin receptors.
When working with clients and their conditions, it’s helpful to categorize common underlying causes of the conditions. When we understand the root of a problem and how to alleviate the cause, we can apply it to other situations with the same root problem. In working with cannabis and the conditions it helps with, we can categorize the conditions in the following ways:
When appropriate, I offer some background information on anatomy and physiology to help you understand how cannabis works with a particular condition (if you just want the top-level facts, you can bypass these sections). I offer a description of each condition; the mechanism of action (some are more in-depth than others); research on effectiveness; and dosage guidelines.
For all conditions, the starting place for dosage is the minimum effective dose (MED) of a 1:1 CBD:THC oral preparation taken every 4 to 6 hours unless otherwise noted. The art and skill will be in determining what the MED is for each client every time they start a new round of medicine. It is a good practice for users to back dosages down when trying something new because the new medicine might have a different potency. Using the MED is cost-effective, too. Why take a whole dropper full when one drop will get the job done?
I have people start with a drop of tincture (yes, one drop), wait 2 hours to see if they get relief, and then take another drop if they aren’t feeling relief. They then wait 4 hours to dose again. This process may seem slow, but that’s good, especially for the cannabis-naïve. You do not need to feel high to receive relief, and if you do feel altered at whatever the MED is, tolerance will occur after a few days, and you won’t continue to feel high.
Keep in mind that if someone is trying your medicine for the first time after having used isolates or something else that is not a full-plant extract, they will likely find they need a much lower dosage than they are used to, perhaps one-third of the dosage of new medicine versus the old. Remember, some studies found whole-plant extracts to be 330 times more powerful than isolates, so it’s wise to start with a one-drop dosage.
In discussing conditions here, I will offer any available evidence based on human or animal studies. For dosage recommendations based on animal studies, I have adjusted for a 150-pound person (the pharmaceutical standard).
Because chronic inflammation underlies all chronic disease, understanding the physiology of inflammation in general will take us a long way toward understanding how cannabis can help in managing disease and how we can apply this knowledge in our formulas for healing. Plus, biochemistry is fun.
Cannabis works when its constituent chemicals bind to specific receptors on or within cells. In nerve cells, this either increases or decreases neurotransmitter release. In immune cells, this mechanism slows the release of inflammatory cytokines and reduces oxidative stress. The only way our body has to heal any injury is through the process of acute inflammation. (Chronic inflammation contributes to chronic disease, not acute.) The process of acute inflammation allows injured cells to call in their own healing. Acute inflammation brings healing to cells and tissues. The complex cascade of events brings in blood with the needed nutrients, oxygen, and immune cells to help heal. When the body fails to shut down acute inflammation, or when inflammation continues in the absence of triggers, the inflammation becomes chronic. Chronic inflammation causes further damage and is present in most major disease. Reducing chronic inflammation is often the root of addressing chronic diseases.
To successfully do this, we need to know and understand the inflammation process.
The inflammatory process begins when a cell is injured. Injured cells begin making and releasing inflammatory chemicals called cytokines. Cytokines act as chemical flares to attract immune cells to the scene, which clean up debris and kill any pathogens present. The immune cells can also release more cytokines to attract still more immune cells to the party. When the acute situation has been handled, the inflammation should cease.
But not always. Chronic inflammation occurs when immune cells or the chronically damaged tissue cells keep signaling inflammation in the absence of an injury. This becomes a self-perpetuating loop that damages, rather than heals, the tissue. Chronic inflammation can be stopped by stopping the production of inflammatory cytokines. This happens through four different pathways: (1) stopping the work of specific enzymes in the cascade; (2) preventing the release of cytokines from immune cells; (3) decreasing the proliferation of immune cells that make the inflammatory cytokines; or (4) killing the immune cells producing the cytokines (a process known as apoptosis). Cannabis and our endocannabinoid system decrease chronic inflammation by interacting with every one of these pathways. Pharmaceuticals work only with the production or release of cytokines.
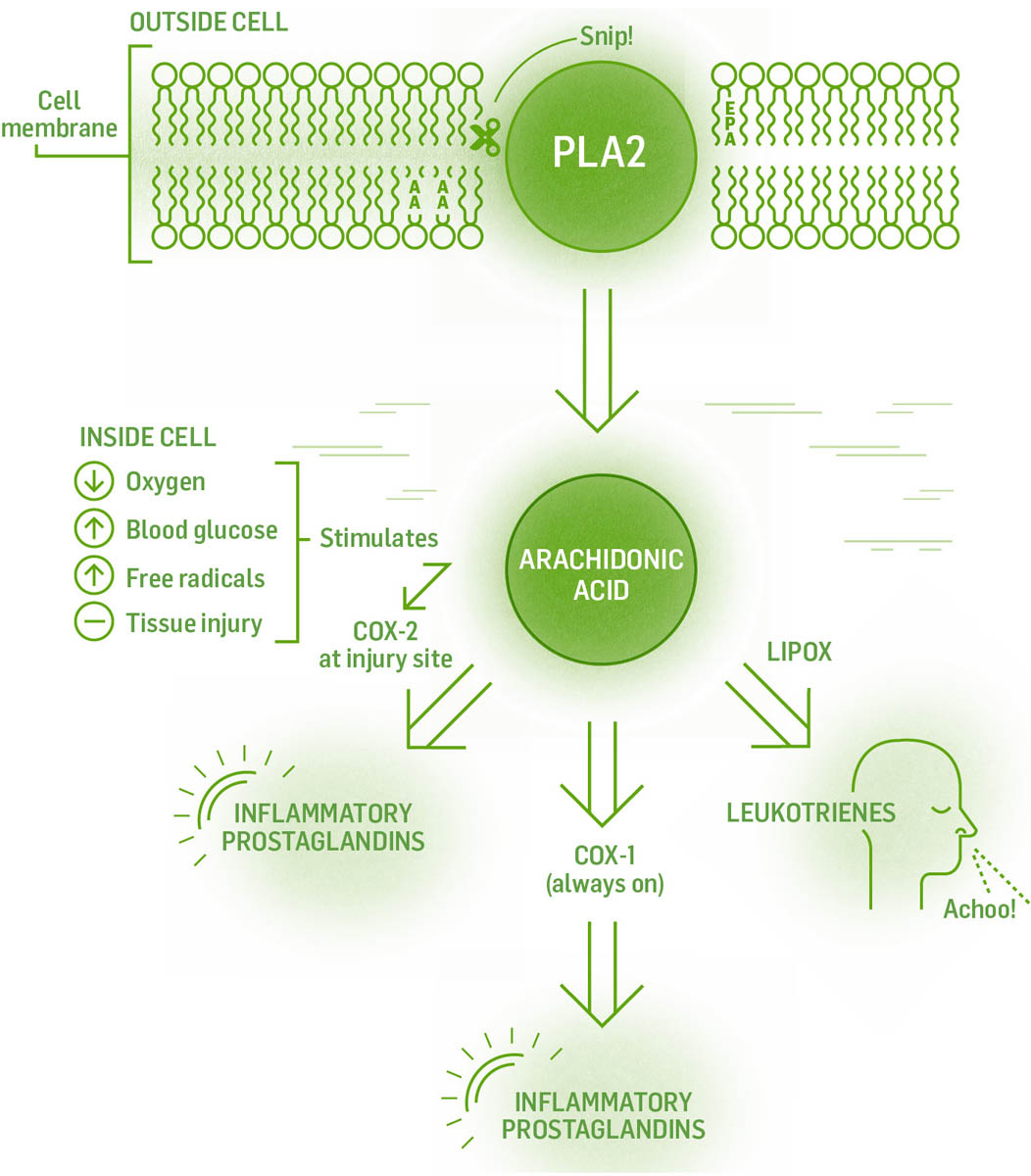
One of the main jobs of the endocannabinoid system is to shut down inflammation after damage has been repaired. Cannabis and our own endocannabinoids accomplish this using a multipronged approach to stop the release of the inflammatory cytokines. The specific methods are: stopping the production of inflammatory cytokines by activated cells; apoptosis of activated immune cells producing cytokines; and preventing activated immune cells from reproducing so they can’t perpetuate the cascade of inflammation.
Western allopathic interventions block or shut down one or all of the enzymes involved in the inflammatory cascade. The problem is that the drugs are not specific to the pathways that cause inflammation; they shut down their target enzymes everywhere. Plant medicine does not completely shut down these pathways; they nudge the enzymes rather than push, so the medicine requires ongoing dosing and requires time to produce effects. Pharmaceuticals tend to push rather than nudge, so they are quicker in producing effects, but their lack of specificity has unwanted adverse effects.
All diseases with a neurodegenerative component, including Alzheimer’s, Parkinson’s, and Huntington’s, share an initiating factor: neuroinflammation, or inflammation of neural cells. Luckily, we can apply our understanding of chronic inflammation and the ways cannabis ameliorates it to the nervous system. Neuroinflammation involves some additional cells: microglia and astrocytes, considered the immune cells of the nervous system.
When we talk about “neural protection,” we mean preventing damage to neurons, which for the most part do not regenerate readily. Our first line of neural protection lies in the natural process of shutting down inflammation by secreting anti-inflammatory cytokines and endocannabinoids. When inflammation begins, immune cells upregulate their CB2 receptors, which leads to increased binding to CB2 receptors by endocannabinoids. The net effect is a decrease of inflammatory cytokine release by the activated cells. As the disease progresses, the immune cells that have crossed the blood-brain barrier also upregulate their CB2 receptors, making them more susceptible to CB2 agonists. Cannabis has four neuroprotective mechanisms:
The beauty of cannabis as an antioxidant is that (similar to its benefits as an anti-inflammatory agent) it interfaces at multiple sites. It is more potent than vitamins A and E as an antioxidant and decreases reactive oxygen species, lipid peroxidation, and nitric oxide (free radical) production during acute inflammation.
Alzheimer’s is the most common form of dementia. It is a progressive neurological disorder characterized by the production of plaques and neurofibrillary tangles in the brain. The plaques are made of amyloid beta peptides, which block neurons from firing, trigger inflammation, and activate microglia. The protein tangles, caused by oxidative stress, block the transport of nutrients and other essential ingredients for neuronal function. The tangles also prevent microglia from moving in and cleaning up debris. Together this leads to the progressive destruction of synapses, neuronal cell death, memory loss, and decreased cognitive function.
Symptoms of Alzheimer’s that may respond to cannabis include loss of sleep, paranoia, anxiety, pain, behavioral disturbances, poor appetite, and weight loss.
Cannabis is neuroprotective, decreases inflammation, neutralizes reactive oxygen species (ROS), and reverses excitotoxicity. These actions mitigate the damage done by inflammation and free radicals to neurons, increasing synaptic transmission.
Rodent studies of early-onset Alzheimer’s have found that preparations of 1:1 THC:CBD decrease inflammation brought on by activated microglia, decrease beta amyloid deposits and inflammatory markers, and improve memory deficits.
CBD-only preparations increased cell survival of embryological neuronal cells, decreased production of ROS, nitric oxide, and neurofibrillary tangles, reduced inflammation, and activated macrophages to remove beta amyloid plaques.
THC-only preparations prevented beta amyloid–induced neurotoxicity and helped reverse memory deficits and cognitive dysfunction. THC binding to CB1 receptors also inhibits production of acetylcholinesterase, which enhances synaptic transmission and stimulates neurogenesis, both of which ameliorate cognitive dysfunction. THC binding to CB2 receptors reduces inflammation and activates macrophages to remove beta amyloid plaques.
Human studies show the addition of THC to existing regimens significantly improved behavior disturbances such as delusions, agitation, irritability, apathy, sleep, food intake, and overall symptom severity.
Minimum effective dose (MED) for alleviation of symptoms.
Parkinson’s disease, the second-most-common neurodegenerative disease, is characterized by a progressive degeneration of dopaminergic (dopamine-producing) neurons in the midbrain, resulting in severe motor impairment and loss of motor control. Current pharmacological therapies consist of administering L-dopa, which gives some relief initially but decreases in effectiveness over time.
Because cannabis offers neuroprotection and lowers oxidative stress, it may also slow the overproduction of ROS in neurons trying to upregulate their production of dopamine. If these neurons experience less damage, they should be able to produce dopamine for longer. Microglial activation may also be attenuated by cannabis’s ability to decrease inflammation.
The ECS regulates dopamine production via the CB1 receptors. Dopamine release is regulated by GABA and glutamate. Both of these neurotransmitters are regulated by endocannabinoids (or phytocannabinoids) binding to the CB1 receptor. Acute THC use increases dopamine release, while chronic THC use decreases dopamine release. CBD is also a partial agonist of the dopamine receptor.
Human studies with 75 mg or 300 mg of CBD per day showed no motor improvement but did improve quality-of-life scores. Dosages of greater than 150 mg of CBD alone did show improvement of psychotic scores, but no motor improvement. Israeli researchers found inhaled cannabinoids improved motor symptoms of tremors, rigidity, and slowness of movement, and a 30 percent decrease in pain intensity, for people with Parkinson’s.
Begin with 25 mg of a high-CBD strain over the day and move up to 150 mg if needed. At 50 mg of CBD, begin doses of THC starting at 1 mg per mL.
Hypoxic damage to the brain from stroke, cardiac arrest, near drowning, birthing complications, or trauma can result in immediate and progressive cognitive decline.
Endocannabinoids and phytocannabinoids both bind to CB1 and NMDA (glutamate) receptors, offering protection against excitotoxicity from excess signaling by glutamate and oxidative stress. The body increases 2AG naturally after injury as a protective mechanism. It binds to CB1 receptors to further help attenuate the excitotoxicity.
Research with mice showed increases in post-injury recognition memory and spatial memory and decreased apoptosis of neurons and decreased inflammation with CBD dose equivalents of 68 mg to 2,040 mg both pre- and post-injury.
Start at 15 mg CBD two to three times per day and work up to a total of 500 mg per day.
Some people with atypical neurological conditions (including autism spectrum disorder, seizures, and epilepsy) have been shown to respond positively to cannabis.
Autism spectrum disorder (ASD) affects 1 percent of the U.S. population; 10 to 30 percent of those individuals have concurrent epilepsy. Forty percent of people with ASD do not respond to standard pharmaceutical treatment. Current pharmaceuticals used — many of them with children — include antipsychotics, mood stabilizers, benzodiazepines, serotonin reuptake inhibitors, and stimulants. We’ve recently begun to question the ethics of giving strong psychoactive drugs to children; especially since the drugs have not been tested for that population.
Children and adults with ASD are deficient in social-reward processing and have atypical responses to facial expressions and emotions. Babies who neurologically feel less reward for gazing into the happy faces of their caregivers will look less often and have fewer social interactions than neurotypical children.
The ECS oversees cognitive function, emotional regulation, social function, motivation, and reward processing. A diminished ECS is a possible mechanism of action for ASD.
Imaging has shown that ASD people have abnormalities in volume, connectivity, and activity in the reward centers of the brain that respond to social stimulus. The phasic release of dopamine in the reward centers is the primary mechanism encoding reward behaviors. Dopamine release is regulated by GABA and glutamate. Both of these neurotransmitters are regulated by endocannabinoids binding to CB1 receptors. This is a possible link between the ECS and ASD. ASD people have fewer CB1 receptors and lower AEA blood levels. AEA levels are also lower in people with temporal lobe epilepsy.
CBD-rich cannabis is known for its anxiolytic, antipsychotic, and immunomodulatory effects and for increasing endogenous AEA. Rodent and human studies show the benefits of CBD-rich cannabis for ASD.
In one human study, adults took 68 to 720 mg of CBD per day (using a CBD:THC ratio of 20:1) for 7 to 13 months; 61 percent showed improved behavior, 39 percent showed improved anxiety, and 47 percent showed increased communication. Thirty-three percent of the participants used fewer meds or decreased their dosage, 24 percent stopped meds altogether, and 8 percent used more. The authors of the study recognized that one flaw was that not all participants used the same CBD-rich cannabis.
Based on the few human studies, start at 50 mg of 20:1 CBD:THC per day and work up as needed.
Anti-epilepsy drugs are the leading treatment for childhood epileptic seizures. One-third of people with epilepsy do not respond to two or more of these drugs and are deemed intractable or drug resistant. Twenty new seizure medications have been developed in the past decades without much improvement, so it’s understandable that parents are looking for alternatives.
A young girl named Charlotte Figi, who has a rare seizure disorder called Dravet’s that caused her to experience 300 seizures per week, and her mother, Paige Figi, breathed life into the paradigm shift we are seeing regarding the use of cannabis as a treatment option for seizures. It’s been a long time coming — cannabis was documented on Sumerian Akkadian tablets in 1800 BCE as being used for “night convulsions.”
The precise cause of epileptic seizures is unknown, but they involve an electrical storm of neural activity within the brain. This electrical activity is caused by an increased release of the excitatory neurotransmitter glutamate leading to excitotoxicity and behavioral deficits.
When neuronal activity (as seen in seizures) increases, endocannabinoids are produced on demand to bind to presynaptic CB1 receptors, decreasing the release of glutamate and increasing the release of GABA and therefore decreasing seizures. Over the long term, CB1 receptors are upregulated. Cannabis modulates excitation in a multitude of ways:
Dosing ranged from 0.02 mg/kg (1.4 mg for a 150-pound person) to 50 mg/kg (3,400 mg for a 150-pound person). The lower range was always used with whole-plant extract, and the higher range was used for purified CBD extracts.
The generalized results of 10 studies on 1,400 people are as follows:
We must remember again to use the minimum effective dose. Reduction of seizures has been found at just 0.02 mg/kg. That’s 1.4 mg for a 150-pound person (pharmaceutical companies recommend a starting dosage at 10 times this amount!).
Pain relief is the most commonly cited reason for cannabis use and is our first known record of use. It is one of three conditions fully endorsed by the National Academies of Sciences, Engineering, and Medicine. To fully understand the multilayered brilliance of cannabis’s interaction in the body to relieve chronic pain, we will take an in-depth look at the pain pathway itself and then look at the mechanisms through which cannabis intervenes.
Chronic pain differs from acute pain in that it lasts longer (at least three months) and is maladaptive (doesn’t shut off automatically). Four categories of chronic pain exist: nociceptive, inflammatory, neuropathic, and dysfunctional. We will discuss nociceptive, inflammatory, and neuropathic.
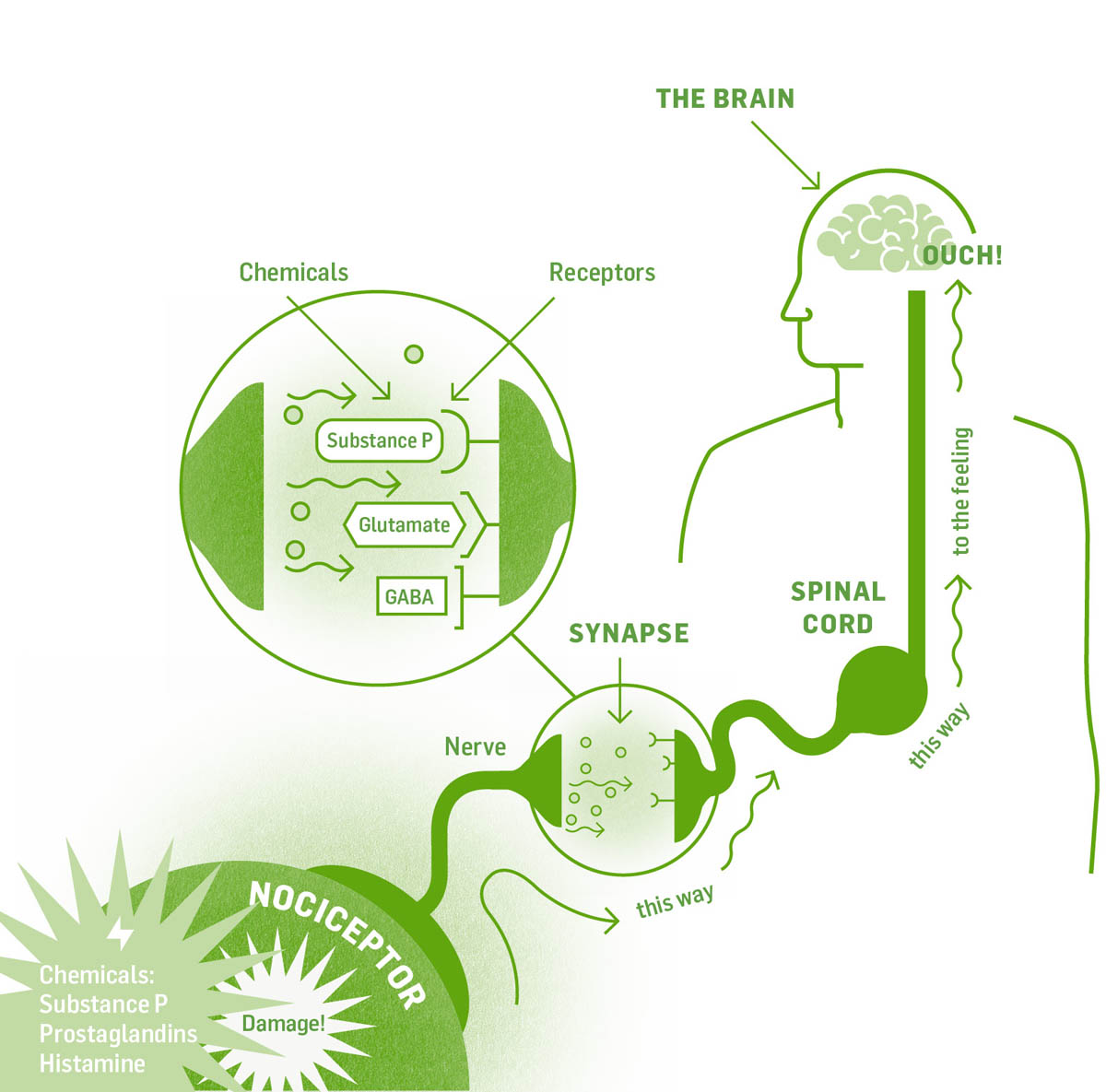
In nociceptive and inflammatory pain, damaged cells release chemicals that initiate inflammation and healing. Substance P (SP) is one such chemical; it binds to NK-1 receptors on sensory neurons carrying pain signals, triggering electrical nerve impulses that carry the pain message up to the brain.
The pathway is not one long, unbroken cord but rather many neurons in a path, separated by synaptic gaps. The nerve impulse cannot jump across these gaps. Instead, chemical messengers (neurotransmitters) are released that cross the gap and bind to receptors on the next neuron, which carries the electric impulse along. Synapses for the pain pathway occur in the spinal cord, brain stem, thalamus, limbic system, and sensory cortex of the cerebrum. Neurotransmitters include substance P and glutamate (both of which are excitatory and keep the impulse going) and GABA, which is inhibitory (it stops the signal). The end point for pain signals is the sensory cortex in the brain. Once the signal arrives there, you become conscious of the pain and know where in the body it is. As you already know from experience, it takes less than a second to stub your pinky toe and feel the pain.
With this understanding of the pain pathway, let’s look at the endocannabinoid system. Endocannabinoids are produced, on demand, in both neural and nonneuronal cells in response to tissue injury and excessive pain signaling. They suppress inflammation and decrease sensitivity to pain.
The endocannabinoids AEA and 2AG, as well as THC and CBN, mediate pain by binding to CB1 receptors, which decreases the transmission of the pain impulse by decreasing the release of SP and glutamate. They also increase the production of endorphins.
CB1 receptors exist on presynaptic neurons within the central nervous system, colocated with vanilloid (TRPV1) receptors. Vanilloid receptors, when bound to by some agonists, trigger the transmission of pain signals. All the agonists listed above bind to and desensitize vanilloid receptors, making them less sensitive to agonists and less apt to transmit a pain signal.
CB2 receptors are found on immune cells, some neurons, and the microglia. Baseline levels of CB2 receptors on microglia are low, but under pathological conditions CB2 receptor production is upregulated. When 2AG, AEA, THC, or caryophyllene binds to CB2 receptors, the release of inflammatory cytokines slows and neurons are protected from oxidative damage.
CBD boosts the effects of AEA by preventing its breakdown by FAAH. (Nonsteroidal anti-inflammatory drugs [NSAIDs] work this way too.) CBD also potentiates the analgesic effects of THC. In the skin, endocannabinoids binding to CB2 receptors on keratinocytes (skin cells) stimulates the release of beta endorphin, which binds to mu receptors and decreases pain.
Microglia contribute to chronic pain via two main mechanisms. First, when trauma occurs in the nervous system, microglia activate and secrete inflammatory cytokines to increase healing and the immune response, causing further inflammation and pain. Activated microglia then increase the number of CB2 receptors on their surface and increase their production of AEA, decreasing pain and inflammation. This mechanism of activated microglia becoming more sensitive to agonists binding to CB2 receptors, and decreasing the release of inflammatory cytokines and relieving pain, initiates the cessation of the inflammatory response.
Neuropathic pain found in people with cancer, diabetes mellitus, multiple sclerosis, and peripheral nerve injuries can be alleviated by CBD’s inhibition of microglia activation and migration within the spinal cord and brain.
The second mechanism is carried out by a separate receptor on the microglia, the TL4-R (toll-like receptor). When the TL4-R is bound to by a class of chemicals called alarmins, immune-stimulating and neuroexcitatory cytokines (IL-1, IL-6, and TNF) are released. Studies have shown that blocking the release of these cytokines decreases pain. Where do the alarmins come from? When any cells of the body are damaged (from inflammation, physical trauma, or chemotherapy) the damaged cells release alarmins into the blood; they eventually end up in the cerebrospinal fluid of the central nervous system. From here they diffuse out to interact with the TL4-R receptors on the microglia, signaling pain.
Opioid and cannabinoid receptors are both present in pain-signaling regions in the brain and spinal cord, and their pathways interact. Administering cannabinoids (as low as 1 mg THC) with opioids results in a greater-than-additive analgesic effect; THC can potentiate opiates by 30 percent.
The prostaglandins that initiate inflammation can also initiate the pain response. AEA, 2AG, THC, THCA, CBD, CBC, myrcene, pinene, and caryophyllene all slow the release of inflammatory prostaglandins. Studies have found that cannabis helps with inflammatory pain due to burns, fibromyalgia, irritable bowel syndrome, atopic dermatitis, and pancreas and pelvic pain.
Chronic or unwanted spastic contraction is another cause of chronic pain. Neurons in the brain initiate a nerve impulse that travels to a synapse at the muscle, where the neurotransmitter, acetylcholine, crosses the synaptic gap, binds to its receptor, and signals the muscle to contract. Spastic pain occurs when the signal continues to fire when not initiated by the brain. We work with this type of pain using antispasmodics and sedatives.
Antispasmodics decrease chronic contraction by decreasing excitatory-neurotransmitter release at the synapse of the neuromuscular junction. THC, CBD, and myrcene work here. Their antispasmodic properties relieve pain related to chronic contraction and multiple sclerosis spasticity.
Sedatives work within the central nervous system by decreasing the excitability of the spinal motor neurons that stimulate muscles. They work on the whole system rather than targeting just motor neurons, so they make people feel sleepy or sedated. Sedatives are also used as anti-anxiety medications and as anticonvulsants. Benzodiazepines, Jamaican dogwood, California poppy, THC and myrcene in cannabis and hops, all increase the release of the inhibitory neurotransmitter GABA to cause sedation.
The National Academies of Sciences, Engineering, and Medicine in 2017 released its report indicating “conclusive high-quality evidence” (its highest rating) for the use of cannabis for chronic neuropathic pain and cancer pain.
The Canadian Pharmacists Association in 2018 released an evidence guide for the medical use of cannabis that indicated “moderate quality evidence” for cannabis with general or acute neuropathic and cancer pain.
A 2015 review of the medical use of cannabis for chronic pain and cancer pain looked at 28 studies totaling 2,454 people. The review found moderate-quality evidence for cannabis as a pain medicine. Smoked cannabis provided the highest reduction of pain (probably due to its whole-plant nature).
In a recent U.S. study of 2,400 people, 80 percent of respondents found CBD to be very effective. More than 66 percent of respondents reported that CBD was more effective than their prescribed medications, and 42 percent reported replacing their prescribed medication with CBD. Fifty-four percent of the respondents used CBD for joint pain, 35 percent used CBD for muscle tension with cluster headaches, and 32 percent used CBD for other forms of chronic pain.
A 2016 seven-month Israeli study of 176 people with chronic pain looked at cannabis, opiate use, and quality of life. Individuals were able to dose as frequently and as high a cannabis dose as they deemed necessary. Forty-four percent stopped opiate use, 39 percent decreased opiate dosing, 80 percent had an increase in function, and 87 percent reported an increase in quality of life!
A 2018 review by the National Cancer Institute’s Cancer Integrative, Alternative, and Complementary Therapies Editorial Board stated that cannabis contributes to pain relief for people living with cancer.
Finally, a summary from a recent review of 20 clinical trials states that using whole-plant extracts has been shown conclusively to provide more effective pain control than THC alone.
Cannabis works well for spinal cord injury, peripheral neuropathy, nerve injury, brachial plexus damage, amputation, phantom pain, rheumatoid arthritis, complex regional pain syndrome, musculoskeletal pain, fibromyalgia, migraine, and cancer.
It is not recommended for acute pain.
Pain-management doses start at a 1 mg oral dose of 1:1 THC:CBD every 4 to 6 hours, working up to 10 to 15 mg per dose if needed. Evidence suggests that increasing the dose above 15 mg does not increase pain relief. Use inhalation methods for breakthrough pain.
Cannabis can provide relief from pain either alone or in combination with opioids. Oral dosing for use in combination with opiates starts as low as 1 mg THC per opiate dose.
Before addressing conditions of the psyche — anxiety, depression, schizophrenia, and posttraumatic stress — it’s helpful to delve into some basic brain physiology and the role of the ECS in stress response, because all of these conditions share some common physiology. Understanding this shared physiology will help us understand how cannabis works with each condition.
The ECS sets an overall tone of safety in the body so the organism can learn new things, express curiosity, rest, relax, eat, and evolve. A healthy ECS decreases our acute response to stress, maintains a healthy response to perceived threats, and allows the extinguishing of fear.
In a healthy person, the amygdala, hippocampus, and prefrontal cortex are full of CB1 receptors and have high levels of endocannabinoids that regulate the tone of the nervous system. The amygdala helps create emotional memory and adapt to fear. It is regulated by the prefrontal cortex and the hippocampus, so keeping these areas healthy and full of synaptic connections is in our best interest. This regulation is diminished in conditions of the psyche. Chemically, the ECS carries out its functions with endocannabinoids binding to CB1, CB2, and TRPV1 receptors, regulating serotonin and glutamate levels.
The physiological mechanism of fight-or-flight is a complicated piece of equipment we are endowed with, and it serves our survival well. The ability to channel resources toward defending the body or running away is appropriate when we are under attack. The issue we face is not the hardwiring but that we can’t log out or disengage and the neurochemistry keeps sending danger messages when in fact there is no danger.
The fight-or-flight cascade begins when the amygdala senses danger in conjunction with changes within the endocannabinoid system. AEA released within the brain promotes a sense of safety and well-being. In order for us to mount our fight-or-flight stress response, AEA levels must immediately drop (the chemical equivalent of “Hey, all is not well!”). AEA levels drop when the amygdala releases corticotropin-releasing hormone (CRH). CRH increases the amount of the enzyme FAAH, which degrades AEA. The stress response proceeds through the hypothalamic-pituitary-adrenal axis when we release norepinephrine (adrenaline) and cortisol and are able to fight off anything our amygdala has perceived as threatening. Eventually, 2AG levels go up as cortisol levels go up, usually 20 minutes to an hour after the threat has passed. The rise in 2AG shuts down the stress response, and our feelings of safety return. 2AG, acting at the forebrain (cerebrum, hypothalamus, and thalamus), also allows us to habituate to a repeated stress; eventually we are able to adapt to it without entering into a fight-or-flight mode.
Mounting evidence exists that early stress and trauma can change the ECS’s stress response. Chronically depressed and anxious people have fewer CB1 receptors and lower levels of endocannabinoids, making the fight-or-flight response more difficult to modulate.
According to the Western medical model, people with conditions of the psyche (depression, anxiety, and PTSD) have one or more of the following: decreased modulatory role of the hippocampus and prefrontal cortex over the amygdala, a decreased production of serotonin or decreased binding of serotonin to its 5-HT1A receptor, or increased binding of glutamate to its receptor. Most pharmaceutical interventions target serotonin or glutamate levels. We will use this model of intervention when examining the role of cannabis in the conditions of the psyche. But first, let’s take a look at each of these neurotransmitters separately.
One function of serotonin is to modulate the amygdala’s response to stressful situations. There are high levels of serotonin receptors in the modulatory regions of the prefrontal cortex and hippocampus. Pharmaceutical antidepressants and antipsychotics work via the serotonin receptors.
Micromolar doses of CBD inhibit the degradation of tryptophan, the amino acid precursor for serotonin. Further studies have shown that CBD may be beneficial in diseases associated with immune activation and inflammation that lead to decreased levels of tryptophan. Evidence also suggests a mechanism whereby CBD’s ability to decrease inflammation and neutralize free radicals and ROS by activating glial cells could prevent neuronal changes leading to depression.
CBD and CBDA (100 times more powerful CBD) bind to serotonin receptors. CBD also binds to dopamine receptors. CB1 receptor agonists AEA and THC activate an increase in serotonin. Studies have shown that the efficacy of AEA is equal to that of pharmaceuticals. Binding of CB1 receptors by its agonists increases the release of serotonin, dopamine, and norepinephrine (all linked to mood) from neurons and decreases their reuptake. CBG is a serotonin antagonist and should not be part of a formula for conditions of the psyche.
Decreasing glutamate is a pharmaceutical target of antipsychotic drugs and obsessive-compulsive disorders. The binding of the endocannabinoids and THC to CB1 and TRPV1 causes a decrease in glutamate. CBD, by increasing AEA and binding to TRPV1 directly, decreases glutamate. CBD blocks THC-induced psychosis via this mechanism.
CBD is a real superstar of amygdala modulation. It increases blood flow to and functioning of neurons in the prefrontal cortex. CBD also stimulates neurogenesis in the hippocampus and increases synaptic connections. All of this allows one to override an overactive amygdala in a fight-or-flight crusade to keep you alive.
Functional MRI shows that CBD activates the regions that modulate the amygdala (prefrontal cortex and hippocampus), quieting the amygdala. CBD and THC show enhanced reward-system activation, and both increase the production of neuron growth factors, which increases the size of the amygdala-modulating hippocampus.
CBD increases the blood-brain barrier function, and there’s evidence of diminished barrier function in depressed individuals. Binding of THC and endocannabinoids to CB1 receptors increases neuron growth factors, increasing neurogenesis in the hippocampus. Further benefits of THC binding to CB1 receptors are inducing euphoria and focusing the mind on the present. Remember, when unopposed, THC can cause anxiety and, at higher doses, psychosis, so when working with the psyche and THC, lower doses are recommended in conjunction with higher-dose CBD.
Anxiety disorders are the most common group of mental illnesses in the United States, and relief from anxiety is one of the most commonly reported motives for working with cannabis. Anxiety is a crucial and necessary emotion. The tension and worry we feel when faced with an unknown or fearful situation causes us to act for our survival. Avoiding predation served our ancestors. It allowed them to survive and reproduce. But continuing to worry after an immediate threat has passed is not beneficial to us. It sets up neural pathways that keep a person in a constant state of fight or flight, never allowing for full rest or relaxation and setting the stage for disease.
Anxiety is a disease of the mind, the emotional body, and the spirit body. To bring about true transformation, we need to work with all components of the person; otherwise we merely manage symptoms. Cannabis has the unique ability to work at all three levels.
Working with cannabis for anxiety requires us to commit to our own learning and healing while we temporarily find relief from maladaptive neural ruts of anxiety. It is good and right to receive relief, to feel what life is like when not constantly listening to the narrative of anxiety. This relief is the gift cannabis offers us. Our co-creative role in this relationship is to commit to our own healing and learning and to create new neural grooves. Curiosity is a good ally.
One area for possible misuse of cannabis and its ability to decrease anxiety is when feelings of anxiety signal the need for a significant change in a person’s life. Our emotions are chemical messages carrying vital information for our health and well-being. If you are in a stressful and demanding situation that requires a lifestyle change, utilizing cannabis (or any anxiolytic herb or drug) will only manage the symptoms, not the underlying causes. Paying attention to our symptoms is a way to tune in to the change we may need in our life — whether you’re changing jobs, leaving an unhealthy relationship, or grieving over the death of a loved one or the current state of the world. Taking an herb or a pharmaceutical that dulls the message is not always helpful. Just because it’s herbal doesn’t mean it can’t be misused to manage the symptom rather than address the cause.
Anxiety may manifest during cannabis withdrawal. During the first three days of abstinence, people may experience nervousness, restlessness, irritability, and sleep disturbances, the very things they may have been hoping cannabis would help with. These symptoms usually peak within one week. After 28 days, all withdrawal symptoms disappear.
Common pharmaceuticals used to work with anxiety disorders are serotonin reuptake inhibitors, tricyclic antidepressants, and benzodiazepines (which work to increase the neurotransmitter GABA). In anxiety it is believed the amygdala is overactivated by a decreased amount of serotonin or GABA.
There are limited human clinical trials for cannabis and anxiety, yet relief from anxiety is one of the most common reported motives for people using cannabis. The National Academies of Sciences, Engineering, and Medicine released a report in 2017 indicating “limited evidence” for the effective use of cannabis for anxiety.
Two separate studies focused on the anxiety of public speaking tested the efficacy of isolated CBD and found that doses of either 300 mg or 600 mg decrease anxiety. An animal study found chronic rather than acute administration of CBD works better for reducing anxious behaviors. An observational study of veterans who self-administered cannabis found a 75 percent decrease in reliving traumatic experiences and avoidance.
Serotonin reuptake inhibitors, tricyclic antidepressants, and benzodiazepines are all strong pharmaceuticals with adverse effects. Less than half of people using these drugs show complete and sustained remission of symptoms, even after long-term treatment.
Given the nature of anxiety and the tendency for THC to cause anxiety, cultivar selection is crucial. High-CBD plants with very little THC are recommended to start. The terpene profile should also address specific anxiety symptoms. For example, if a person is anxious with low energy (depressed), stimulating terpenes like terpinolene and pinene are good choices, as are the uplifting floral (linalool) and citrus (limonene) terpenes. Higher myrcene levels will tend to be more sedating and grounding.
A good protocol is to start with 2.5 to 5.0 mg of high-CBD tincture every 6 to 8 hours. Increase the dosage to 50 mg if needed over a few weeks. When at an anxiolytic dose of CBD, THC can be added, starting with 1 mg of THC per dose. Remember the rule of minimum effective dose: more is not necessarily better. If you gain relief with 5 mg of CBD there’s no reason to use more.
Depression is defined as a feeling of sadness and/or loss of interest in activities once enjoyed, low mood, hopelessness, trouble concentrating, and pain. It affects 6.7 percent of adults, with 15 percent of men and 30 percent of women experiencing depression at some time during their life. There are many theories about how and why people develop depression — from genetic predisposition to trauma to environmental factors. Other illnesses such as chronic fatigue and hypothyroidism, and some pharmaceuticals, can mimic or cause depression.
Cannabis use for melancholia dates back 150 years in America. Fifty percent of people using pharmaceuticals for depression do not achieve long-term relief. Given that statistic, it’s not surprising that 30 to 50 percent of people using medical cannabis said they came to cannabis for relief from depression.
The classic pathophysiology of depression is thought to stem from three areas: neurochemical deficits (serotonin levels), neurodegeneration of the hippocampus and prefrontal cortex, and disturbances in the hypothalamic-pituitary-adrenal axis (the pathway of the fight-or-flight response). Chronic inflammation, a disrupted gut biome, and dysregulation of the immune system can also be causative factors.
A heightened stress response initiated by the amygdala’s perception of threat translates to an increased fight-or-flight response. This is often coupled with impaired functioning of the prefrontal cortex and hippocampus, which modulate the amygdala’s response.
Neurotransmitters that signal reward and pleasure and allow us to concentrate, such as dopamine, serotonin, and norepinephrine, may be at lower levels in depressed people. Decreases in motivation, pleasure, and reward can stem from a decrease in neurological firing in the reward centers of the brain.
It’s interesting to note that depressed, anxious, and stressed people have a smaller hippocampus than healthy individuals. Remember, the function of the hippocampus is to regulate mood, memory, and the amygdala.
The endocannabinoid system relates to depression in a few ways. People with a genetic variance for fewer CB1 receptors may be predisposed to develop depression, especially after stressful events. Mice bred without CB1 receptors become sensitive to depression, have a heightened amygdala response, experience less reward, have decreased serotonin levels and neurotrophic factors (which grow nerves), and have smaller hippocampal regions.
To maintain psyche health, we need a healthy, functioning ECS. You’ll remember that CB2 receptors, when bound to by 2AG and AEA, decrease inflammation, increase neuron growth factors, and modify the immune system. Tonic release of AEA within the brain promotes emotional homeostasis; 2AG shuts down the fight-or-flight process. With these functions in mind, it’s interesting to note that chronically depressed people have less 2AG and AEA and fewer CB1 receptors able to respond to the decreased amounts of 2AG and AEA. This results in a decreased ability to navigate the stresses of life. A smaller hippocampus and decreased firing from the prefrontal cortex results in a diminished ability to calm the overactive amygdala.
Mounting evidence exists that early stress and trauma can change the ECS’s response to stress. Functional MRI shows that CBD activates the prefrontal cortex and hippocampus, quieting amygdala activation. CBD and THC show enhanced activity in the reward system region of the brain, and both stimulate the production of neuron growth factors, increasing the size of the amygdala-modulating hippocampus.
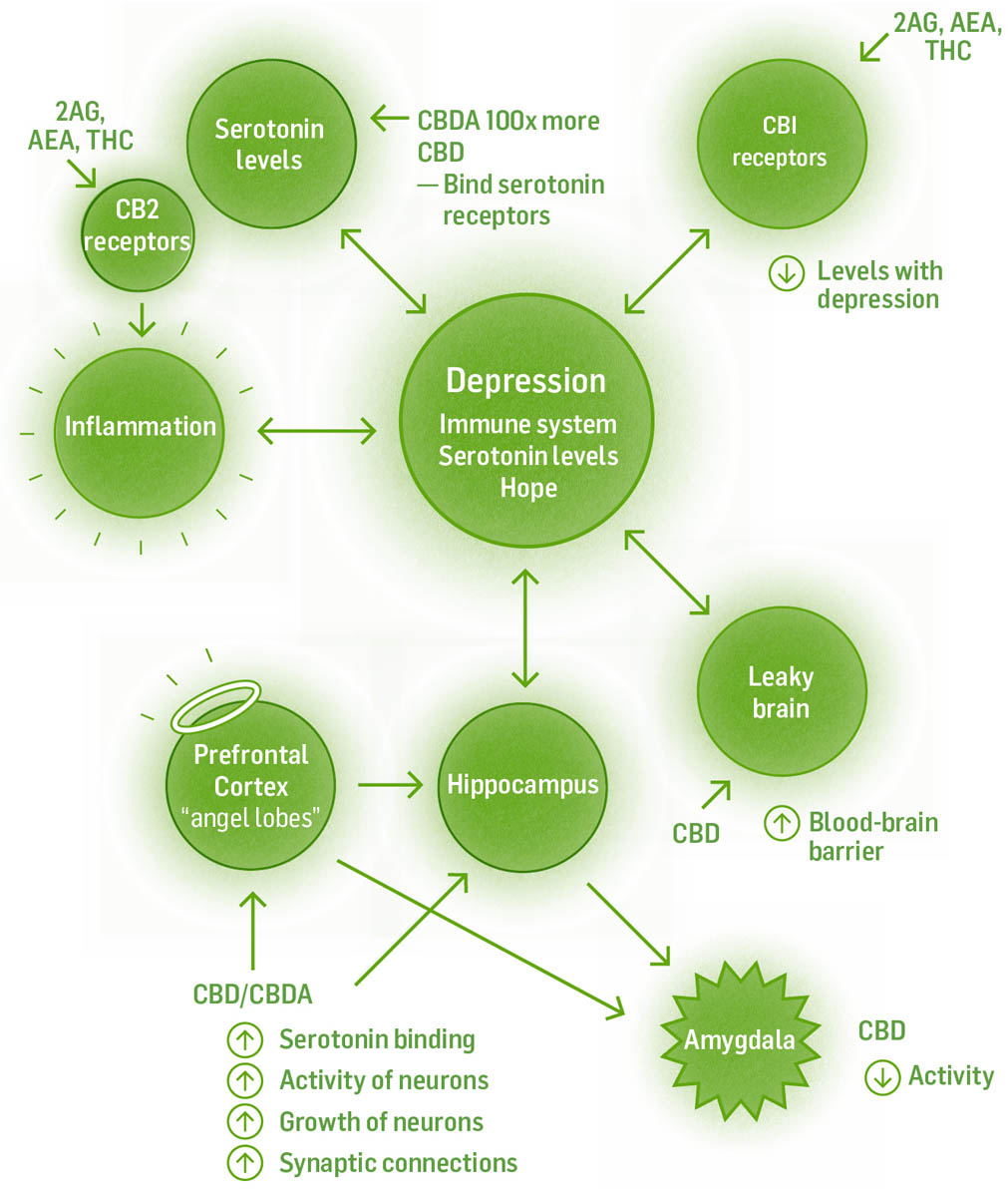
Cannabis interacts with depression through a multipronged approach. It activates modulatory regions for the amygdala, calms the excited amygdala, increases mood-controlling neurotransmitter levels, binds to serotonin and dopamine receptors, and stimulates neurogenesis. The Western medical model works with depression mostly by one mechanism: utilizing reuptake inhibitors, which keep serotonin or norepinephrine in the synapse longer.
CBD increases the blood-brain barrier function, and there’s evidence of diminished barrier function in depressed individuals. THC binding to CB1 receptors increases the release of serotonin, dopamine, and norepinephrine from neurons and decreases their reuptake. It also increases neuron growth factors, which increases neurogenesis in the hippocampus. CBD and CBDA (100 times more potent) both bind to serotonin receptors. CBD also binds to dopamine receptors. Linalool, found in cannabis, is another uplifting terpene, both anxiolytic and antidepressant.
Some of what we know about the ECS in relation to depression and anxiety comes from pharmaceuticals gone wrong. The drug rimonabant, approved by the FDA and put on the market for weight loss, was pulled when people using this CB1 receptor blocker were 2.5 times more likely to become depressed and two times more likely to commit suicide.
Rodent models of depression showed THC and CBD equal in efficacy for treating depression to pharmaceutical antidepressants. One study of depressed people showed 200 mg of CBD taken for 10 weeks showed an increase in hippocampus size, while no change was seen in healthy people. Limonene increases dopamine and serotonin levels in the prefrontal cortex; in a Japanese study, 9 out of 12 depressed people found enough relief with limonene to stop using pharmaceutical antidepressants.
Start with 5 mg of a high-CBD strain every 6 hours. Remember, some people find CBD stimulating; they should not take a dose after 5:00 p.m. A high-THC strain in doses of 2.5 to 5.0 mg lifts mood. High doses of THC can be anxiety promoting, so it’s best to stay at this low dose range.
Cultivars high in pinene should be avoided because some evidence exists that pinene may interfere with memory remodeling and fear extinction. Cultivars high in myrcene should also be avoided because they tend to sedate.
PTSD is a disorder some people develop after an intense experience of terror or helplessness. Symptoms of PTSD can include insomnia or nightmares, anxiety, an inability to tolerate frustration, anger issues, flashbacks, and hyperarousal.
Everybody experiences stressful or dangerous events at some point in life, but with time we usually forget to be afraid of them. Normally we can eventually learn to dissociate components of the event (say, a sound or smell) with the painful event itself. Healthy nervous systems don’t go on high alert with the slightest memory of the event. The ability to forget to be afraid and the uncoupling of the event with the strong emotions and sensations that occurred during it are all functions of a healthy endocannabinoid system and a healthy, functioning relationship between the amygdala and its modulators, the prefrontal cortex and the hippocampus.
People with PTSD are unable to forget to be afraid. They experience memories of a traumatic event as if the event is happening in present time. Benign stimuli can trigger painful memories of the event. Evidence exists that endocannabinoid deficiency and an atrophied hippocampus contribute toward developing PTSD after a traumatic event.
The primary goals in the treatment of PTSD are the extinction of the fear memory and the uncoupling of the fear memory from triggers. Another goal is a sense of safety and well-being. Cannabis is well equipped to support both these goals. However, even with all the benefits of cannabis, it is important to incorporate other modalities of healing along with cannabis when addressing PTSD.
PTSD sufferers show changes in the structure and function of the amygdala with a concurrent decrease in hippocampus and prefrontal cortex function. They also show a decrease in endocannabinoids and diminished CB1 receptor numbers in those areas.
Cannabis has been shown to be helpful with PTSD in a number of ways. CBD has been shown to:
THC helps with:
Take 5 to 10 mg CBD every 6 hours to start. If more relief is needed, use a 10:1 ratio of CBD:THC, 5 to 10 mg CBD plus 0.5 to 1.0 mg THC every 6 hours; 5 mg or less of THC smoked twice per day helps to increase sleep and decrease nightmares. Strains high in pinene should be avoided, as they may interfere with memory remodeling and fear extinction.
Schizophrenia is a chronic neurodevelopmental disorder with three main symptom domains: positive symptoms, negative symptoms, and cognitive defects. Positive symptoms are psychotic behaviors not generally seen in healthy people, including hallucinations, delusions, and paranoia. Negative symptoms are disruptions to normal emotions and behaviors: social withdrawal, flattened emotional expression, and lack of motivation. Cognitive defects can include an impaired ability to understand information, problems with working memory, and trouble focusing. Existing pharmaceutical medications offer minimal relief from positive or negative symptoms and no benefits (or negative effects) to cognitive deficits. Adverse effects of pharmaceuticals include diabetes mellitus and weight gain.
Schizophrenics are more sensitive to THC’s psychomimetic effects — those that mimic the disease. Psychomotor skills, short-term memory, working memory, verbal learning, and decision making are all negatively affected. The regions in schizophrenics’ brains responsible for information processing show lower levels of GABA and glutamate. When THC binds to CB1 receptors, glutamate and GABA levels decrease even more, which may be the basis for THC sensitivity. Pharmaceutical-induced GABA deficits replicate the psychomimetic effects of THC.
Cannabis rich in CBD, on the other hand, has been shown to alleviate symptoms with its ability to increase AEA by decreasing the degrading enzyme FAAH. CBD has also been proven to be anxiolytic and antipsychotic via different mechanisms.
Rodent models of schizophrenia showed improvement of both cognitive impairment and social interaction deficits with a 680 mg dose of CBD two times per day. One human study showed that 200 mg CBD four times per day improved both positive and negative symptoms as effectively as pharmaceuticals, without the adverse effects. Another human study showed an increase in positive symptoms and cognitive performance when 1,000 mg CBD per day was co-administered with existing medications.
Based on the literature, dose 200 mg CBD four times per day.
Sleep is essential for our health and well-being. Lack of sleep is linked to chronic disease, including chronic inflammation and pain. Our bodies, brains, and psyches need 8 to 9 hours of sleep per day to rest, rebuild, recover, assimilate the day’s events, and clear the circuitry of our neurochemistry so we can start fresh. Just one night of poor sleep decreases reaction time, decreases cognitive performance, lowers energy, aggravates pain and inflammation, and stimulates overeating.
Insomnia, the inability to fall asleep or stay asleep, ranks as a top reason people turn to cannabis. It’s not surprising that people turn to cannabis for help with sleep, given that 50 percent of insomnia sufferers are dissatisfied with pharmaceutical interventions. Moreover, many sleep drugs have serious long-term adverse effects, including dependency. Pharmaceutical sleep aids are meant to be used short-term, and when people stop using them, they can experience rebound insomnia and anxiety. And many pharmaceuticals used for other conditions can cause insomnia or sleep disturbances, especially antidepressants. Because inflammation and endocannabinoid deficiency are linked to many chronic diseases, and sleep helps in both of these situations, getting consistent, sound sleep is not a luxury but an essential component to our health.
Cannabis helps with both anxiety and depression, and people feeling less anxious or depressed are better able to sleep. However, here are some things to keep in mind:
The sleep cycle — falling asleep, staying asleep, waking up, and remaining awake — is governed by complex circadian rhythms and our endocannabinoid system. The ECS functions to maintain wakefulness and to generate sleep. (Doesn’t that sound delicious!) AEA levels increase at night, quieting the nervous system so we can fall asleep. 2AG levels increase during the day to maintain wakefulness. AEA and 2AG both bind to CB1 receptors to enhance wakefulness during the day and maintain deep (non-REM) delta sleep at night (a highly restorative sleep phase).
THC can be initially stimulating, but after 90 minutes tends to be sedating. All studies on sleep and cannabis show THC to be most effective either by itself or in a 1:1 ratio with CBD. CBD at low doses is stimulating and helpful for maintaining wakefulness. High-dose CBD (160 mg in one study) can help increase length of time sleeping and decrease sleep interruptions. One study also showed high-dose CBD to decrease REM behavior disorders (the acting out of dreams). CBD also decreases anxiety and helps excessively fatigued people fall asleep without providing too much stimulation for well-rested people.
CBN, the oxidized product of CBD and THC, is sedating and potentiates the sedative action of THC. Sleep-promoting terpenes include terpinolene, nerolidol, phytol, linalool, and doses of myrcene above 0.5 percent, because levels below that are mildly stimulating.
Forty percent of insomniacs are also depressed or anxious. Ninety-three percent of anxious or depressed people sleep better with cannabis. Many studies on cannabis and pain show an added benefit of helping with sleep. Pain levels decrease with increased sleep due to decreased substance P levels.
Dose and delivery method are important in helping with sleep. In general, 1:1 extracts or high-THC extracts work better for sleep in the short term. For most people, CBD after 5:00 p.m. is too stimulating and should be avoided unless in a 1:1 ratio with THC. The dosing range is 1.5 to 15 mg of THC. Here are some specific dose recommendations.
For Trouble Falling Asleep
For waking in the night
A note about edibles here. Remember, go low and slow with edibles, especially if you are new to cannabis. THC’s psychoactive and intoxicating properties may interfere with restful sleep.
Nausea is a feeling of sickness with an inclination to vomit. Vomiting is a protective mechanism that is easily provoked to ensure that you expel toxins quickly; when you eat something poisonous, getting it out of your body quickly offers the most protection. Delayed-onset nausea and vomiting, on the other hand, usually appears hours to days after the introduction of something noxious. This most often occurs during chemotherapy.
Delayed-onset nausea and vomiting are difficult to treat with conventional pharmaceuticals. This complex process can be initiated in the brain, the gut lining, or the inner ear, and it’s caused by a wide range of conditions: external stimuli like poison, toxins, or odors; and many pharmaceuticals, including antibiotics and opiates.
Cannabis helps with nausea and vomiting via three mechanisms: (1) CBD boosts AEA; low levels of AEA lead to nausea and vomiting; (2) THC and CBN bind to CB1 receptors, which decreases nausea and vomiting; and (3) CBDA and CBD bind fully to 5-HT1A receptors, and THC is a partial agonist.
Low-dose CBD and CBDA binding to serotonin receptors mediates nausea and vomiting and also helps mediate anxiety, especially in people with anticipatory nausea and vomiting.
The endocannabinoid system regulates nausea and vomiting. A high density of CB1 receptors are found in the vomit centers of the brain, the gut lining, and the inner ear. Lower levels of AEA and 2AG or low levels of CB1 receptors lead to nausea and vomiting. This may also play a part in motion sickness.
Serotonin is a neurotransmitter involved in the mechanics of nausea and vomiting, specifically when binding to its 5-HT1A receptors. Binding at this receptor is also anxiolytic, antidepressant, and provides help with migraines.
Cannabis has been proven repeatedly to alleviate nausea and vomiting, even in hard-to-treat anticipatory cases. In the broad study by the National Academies of Sciences, Engineering, and Medicine, cannabis, when used as an antiemetic in the treatment of chemotherapy-induced nausea and vomiting, received the highest ranking of “conclusive evidence for therapeutic use.”
Whole-plant medicine ranks as most effective. THC working at the CB1 receptors combined with CBD or CBDA binding to the serotonin 5-HT1A receptors both decrease nausea and vomiting. CBDA has been shown to be 100 times more effective than CBD and doesn’t show the potentiation of nausea and vomiting that CBD does at higher doses. Because of CBD’s potential to increase nausea and vomiting at higher doses in animal models, I recommend keeping the dose low (below 2,000 mg, which is quite high).
It is worth noting we are in the early stages of understanding CBDA, but given that it is 100 times more powerful at binding to serotonin receptors, it might be excellent for relieving nausea and vomiting and worth adding into formulations. THCA has also shown antiemetic and antinausea properties.
Dosing for THC has been studied more than CBD or CBDA. For acute nausea, start at a 2.5 mg dose of 1:1 THC:CBD — or replace some CBD with CBDA — and if needed, work up to the sweet spot of 10 to 12.5 mg for symptomatic relief. This can either be inhaled or used as an oral tincture.
For chemo-induced nausea, start two weeks before chemotherapy and work up to the sweet spot of 10 to 12.5 mg per day. The cannabis-naïve should start at 2.5 mg per day and work up to 10 mg per day to avoid intoxication. Avoid cultivars with CBG because it is a 5-HT1A receptor antagonist.
Although irritable bowel syndrome (IBS) and migraine headaches may not appear to have anything in common, they do share one common causative factor: deficiency in the ECS. So while we may have different herbal protocols for helping with these conditions, all clients will benefit from boosting the ECS.
IBS, also sometimes called spastic colon, is characterized by gastrointestinal (GI) pain, spasms, discomfort, and diarrhea or constipation. It can be brought on by anxiety, specific foods, overeating, and an unhealthy gut biome. IBS is the most frequently diagnosed condition by gastroenterologists in the United States, at 10 to 15 percent of GI patients.
The endocannabinoid system maintains tonic control of gastrointestinal motility and modulates secretion, inflammation, and pain along the GI tract. Thus, maintaining healthy levels of endocannabinoids or modulating this system using cannabis makes physiological sense for working with IBS.
When there is an injury or chronic inflammation of intestinal tissue, the body increases production of inflammatory cytokines. Cytokines signal an increase in smooth muscle contraction along the digestive tract, which increases fecal transit time and decreases a person’s ability to digest and absorb food. In response, the endocannabinoid system increases production of AEA, CB1 receptors, and CB2 receptors to alleviate the inflammation.
An individual with endocannabinoid deficiency syndrome (ECDS) loses some ability to upregulate the ECS to compensate for the inflammatory injury to the GI tract. So a partial mechanism of action for alleviating IBS is boosting the endocannabinoid system.
CB1 receptors are found within the enteric nervous system and in nonneuronal tissue, including intestinal mucosal cells, enterocytes (cells lining the intestine), immune cells, and enteroendocrine cells. Binding of the CB1 receptors by AEA, THC, or other agonists delays gastric emptying, decreases peptic acid secretion, and slows peristalsis.
CB2 receptors are found on immune cells along the GI tract and neurons of the lining of the tract. Binding of CB2 agonists to CB2 receptors modulates nausea, pain, and inflammation induced by increased transit time. TRPV1 nerve fibers, which are found at 3.5 times normal levels in IBS sufferers, are responsible for visceral hypersensitivity and pain. High-CBD strains boost endogenous AEA and directly desensitize TRPV1 nerves, helping to alleviate the discomfort.
Limitations of the few clinical trials with humans include (1) the use of synthetic cannabinoids in isolation; (2) single-dose administration rather than a longer regimen; and (3) the very few numbers of clinical trials.
Cannabis was the first effective intervention for the intense secretory diarrhea associated with cholera in the 19th century. Mouse models of cholera have proven that AEA binding to CB1 receptors alleviates secretory diarrhea. Pharmaceutical interventions targeting the 5-HT3 and 5-HT4 receptors were discontinued due to the adverse effects on the cardiovascular system and the development of ischemic colitis in people.
Conventional treatment utilizing anticholinergics, opioids, and antidepressants do not offer much relief. In 34 out of 42 studies on IBS, beneficial effects of probiotic supplementation were shown for one or more of the following: pain, discomfort, bloating, and distension. A healthy population of lactobacillus acidophilus stimulates an increase in mRNA for CB2 receptors in the gut.
I recommend dosing with 1:1 cultivars higher in anxiolytic and antispasmodic terpenes. If alcohol exacerbates symptoms, use infused olive or MCT oil rather than alcohol tincture.
Migraine presents as a hemicranial, beating headache that can be recurring and usually lasts from 4 hours to 3 days. It is often accompanied by nausea, vomiting, and sensitivity to light (photophobia) or sound (phonophobia), and it is sometimes preceded by an aura and is often followed by fatigue. Fourteen percent of Americans suffer migraines, with women having a threefold chance over men of experiencing them. Migraine may have several possible etiologies, including endocannabinoid deficiency, decreased serotonin levels, and trigeminal vascular system dysfunction.
Cannabis works with migraine via four known mechanisms: (1) increasing serotonin levels or acting as an agonist at serotonin receptors; (2) quieting overactivity of the trigeminal vascular system; (3) acting directly as an analgesic; or (4) boosting a deficient endocannabinoid system.
Low serotonin levels (40 percent below normal) are found in migraine sufferers. CB1 receptor agonists AEA and THC directly activate an increase in serotonin. Accepted pharmaceutical migraine interventions at serotonin receptors 5-HT1A (for potentiation) and 5-HT2A (for inhibition) can be reproduced with AEA. AEA also antagonizes the 5-HT3 receptor, known for emesis and pain. CBD and CBDA directly activate 5-HT1A receptors as full agonists, while THC acts as a partial agonist initiating the effects of serotonin. CBG is an antagonist and should not be part of a formula for migraines.
The trigeminal vascular overexcitation theory of migraine begins with a stimulating event (such as stress, trigger foods, or hormone levels) that activates axons of the trigeminal nerves. The activated nerves release vasoactive neuropeptides such as SP, neurokinin A, and calcitonin gene-related peptide, which cause neurogenic inflammation, vasodilation, leakage of plasma proteins, and mast cell degranulation (more inflammatory cytokines released and leaking into the brain). This vasodilation and the leakage of cytokines is thought to be responsible for the pain of migraines.
CBD strengthens the blood-brain barrier and lowers inflammatory cytokines. CB1 agonists AEA, 2AG, and THC inhibit A and C fibers of the trigeminal nerves, which decreases overall neuronal activity or the cortical spreading depression found in migraine sufferers.
AEA is tonically active, and its continued release in the periaqueductal gray (PAG) area (pain region) of the midbrain provides analgesia and prevents dilation of blood vessels of the dura in response to the release of vasoactive neuropeptides. It also prevents the hyperalgesia of phonophobia and photophobia that sometimes accompanies migraine. Deficiency of AEA prevents analgesia and vasodilation (the ability to vasodilate and vasoconstrict are necessary for healthy blood flow), and indeed in numerous studies migraine sufferers showed lower AEA levels due to increased functioning of the degradation enzyme FAAH and the AEA transporter. CBD decreases transporter function and FAAH activity to increase AEA levels.
TRPV1 receptor activation has also been implicated in the hyperalgesia of migraine. Desensitizing it with either AEA or CBD would be an effective intervention. Intranasal capsaicin has been used for migraine by brave souls. Oral CBD would be a less noxious and friendlier intervention.
Because the endocannabinoid system maintains tonic control of analgesia/hyperalgesia at the PAG area of the brain, low levels of AEA predict a tendency for migraines because this area is thought to generate migraine and pain in the brain. Photophobia and phonophobia are expressions of sensory hyperalgesia, which is modulated by the PAG. Studies where CB1 receptors are blocked show increased hyperalgesia. AEA is tonically released to modulate the trigeminovascular system, causing a decrease in vasodilation induced by calcitonin gene-related peptide and nitric oxide (NO), two vasoactive neuropeptides released upon nerve stimulation and known to induce neurogenic inflammation that leads to migraine. AEA causes a dose-dependent vasodilation as well. Migraine sufferers tend to have lower serum AEA levels due in part to increased FAAH enzyme levels and AEA transporter function. Cerebrospinal levels of AEA are also lower in chronic migraine sufferers. THC at low doses stimulates AEA biosynthesis.
The main treatment for migraines in Europe and the United States from 1843 to 1943 was cannabis. A Colorado-based survey of 120 self-reporting cannabis users for migraine found a decrease from 10.4 headaches per month to 4.6 per month. Numerous studies find evidence of endocannabinoid deficiency in migraine sufferers.
Lifestyle change is a large factor in managing migraine. Removing triggers and boosting the ECS form the foundation of working with migraine. Consider cultivars devoid of CBG and containing linalool and other soothing terpenes. Also consider CBDA for its increased capacity to bind to serotonin receptors. Mix CBD and CBDA in a 1:1 ratio. Work up to 80 mg total per day. Then work in a low-dose THC tincture. Use drops of 10mg/mL THC tincture for accute migraine relief.
Cancer and multiple sclerosis (MS) are grouped together here because at the root of both diseases is immune system dysfunction. In the case of cancer, the immune system cannot recognize cancer cells as foreign and kill them or it can’t keep up with killing them. With MS, the immune system is attacking its own cells, causing disease.
Cancer is a disease in which abnormal cells divide out of control. These abnormal cells no longer carry out their intended function but rather become freeloader cells, consuming nutrients, growing, and taking up space. In some cases, the cancer can metastasize, spreading to other tissues and consuming more resources. The issue for the healthy cells is that the cancer cells (I like to think of them as blob cells) don’t do the job of the tissue, and they consume resources the healthy functioning cells need. Further, cancer cells secrete chemicals that promote blood vessel growth (angiogenesis) so they can continue consuming and growing. Eventually, the tumor or tumors become so big and widespread that there aren’t enough resources for healthy tissue to carry out their functions. When we die from cancer, we die from the inability to perform the necessary tasks of the organ or organs with the cancer.
Our bodies make 10,000 DNA “mistakes” every day — we are essentially always precancerous. Luckily, we have ways of keeping these rogue cells in check: the immune system and the endocannabinoid system. Our immune system destroys rogue cells, and the ECS decreases tumor cell growth.
Our natural cancer prevention has a four-tier response. Our body can (1) kill rogue cells directly; (2) induce autophagy (cell eating) and apoptosis (natural cell death) of cancer cells; (3) prevent angiogenesis, metastasis, and tumor invasion; and (4) slow tumor cell growth.
The endocannabinoid system is also antitumorigenic. Endocannabinoid levels increase in tumors as a homeostatic mechanism to decrease their growth. Endocannabinoid degrading enzymes are downregulated (prolonging the effects of endocannabinoids), indicating the body’s natural healing response to cancer. Tumor growth rates decrease when fewer degradation enzymes of ECS chemicals are present.
The body’s immune system in its wisdom has developed a method of removing rogue cells. It produces natural killer cells (NKCs) that circulate in the blood, lymph, and connective tissue looking for foreign cells, viral particles, harmful molecules, or your own cells gone rogue. When NKCs find cancer cells, they send out cytokine signals to other immune cells for help, then go about poking holes in the rogue cells to kill them.
CBD increases intercellular adhesion molecule (ICAM) expression in cancer cells. ICAM increases NKCs’ adherence to cancer cells, thus increasing their effectiveness. CBD does not increase ICAM expression in healthy cells, unlike chemotherapy, which kills all rapidly dividing cells, healthy and cancerous alike.
Immune cells can also induce apoptosis (natural, programmed cell death) or autophagy in cancer cells. In the ECS, binding of CB receptor agonists on cancer cells induces apoptosis and autophagy via three different intracellular pathways: MAPK, PI3K, and inhibition of adenylate cyclase. Remember, endocannabinoids and THC bind to CB receptors. CBD, by inhibiting the degrading enzyme FAAH, increases AEA, which allows increased binding of CB receptors. CBD binding to GRP55 and TRPV1 receptors induces autophagy and apoptosis in tumor cells as well.
THC also decreases angiogenesis, the process whereby cancer cells encourage blood vessels to grow toward them. If the blood supply is cut off, the cancer cells will wither and die and won’t outcompete the healthy cells for resources.
THC inhibits angiogenesis by decreasing vascular endothelial growth factor and its receptors. THC also decreases the metastatic movement of cancer cells. In animal models, CBD also decreases metastasis. And CBD increases ICAM expression in unhealthy cells, which leads to decreased invasion by cancer cells.
When endocannabinoids bind to CB receptors, cell growth decreases in many tumor cell lines, including glioma, melanoma, breast carcinoma, hepatic and pancreatic cancers, thyroid epithelioma, uterine carcinoma, biliary tract cancer, cervical carcinoma, colorectal carcinoma, gastric adenocarcinoma, leukemia, lung carcinoma, lymphoma, oral cancer, pancreatic adenocarcinoma, prostate carcinoma, and skin carcinoma. It’s important to note that these are cell lines, cancers grown in petri dishes, not living tumors in humans. We can extrapolate that they would work in humans, but the research has not been done.
THC decreases cell growth and tumor growth by blocking epidermal growth factor (EGF). Cancer cells possess an unusually high number of receptors for EGF. Decreasing the amount of this growth factor makes it less available to bind to cancer cells, thus decreasing signals to keep growing.
CBD does not directly bind to CB receptors but acts via different mechanisms to decrease tumor growth. CBD has also been shown to decrease cancer cell viability in neuroblastoma, glioblastoma, melanoma, leukemia, colorectal, breast, lung, and prostate cancers.
THC does all of this anticancer work while protecting healthy, noncancerous cells. It has also been shown, along with CBD, to potentiate chemotherapy meds. Potentiating chemotherapy medications means you can take less of them, which results in fewer adverse effects of the chemotherapy medications themselves.
After injury or disease, the body’s response for healing is local inflammation. This can be shut down by increasing endocannabinoid production and/or increasing CB2 receptors. Both help decrease production of inflammatory cytokines and increase production of anti-inflammatory cytokines, ultimately decreasing inflammation. The level of CB2 receptor numbers on immune cells correlates with their activation state. When the body cannot stop inflammation, the inflammation becomes chronic. The ECS signals cytokine production, which directly signals activating or quieting of the immune system.
When endocannabinoids or phytocannabinoids bind to CB receptors, inflammation and the production of T cells (which make inflammatory cytokines) are both suppressed, T cell apoptosis is induced, and fewer immune cells migrate to and adhere to the area of injury. But if the inflammatory process shuts down too soon — for example, when there is a live pathogen or with certain cancers — increased damage may occur because the protective role of the immune system has been compromised. Understanding which cancer cells cannabis is effective against is important when working with cannabis to fight cancer.
There are also some cases where the ECS’s suppression of the immune response can lead to an increase in tumor growth due to an increase of cytokines IL-10 and TGF beta and a decrease of IFN gamma. Here, the balance between the antitumor actions of CB agonists and their immunosuppressive actions may shift in the direction of an increase in tumor growth. This is especially true in tumors with low CB receptor expression. They are less sensitive to the anticancer actions of the CB receptor agonists on the tumor while the immune system is still susceptible to the suppressive effects of CB agonists, with a net effect of encouraging tumor growth. In short, not all cancers are stopped by cannabis. The antitumorigenic properties of CBD via noncannabinoid receptor mechanisms or other palliative benefits listed for cannabis could still occur.
The following is a list of tumors known to respond to cannabis: hepatocellular carcinoma, endometrial carcinoma, glioblastoma multiforme, meningioma, pituitary adenoma, Hodgkin lymphoma, chemically induced hepatocarcinoma, mantle cell lymphoma, neuroblastoma, melanoma, leukemia, breast carcinoma, thyroid epithelioma, uterine carcinoma, biliary tract cancer, cervical carcinoma, colorectal carcinoma, gastric adenocarcinoma, lung carcinoma, oral cancer, pancreatic adenocarcinoma, prostate carcinoma, and skin carcinoma.
Currently, cannabis is recognized by Western science and medicine for use in palliative (quality of life) cancer care to decrease nausea and vomiting, alleviate pain, stimulate appetite, elevate mood, and relieve insomnia.
Research in tumor cell line studies, in vitro animal studies, and human trials suggests cannabis can directly and indirectly decrease tumor growth. Cannabinoids in nude mice curb tumor growth in lung carcinoma, breast carcinoma, skin carcinoma, melanoma, thyroid epithelioma, lymphoma, and glioma, while normal glial cells were unaffected.
A 2018 review by the National Cancer Institute’s Cancer Integrative, Alternative, and Complementary Therapies Editorial Board stated that cannabis contributes to pain relief for people living with cancer along with being an antiemetic, appetite stimulant, and sleep aid. All but 1 of 34 in vitro studies showed cannabis kills tumors selectively via antiproliferation, decreasing cell viability, and initiating cell death via toxicity, apoptosis, necrosis, and autophagy; cannabis is also antiangiogenic and antimetastatic.
In a study of 21 people with recurrent glioblastoma multiforme taking Sativex (a 1:1 CBD:THC whole-plant extract) with temozolomide, the one-year survival rate went from 53 percent with a placebo to 83 percent using Sativex; the median days of survival was 550 with Sativex versus 369 with placebo.
In an Israeli study of 2,000 people with cancer, improvements in reported symptoms were:
About half of these patients reported a 35.1 percent decrease in concomitant medications. Of 344 people using opiates, 36 percent stopped and 46 percent decreased their daily dosages.
A study on the murine model of glioma in mice found a dramatic decrease in tumor growth in vivo with a 1:1 extract used concurrently with ionizing radiation. Further, 50 percent less THC and CBD were needed when they were combined in the extract. The factored equivalent dose in humans was 136 mg of each given three times over one week.
Studies in murine models of cancer with silenced CB1 receptors show accelerated cancer growth. After the same CB receptors are activated, a decrease in growth is seen in mouse colon cancer models.
If working with palliative care, the dose is still MED of 1:1 full-plant extract. For specific symptoms (nausea and vomiting, alleviating pain, stimulating appetite, mood elevation, and relief from insomnia), refer to the dosing considerations listed for each separate condition in chapter 4.
Working with cancer-fighting aspects of cannabis is controversial, with limited human trials but many anecdotal reports. The dosing in animal studies ranges from 700 to 3,400 mg of THC per day for 10 days to 2 years. The regimen needs to be coordinated with a person’s primary care physician. The protocol consists of a regimen working up to 1,000 mg per day of THC and 400 mg per day of CBD. This is one of the few situations necessitating resin extracts. Please do not attempt to implement this protocol by yourself. Supervision with a provider who is experienced working with this medicine is important; depending on your cannabis tolerance level, you will need supervision at home for at least a month. A 1,000 mg daily dose is extremely high, and most people must work up to it. Tolerance of a level to return to “unsupervised life” takes three to five weeks.
Multiple sclerosis is an autoimmune disorder involving specific reactive T cells, macrophages, microglia, and astrocytes within the central nervous system. The body’s immune system begins to recognize the insulating fat surrounding the neuron (myelin sheath) as foreign and initiates inflammation. The secondary response occurs when specific T cells begin attacking the myelin, the cells that make myelin (oligodendrocytes), and the neurons themselves. This process leads to the demyelination of the neurons, making them less and less effective at conducting nerve impulses. The body can resynthesize myelin initially, but over time the neuron becomes scarred, giving the disease its name (“sclerosis” means “scarring”). Scar tissue, by definition, does not function like healthy tissue.
Because the disease targets neurons within different regions of the brain and spinal cord, there can be a spectrum of signs and symptoms depending on where the demyelination occurs. These signs and symptoms may include muscle spasms, tremors, ataxia (loss of muscle control), weakness or paralysis, constipation, and loss of bladder control.
Under healthy conditions, the blood-brain barrier blocks immune system access to the cells within the central nervous system. In pathological conditions, the blood-brain barrier becomes leaky, and helper T cells enter the extracellular space and gain access to neurons. The T cells mistake the myelin as foreign material and begin making the inflammatory cytokines IFN-gamma and TNF-gamma. Inflammation builds as astrocytes and microglia begin making their own inflammatory cytokines (IL-12, IL-13, IL-23, NO) and the excitotoxic neurotransmitter glutamate.
The next phase of the immune response continues when specific, activated T cells (cytotoxic T) — normally not allowed access to myelin — begin to attack the myelin. The body responds by upregulating the ECS, increasing CB2 receptor expression on astrocytes and microglia and making them more responsive to the inflammatory dampening effects of endocannabinoids. Circulating levels of AEA, OEA, and POA increase as well.
Another mechanism of healing is the activation of CB1 receptors. Agonists of the CB1 receptor work in myriad ways on the T cells themselves, including:
Agonists of CB2 receptors induce apoptosis of microglia and helper T cells, decrease TNF-alpha, decrease NO production, decrease IFN-gamma, and increase IL-6. CB2 agonists decrease the replenishment of microglia at the bone marrow, where they are made.
The net effect of cannabinoid receptor binding is a decrease in inflammation, decreased functioning of the T cells causing damage, and a reduction of activated microglia.
The National Academies of Sciences, Engineering, and Medicine published a report in 2017 citing conclusive evidence for the use of cannabinoids in improving spasticity in multiple sclerosis.
A survey of 112 cannabis-self-medicating people in the United States and United Kingdom found improved symptoms for spasticity, pain, tremor, and depression in more than 90 percent of cases. One study of 37 people utilizing smoked cannabis for three days found relief for pain in multiple sclerosis. A study of 279 individuals for 12 weeks utilizing oral cannabis and Sativex reported relief from muscle stiffness.
A large child/adolescent anxiety multimodal study (CAMS) showed decreased pain and increased mobility over 15 weeks at 2.5 to 5.0 mg THC. Numerous Sativex studies have shown cannabis to be effective at lowering spasticity and sleep disturbances as well as providing relief from pain and bladder incontinence; Sativex is now approved for use in Europe and Australia for these symptoms.
Low doses of THC were not effective. It wasn’t until THC levels increased above 7.5 mg did people see positive results. I recommend 1:1 MED, with the possible need to build tolerance in order to work up to at least a 7.5 mg dose.