High pressure and low food supply make for a difficult daily life.

On June 6, 1930, two men climbed into a hollow steel sphere, sealed themselves inside, and dropped headlong into the Atlantic Ocean. A few hours later, they returned to the blinding Bermuda sunlight, forever changed by what they’d seen. Naturalist William Beebe and an engineer named Otis Barton had descended into the blue-black depths in what would be the deepest dive in human history. Their vessel, a bathysphere, measured less than 5 feet across and offered only a porthole of fused quartz to look through. A single steel cable and a rubber air hose ran up to the surface, narrowly distinguishing the bathysphere from a tomb. They reached 800 feet—a monumental achievement that placed their names in history books—and came back because “some mental warning . . . spelled bottom for this trip.”1 But the bathysphere had proven itself, despite just the smallest of leaks, and Beebe and Barton kept pushing their dives deeper. Every new dive was a life-or-death experiment, allowing them to refine both their equipment and their methods. On August 15, 1934, Beebe and Barton reached a depth of 3,028 feet— inspiring the title of Beebe’s wildly popular account, Half Mile Down.
William Beebe was more than a scientist. He had an explorer’s soul and a poet’s pen. He unshipped them both to introduce the lumbering surface dwellers of planet Earth to the delicate ballet and the toothy terrors of the world’s darkest deepest places. What Beebe saw beneath the waves was completely alien to the human experience: pitch-black water, monstrous-looking creatures, delicate jellies, and the unending flicker of tiny lights in the darkness. A half mile down under tons of barometric pressure, Beebe felt more than anything else the weight of absolute loneliness: the knowledge that he was past the human world, viewing sights never before seen:
nowhere have I felt so completely isolated as in this bathysphere, in the blackness of ocean’s depths . . . we seemed like unborn embryos with unnumbered geological epochs to come before we should emerge to play our little parts in the unimportant shifts and changes of a few moments in human history.2

William Beebe and Otis Barton standing next to the bathysphere.
The dark unnerves us. Whether it’s in the space below the stairs or beyond the campfire’s edge, people are nervous about surprises that lurk unseen. The deepest reaches of the sea really are akin to another planet, subject to conditions unimaginable on Earth’s surface. Crushing pressure, deep cold, and eternal darkness rule the world’s basement.
Beebe immediately understood what he had accomplished. The bathy-sphere was a simple craft—a tiny helpless sphere with nothing but an open vial of soda lime to absorb CO2 from their breath—and yet it transported the adventurous pair to another world, entirely beyond human experience. One simple communication from 600 feet, sent by copper telephone wires up the air hose, conveys the weight on Beebe’s mind, like so many tons of salt water overhead: “Only dead men have sunk below this.”3
Deep-sea creatures are hugely varied, displaying a fascinating (and occasionally nightmarish) array of forms and adaptations that mold their lives to the pressures and temperatures around them. In the abyssal deep where the Sun never rises, razor-toothed fish hunt by deception, and giant worms dine on a soup of boiling chemicals. There is a quiet flow of simple beauty— delicate bodies built of jelly spinning their gossamer lives unseen and alone. And always, as Beebe observed, there is the flashing green glow of bioluminescence—light created by living creatures. Jellies sport glowing aquamarine racing stripes, and fish dangle lit lures that entice prey with a calculated spasticity. Helpless creatures set off their own fireworks when attacked, drawing even bigger predators in desperate life-or-death gambits.
Though Beebe’s journeys were pioneering, and his discoveries fundamental, the science was simple: observe, report, survive. His scientific assets were a camera, a good photographer, and an encyclopedic knowledge of deep-sea species brought up by nets and dredges.4 In the 80 years since Beebe’s dive, with a wealth of modern technology, we’ve come to understand a great deal more about the lives of deep creatures that the great naturalist couldn’t begin to know.
The most valuable food source in the ocean—the Sun—is completely absent in the depths. Photosynthesis is no longer an option where light is missing. And so no deep-sea mother ever demands “Eat your vegetables,” because there are none.5 Instead, in the abyssal dark, every species is a killer, a scavenger, or a landlord.
The landlords live in the neighborhood of Earth’s cracked floor. Boiling water gushes from continental wounds, agitated by the planet’s tremendous heat and laced with sulfurous poisons. The cracks are called hydrothermal vents, or more colorfully, black smokers.
The first hydrothermal vents were investigated in 1976, stunning the expedition scientists with their dense and diverse populations of new species.6 The slow delivery of food means that life generally lives on thin rations in the deep sea, and any one species usually has low abundance. Vents did not appear at first to be different: under a modern submersible’s floodlights, the vents appear as black clouds spewing from spires of accumulated sediment.7 The chemicals are poisonous to most terrestrial life and smell like rotten eggs.8 Yet hydrothermal vent communities were lush life in the deepest ocean darkness, hidden oases in the world’s most barren desert. Where was the food supply for this bonanza?
The food and the rotten egg smell both come from a simple chemical, hydrogen sulfide. For all its toxicity, the molecule’s sulfur bonds practically crackle with energy. Living in and around hydrothermal vents are bacteria that have mastered chemosynthesis: the conversion of the chemical energy of hydrogen sulfide into raw cellular energy. Chemosynthetic bacteria break the noxious sulfide molecules apart and use the resulting release of chemical energy to fuel microbial growth.9 That mastery lets them build new cells and power their metabolism by cracking the chemical energy bubbling from Earth’s crust.
Hydrothermal vents house a fascinating collection of bacteria living in a very odd place. But these deep communities consist of much more, because a unique set of animals has evolved to exploit this microbial abundance. Worms and bivalves and shrimp all live off the microbes that live off the sulfur. Shannon Johnson, an ecologist studying deep-sea organisms off the California coast, puts it succinctly: in the blackened deep, “Everything has to live with or off of bacteria to survive.”10 But they do not merely consume the bacteria—they rent out living space for them.
The microbes’ most visible beneficiary—the abyss’s most prosperous land-lord—is Riftia pachyptila, the giant tube worm. Nicknamed “living lipstick,” the worms’ white bodies are capped with vivid red plumes like folded flower buds. Worms take root near vents, building pale chitinous tubes to house themselves and growing up to 5 feet in length.11 The plumes resemble a pursed pair of lips, but Riftia has no mouth—these worms lack even a gut. Instead, they have organs near their plumes called trophosomes: fleshy sacs containing so many microbes they account for a good chunk of the worm’s body mass. The worms are sustained entirely by their resident bacteria, which process hydrogen sulfide from the hot water and pipe the excess product to the tube worm’s systems.12
A new species of crab clusters around hydrothermal vents in the Southern Ocean near Antarctica. From Rogers, A. D., P. A. Tyler, D. P. Connelly, J. T. Copley, R. James, et al. 2012. “The discovery of new deep-sea hydrothermal vent communities in the southern ocean and implications for biogeography.” PLOS Biology 10(1):e1001234. doi:10.1371/journal.pbio.1001234.
The lip-like structures are the deep-sea equivalents of leaves gathering sunlight. Absorbing huge quantities of hydrogen sulfide, CO2, and oxygen into tissue of the red plume rich with capillaries, they bind the molecules to a familiar protein: a hemoglobin like that found in our own blood. From the worm’s plume, the hemoglobin transports these molecules to the bacteria in the trophosome.13 Sulfides, oxygen, and CO2 are the ideal metabolic fuel to keep the trophosome factories running and the tenants content.14
Because Riftia tube worms have no mouth and no gut, they depend entirely on microbes for their food supply. But young worms are not born with on-board microbes, instead acquiring them during the larval stage. For decades, biologists thought the larval tube worms just swallowed the bacteria they needed, using a tiny mouth-like aperture that only the larvae had.15 Newer research reveals the truth: bacteria invade the young worm through its skin; later, the worm forms the trophosome to hold them. With the right microbes aboard, juvenile worms jettison their digestive tracts and live by digesting the excess bacteria growing in the trophosome.16
Like many rent-seeking arrangements, this is a fantastic deal for the landlords. Most deep-dwelling organisms grow and reproduce slowly, stunted by cold and deprivation. Riftia tube worms defy the trend, growing at astounding speed. Cameras on the Pacific floor have recorded tube worms colonizing a new site; they settled, reproduced, and grew to a thicket nearly 5 feet high in less than 2 years.17 They are among the fastest-growing marine invertebrates on the planet, standing in stark contrast to some of their own relatives. Other tube worms eschew hot vents for quiet deep-sea “cold seeps”: cold, slow hydrothermal vents with similar chemical output. They grow to equally impressive size, but take more than 200 years to do so.18 The extreme conditions at hydrothermal vents are hazardous, but they catalyze spectacular growth at Earth’s most inhospitable sites. More than 500 new species have been identified at hydrothermal vents in the few decades since their discovery.19 Many more will likely be found in the Southern Ocean near Antarctica, where the first expeditions have already uncovered a zoo of new species living at the hottest places in the coldest sea.20
Hydrothermal vents aren’t the only deep-sea oases bursting with unexpected life. Life blooms wherever resources exist, and not all bottom-dwelling communities are based on chemosynthesis. Some are fed by the riotous productivity of the sunlit layers thousands of feet above. Surface-water detritus, flakes of tissue, pieces of algae, and fecal matter, drift down in spiraling white flakes, solitary and slow, whimsically dubbed “marine snow.” The descent can take weeks, and most of the material is consumed high in the water column before it reaches the bottom. Bacteria are a major beneficiary of this trickling snowfall (see Chapter 3’s discussion of dissolved organic carbon), but snow is also a valuable food source for scavengers when it reaches the bottom. Still, living on marine snow is a thin diet for larger animals and does not generally support abundant species in dense communities.21
There is, however, an occasional bonanza for denizens of the deep, like winning the lottery for worms. A dead whale is an oasis on the sea floor.
Whales don’t fall like snow.22 Instead, they deliver huge lumps of meat to the ocean’s bottom, falling rapidly in one place and all at once. The largest whales are open-ocean swimmers, so they tend to die in cold, deep water. When they strike the muddy bottom with a soft gurgling thump, the scavengers of the deep go to work.23
An armada of hagfish, sharks, and squid arrives, somehow rapidly locating the carcass in the sea’s vast wasteland—a mysterious process akin to finding the only open coffee shop at midnight at a giant airport. A multi-ton whale carcass is stripped of most of its soft tissue within a few months.24 True bottom-dwelling organisms—mollusks, worms, crustaceans, and other simpler life forms—are largely absent, while fast swimmers tear through the carcass. This is the mobile scavenger phase, the first of three generalized stages of decomposition of whale falls.25
Once the meat is gone, it’s time for the next meal. Then the enrichment opportunist stage begins, when communities of bottom-dwelling scavengers colonize the carcass. Crustaceans and polychaete worms make up this phase, but a second wave of mobile predators also arrives to set up shop, ignoring the carcass to feed on the sessile scavengers. A community of scavengers and their predators feeds on the whale for months or years. Eventually, only the leviathan’s skeleton remains.
During the third and final period, what was a grim (if busy) boneyard transforms into an unexpectedly flourishing oasis. The whale’s flesh may be long gone, but its bones are filled with valuable oil. Bacteria take the lead, dissolving the bones while feasting on the lipids inside. These microbes aren’t Archaea, but they employ similar chemosynthetic processes, using dissolved sulfates to digest the whale bones.26
Whale-fall oases may fill a special and heretofore-underappreciated role in the ecosystem of the sea floor; they may act as stepping-stones between larger oases. Hydrothermal vents are ephemeral. They start and then stop, sometimes over the space of mere years. They may be separated by thousands of miles; only Earth’s fickle crust can say. Because some of the same species are seen at vents all over the world, some brave souls must have traversed the desert to populate new sites. Whale falls may fill in the gap: tiny isolated pockets of vent-like life peppering the gulfs between the more robust vent communities. Scientists estimate that more than a half-million whale falls exist in the planet’s oceans at any given time, deposited every few miles along the biggest migration routes.27 These are outposts, way stations in the darkness where sojourners may settle and reproduce. Their descendents will push farther into the desert, long after the original colonizers are dead.28
If organisms in the deep ocean have always been concentrated at vents and falls, their presence raises a troubling question. How has whaling affected deep-sea ecosystems? Human beings have, by conservative estimates, destroyed three-quarters of the planet’s great whales. Extrapolating from that hideous figure, the ocean floor must receive only a small fraction of the whale falls it used to. Oases, rare now, may have been far more common in the past. By hunting the great whales, we’ve spent centuries starving and fundamentally altering ecosystems that relied on their dead bodies.29
In February 2002, a research scientist at the Monterey Bay Aquarium Research Institute stumbled on a whale fall in a deep chasm offshore. Piloting the remotely operated submersible Tiburon, Robert Vrijenhoek sighted a gray whale skeleton on a ledge nearly 2 miles down. Though perfectly and magnificently complete, the bones didn’t glow the typical eggshell white under the Tiburon’s lights. They were mossy and gray, carpeted by a mysterious red growth. Moving closer, Vrijenhoek could see the color came from hundreds of crimson filaments, swaying slowly, even though the water was still. At the touch of a metal claw, they retracted instantly into the amorphous gray. This was not vegetation. Extracting a sample to the surface, the intrepid little robot introduced science to an entirely new species: Osedax mucofloris, the zombie bone worm.30
Whale-fall communities are highly diverse; up to 200 species may populate a single oasis.31 Osedax stands out among them all for its sheer eye-popping weirdness. The Latin name of Osedax mucofloris translates to “bone-eating snot flower,” and rarely has taxonomy been so descriptive. An irregular mass of brightly colored gelatinous tissue about the size of a fingernail, Osedax looks like nothing so much as the contents of a tissue after a sneeze. A single, delicate stalk of flesh extends into the water column, absorbing oxygen like a single strange gill.32
Osedax has no mouth and no digestive tract. Like its relative the great tube worm, it relies on symbiotic bacteria to survive. But whereas a Riftia worm pulls sulfur compounds from the water near vents, Osedax extracts nutrients from whale bones. The gelatinous body conceals its most crucial adaptation: specialized tendrils on the underside. Like tiny drills, the roots of the worm bore relentlessly into bones. A potent pairing of enzymes converts seawater into a powerful acid as the tendrils branch out like tree roots, scouring out lipids and conveying them to symbiont bacteria inside the Osedax. The end result is a highly efficient eating machine. Colonies of Osedax spread across the whale bones, riddling them with tiny Swiss-cheese apertures.33 They’re much faster than bacteria alone, and before long the skeleton is broken apart: scattered across the sea floor like so much shattered marble.
The little snot flowers had one more surprise in store for zoologists, who wondered why they could never capture an adult male. As it turned out, the answer was simple: there were none to find. Adult male Osedax do not exist. Only females grow to true sexual maturity; males remain stunted larvae. Nearly microscopic, they’re condemned to live as sperm-producing dwarves serving the vastly larger females. A typical large female holds dozens of males near her, protecting them so they can churn out sperm to fertilize eggs. But they are never fed. Males live their entire short lives off the egg yolk they were spawned with.34
At first, it appeared Osedax needed whale falls to survive. Their adaptations are so incredibly specialized that it seemed unlikely they would work well with other food sources. But scientists have convinced the worms to consume cattle and seal bones in a laboratory, and the eerie, branching tunnels typical of Osedax “roots” have been observed in the fossilized bones of prehistoric marine birds.35 Recently, Greg Rouse and his colleagues dropped fish bones into the deep sea to see what life they attracted. Osedax appeared in these piles, showing more initiative than previously supposed.36 The ocean’s floor is an endless catacomb, and Osedax work tirelessly, sweeping the darkened halls.
Away from whale falls and outside hydrothermal oases, the abyssal ocean is barren and lonely. Cold, darkness, and starvation slow life to a crawl. But it’s not just the cold, the dark, the emptiness—it’s the pressure. Anyone who’s jumped into a pool’s deep end knows the sensation: steely pneumatic fingers digging into your ears and pressing on your cheekbones. You feel the weight of every water molecule between you and the surface. Although the pressure is manageable at 12 feet of depth, an abyssal environment is something else entirely. With miles of water overhead, pressure in the deep sea can reach up to 1,000 atmospheres, or nearly 15,000 pounds of force per square inch of body surface. Even the most sophisticated submersibles must carefully hover above crush depth—the level at which the pressure will sunder their titanium hulls.
One of the biggest problems with pressure is that gas under high pressure compresses into a much smaller volume. Take the example of a diving seal. A human would take a full breath before a long plunge, but the seal does the opposite: she exhales before a long, deep dive. Pressure on her lungs increases from 1 to 10 atmospheres as she drops 400 feet from the surface. Boyle’s Law decrees that air will compress to a tenth its previous volume during this dive.37 The seal’s lungs obediently shrink. Because they were not initially full of air, the lungs compress essentially to a solid mass.38 This prevents nitrogen from dissolving into the seal’s blood supply—too much nitrogen in the blood causes “the bends” in human divers who descend too long and ascend too quickly. The other advantage of collapsed lungs is that they’re no longer buoyant, allowing the seal to spend less energy powering down from the surface to ever deeper depths.

A Styrofoam cup that has been taken into the deep sea by the Johnson Sea-link. Image courtesy of Ross et al., NOAA OE, HBOI.
By and large, scientists who work in submarines are not a happy-go-lucky kind of group. There is one whimsical tradition, though, completely in accord with Boyle’s Law, that deep sea biologists are known to follow: before a dive, they’ll strap a simple Styrofoam cup to the hull. The tiny sequestered gas bubbles that make Styrofoam such a good insulator surrender to Boyle’s Law, shrinking the cup both dramatically and permanently.39
There is no particularly clever point to this ritual. It may be simply that the souvenir reminds each deep-sea explorer that they were in an extraordinarily odd and different place.
When pressure reaches extremely high levels, it interferes with more than Styrofoam cups—it begins disrupting the very working of cells, because pressure alters how molecules react to one another. Animal cells are swaddled in an outer membrane made of lipids: hydrocarbon molecules loosely classified as fat. Normally, portals made of proteins in the membrane move nutrients and ions in and out, regulating the cell’s function, feeding it, removing wastes. But when membrane lipids are subjected to extreme pressure, they thicken and harden like bacon grease congealing in a mason jar. Cell membranes become congealed, and their gates shut down. The cell is unable to get what it needs, is unable to communicate with its fellows, and unable to function properly.40
In response, the deepest animals have re-engineered their membrane chemistry. A different lipid is used for membranes in the deep, one that remains fluid even under enormous pressure. One of the ways deep-sea animals accomplish this is by decreasing how much saturated fat they put in their cell membranes. Saturated fat is “solid fat,” made up of straight carbon chains with only single bonds between carbon atoms. This arrangement lets these molecules stack up solidly under pressure or cold temperature, like pieces of lumber stacked up in a lumber yard. Butter, meat, and dark chocolate are high in solid saturated fats and congeal easily, clogging up human arteries.41 In contrast, unsaturated fats have one or more double bonds between adjacent carbon molecules, which introduces a kink in the chain and keeps these compounds more fluid under pressure or low temperature. These molecules are more like bent tree branches than straight lumber—they do not stack up easily.
Margarine is low in saturated fats—the straight molecules, which constitute only 10–20% of the lipid total—and has a high proportion of unsaturated fats (the bent molecules). So, it does not congeal as easily as butter. As a result of this chemistry, an abyssal membrane is made more like margarine and less like butter: abyssal membranes contain fewer saturated fats. Surface fish like salmon tip the scales at about 35% saturated fat. Three miles down, evolution under high hydrostatic pressure has fundamentally altered the same tissues: fish contain only 10% saturated fats.42 At depth, the loose lipids are compressed by pressure but retain the right fluidity for function. And of course, the process works in reverse. Deep-sea animals brought to the surface don’t behave normally, even if treated with care. Chaos ensues as their unsaturated lipids melt and proteins fail to function at low pressures. As a result, modern deep-sea biologists must collect specimens very carefully. Organisms captured at extreme depth survive poorly unless they are brought to the surface in super-pressurized containers.
But many of the most interesting creatures will not fit into the small “upmersibles” that shipboard biologists use to retrieve deep-sea specimens. Because, despite the cold and the pressure and the lack of food, over the eons of evolutionary time, a few species have skewed the calculus of survival in their favor—by simply growing larger.
Most deep dwellers have cousins in more shallow waters, displaying differing colors, behaviors, and genetics.43 Along the west coast of the United States, the deep sea urchin Allocentrotus fragilis is a pale, fragile-shelled sister species of the common, tide pool purple sea urchin, Strongylocentrotus purpuratus. The fragile urchin was one of the first deep-sea species to have its whole genome sequenced and compared to a shallow-water relative. It’s long string of 28,000 or so genes is marked by change after change that signal the evolutionary transformations needed in the deep sea. It was not just a few genes that needed altering, but many.44
Some evolutionary changes adjust the basic size and growth patterns of a species. Many deep-sea animals have evolved small size, probably as a consequence of deep sea’s eternal famine.45 But some have separated themselves from their shallower cousins by paradoxically evolving in the opposite direction—a class of adaptations broadly termed deep-sea gigantism. Animals of astonishing size have been dredged from the darkness; movie-monster variants of common creatures.
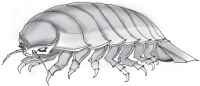
The giant isopod, Bathynomus giganteus, is one such species. Isopods are crustaceans with layered armored plates on their backs. They exist on land as well—the pill bug or potato bug, known for curling itself up into a ball is an isopod. The giant isopod is essentially a 20-pound pill bug. The largest measure about the size of a bag of Doritos corn chips, about 2 feet long from mandible to tail.46 Twelve scrabbling legs adorned with hooks and pincers sprout from beneath a broad, armored shield. Large compound eyes are set deep in the peach-colored plates of its face; head-on, it displays a menacing glare.47 This horror-movie beast, for all its creepiness, leads a simple life. It’s a scavenger and opportunistic predator, content to gnaw on a corpse or to devour slow-moving benthic invertebrates.
The giant Isopod Bathynomus giganteus. From the National Oceanic and Atmospheric Administration’s Ocean Explorer program. Photograph by Ryan M. Moody.
The precise factors behind deep-sea gigantism are a contentious subject among biologists.48 The giant tube worm, thankfully, is a relatively easy case: a simple, sedentary animal force-fed huge amounts of energy by symbiotic bacteria.49 For other deep-sea animals, gigantism occurs in cold, well-oxygenated waters—especially at the poles. More oxygen in the water may mean it is easier for creatures to supply oxygen to tissues deep in their bodies, especially for crustaceans that have relatively poor gills. But giant isopods also occur in poorly oxygenated water. This and other inconsistencies have presently shelved gigantism’s “oxygen hypothesis.”50
Two other features of the deepest sea might encourage gigantism: low temperature and environmental stability.51 Low water temperature is associated with larger cell size and larger overall bodies, but every cold-water species should theoretically benefit. Environmental stability should also be experienced by all species in the deep sea, making long life spans a better evolutionary bet than in shallower, more volatile waters. But when it comes to betting on survival, different evolutionary tactics can succeed even in the same environment.
Some species delay sexual maturity and continue to grow late in their lives before reproducing profusely.52 Funneling food energy into growth at the cost of delayed reproduction may yield greater success down the road if the animal is larger and can produce more young.53 But delayed reproduction is always a gamble, because any animal can die at any moment. So some species with high mortality rates breed like guinea pigs, growing only to the minimum reproductive size before throwing themselves into parenting. The deep sea fosters both strategies. Fast reproducers colonize oases near vents or whale falls, exploiting temporary resource booms to spread their genes. Other species take advantage of the deep sea’s slow-and-steady environment, living long and hoping to prosper.
For centuries, authors from Melville to Verne to Beebe romanticized the abyss. The edges of maps crawled with sea monsters: fantastic apparitions born from wonder and fear. As modern science directed its bright light into these dark places, the mysteries have faded one by one. But a demon of the Dark Ages survived, a legend never totally laid to rest. It is perhaps the only organism on the planet to inextricably bind together its own fact and fiction: the giant squid.
It is one of the ironies of the vastness of the sea that two types of behemoth cephalopods remain largely hidden from human view: Architeuthis dux and Mesonychoteuthis hamiltoni, the giant squid and colossal squid, respectively. The giant squid may grow a bit longer, though the colossal squid is broader, thicker, and much heavier.54 Casual accounts from the nineteenth and early twentieth centuries measured dead Architeuthis at up to 60 feet: Mesonychoteuthis might reach 80. But these numbers have never been confirmed. The largest captured colossal squid was about 33 feet in total length, from the tip of the mantle to the tip of the longest tentacles.55 Giant squid likely reach a maximum of about 40 feet: two animals of roughly this size reportedly washed up in Newfoundland in Canada in 1870 and are entered in the Smithsonian’s records of Architeuthis from published reports.56 A squid’s body is just over half its total length (the rest is tentacles),57 so a 30-foot specimen has a 16-foot body—about the size of a minivan. No other animal this size on Earth remains so poorly known (we think).58
Architeuthis prowl open ocean waters the world over. They are active predators, eating fish and other cephalopods. But they are also prey: their young are eaten by dolphins, fish, and even seabirds.59 But sperm whales (Physeter macrocephalus) are better squid hunters than any species, including humans (see the Prologue). Sperm whale stomachs often hold undigested beaks the size of softballs, and their flanks are adorned with battle scars from the hooks and spinning saws of mighty squid.60
Recently, a band of scientists pooled samples from around the world to examine the genetics of giant squid.61 They found two odd patterns: first, even though they had samples from all the world’s oceans, the squid’s genetics suggested a global species with no separate populations in different parts of the world. Second, there was little genetic diversity among giant squid. It seemed as though the global population had only recently expanded from a bottleneck in just the past few hundred thousand years. These genetic history patterns eerily resemble those of the giant squid’s chief predator, the sperm whale, which also has low global population differences and low genetic diversity consistent with recent population expansion.62
The colossal squid masses higher than the giant squid—up to 1,100 pounds—and sports vicious hooks along its arms and tentacles. It is restricted to the remote Southern Ocean around Antarctica, and so few specimens have been recovered. The largest on record was snared by a fishing boat in the Ross Sea in February 2007. The squid was chomping on Patagonian toothfish (usually called Chilean seabass) in the boat’s nets, stubbornly and foolishly refusing to abandon its fine meal. Once frozen, the squid was given to the Museum of New Zealand, where it can still be seen today.63 Steve O’Shea of the Auckland University of Technology quipped it would make “calamari rings the size of a truck tire.”64
Until 2004, neither giant nor colossal squid had been seen alive in its natural habitat; all the world’s knowledge came from beached or floating carcasses, from an occasional animal near the surface, and from the squid beaks that accumulate in sperm whale stomachs like so much loose pocket change. But then a Japanese remote submersible captured one of the world’s most elusive images: a 15-foot giant squid in its habitat, hunting 3,000 feet below the waves.
Tsunemi Kubodera of Tokyo’s National Science Museum and Kyoichi Mori of the Ogasawara Whale Watching Association had spent countless hours trolling the deep ocean for giant squid with little success. When luck finally broke their way, the cameras were ready. Video from the dogged pair shows a squid in action, a single floodlight illuminating silver skin as the animal advances toward a nugget of bait. Arms extend and blossom with geometric perfection, studded with white suction cups lined with serrated teeth. There is an amazing grace to this apparition, arms swirl in a ballet of exploration of the caged bait, and then the animal moves gently away as if spooked. Grainy pictures do no justice to the scale of this apparition; those arms could enfold a family sedan. Yet the animal was never aggressive except in the businesslike way of predators. Popular images of giant squid show them cherry red with rage (as happens when at the surface, fighting for their lives) and thrashing their tentacles like whips. Kubodera and Mori captured Architeuthis in its element, breathtaking and utterly in control.65
Where are the truly huge specimens, like those Captain Nemo fought on the deck of his Nautilus? It is possible that exceptional individuals exist, somewhere in the ancient blackness beneath the planet’s seas, hidden under ice caps, or among the volcanoes of the vast Pacific. If a creature grew beyond the strength of the largest whales, no mysterious squid beaks would appear as gastric evidence of the existence of a third giant squid species. If it lived its life in the hidden vastness of the deep sea, avoiding submarines, we’d have no way to know about it. Imagination will always tempt us more than reality: we’ll always draw monsters on the margins of maps.
We know about species because we drag them up to the surface and name them. But in the deep sea, these species know one another as well. They do not have name tags like you might at a tedious high school reunion. Instead many of them are bedecked with lights.
Imagine yourself a tiny, helpless fish in the limitless darkness of the deep sea. The blue-black water has neither ceiling nor floor: it is like dark moonless sky stretched above and below. Yet the endless night is not peaceful. You are forever being watched by hundreds of eyes anxious to grasp just a few slivers of light. Predators lie concealed in the dark all around, gnashing needle teeth in untold numbers. And at any moment, a tiny trickle of sunlight leaking in from up above might betray you.
But there is other light—like the moonless sky has stars. A constant flickering of blue and green surrounds you, faint and furtive spikes of light that could mean anything from a fresh meal to a gruesome death. The deep sea is the only ecosystem on Earth—barring deep caves where only fungi grow— where the main source of light is not the Sun, but an organic protein.
Luciferases, or photoproteins, generate light by splitting high-energy molecules—producing photons instead of metabolic energy.66 Some fish have luciferase genes and array these glowing proteins inside a small pit in the skin, a specialized light-producing organ called the photophore. Most fish secrete their own luminous compounds, but some grow sacs packed with symbiotic light-producing microbes.
Bioluminescence is the sea’s most important tactical adaptation. Deployed in careful patterns on the underbelly, photophores of some fish match their output to the faint light coming from above. Thus a fish below them cannot detect their passage as they cruise above, giving them stealth as predators or prey.67 Simple plankton make a great deal of light noise, blaring photons at the slightest disturbance and filling the deep sea with inane visual chatter.68 This chatter may actually serve a purpose—experiments have shown that when a shrimp consumes some types of plankton,69 the plankton shoot off bioluminescent sparks. Predatory fish rush in like a SWAT team, attracted by the alarm call, devouring the shrimp and ignoring the tiny plankton. Marine biologist Steve Haddock and his co-workers have recently documented as many as seven different defensive roles for bioluminescence in deep-sea fish.70 There are offensive roles as well: bright lights can stun or confuse prey, attract them to dangling lures held in front of monstrous jaws, or serve as high-beam headlights to find morsels drifting in the water column.
Perhaps the most famous of these innovators is the anglerfish, which hunts with a long fleshy lure. The generic “anglerfish” describes an entire family of spectacularly ugly animals. Lacking dorsal fins, the anglers have moved the spines that would typically form these fins forward to a spot just above the eyes. The first spine is thickened and greatly elongated into a protruding digit and crowned with an irregular bulb of tissue called an esca: a bioluminescent lure.71 The lure’s spongy tissue is thoroughly inhabited by hard working, light-producing microbes. They cause the esca to glow enticingly in the dark water, as the host fish sells the illusion. Like a seasoned bass fisherman, the angler makes its lure unbearably enticing. Twitching, bobbing, turning in loops like a frantic frolicking worm, a glowing blob becomes an irresistible target. Little predatory fish, dwarfed by the angler, approach the esca and strike with all the force they can muster. They vanish instantly, without sound and barely a perceptible movement: sucked into the huge mouth and impaled on a flash of needle teeth. Every anglerfish species has its own unique esca, some longer than the fish’s body, all luminescent.72 How the anglerfish detects nearby prey isn’t clear—their eyes are small and poor. Some have suggested the killing reflex is triggered by the slightest touch on the lure. What has become clear is that anglerfish will attack fish of almost any size. The record is a 4.5-inch anglerfish with a foot-long rattail fish in its mouth. Both predator and prey were dead when they were caught floating off the shore of Papua New Guinea.73

Stoplight loosejaw, Malacosteus niger. Published in Goode, B. G., and T. H. Bean. 1896. Oceanic Ichthyology. Special Bulletin 2. Washington, D.C.: Smithsonian Institution, plate 37.
Most luminescence is blue-green to match the deep sea’s weak sunlight. But the loosejaw group of deep-sea dragonfish (family Stomiidae) project a unique hue.74 Large and powerful photophores just beneath their eyes beam red light through the water. They accomplish this through a unique fluorescent protein in some species and a red-brown filter over the photophore in others.75
Red is an unusual color in the deep sea. Seawater absorbs red light and more easily transmits blue, and so most of the bioluminescence in the sea is in a far-reaching blue-green hue. The predators and prey of loosejaws have eyes particularly sensitive to this blue and green light, having evolved beneath a mile of seawater.
The loosejaws are a rare exception, specially evolved to see the red light that they themselves produce. The protein that fish use to capture light in their eyes, opsin, is specially modified in the loosejaws. At position 261, a unique mutation changes an amino acid that is critical to the way the protein absorbs light. As a result, this protein in loosejaws absorbs much more red light than do other deep-sea fish, and they can see the prey reflected in their own unique spotlight.76 Most deep-sea creatures can only flicker their lights—quick on/off bursts lest they are discovered and devoured by predators. In a dark world of killers, bright signals illuminate food but also summon death. The loosejaws—small and feeble compared to surface predators—have beaten this game, seeing red without being seen, prowling the abyss with crimson impunity.
The true character of the deepest ocean isn’t embodied by a stalking automobile-sized squid, or hundreds of 6-foot tube worms sprouting like weeds around a black smoker. When we imagine these things, we miss the vast bulk of the deep ocean. We typically think of water as clear, of light as omnipresent, of big things moving through empty spaces. The nature of the real abyss was captured by William Beebe. As he sat in his tiny globe, in the night of the sea, what stuck with him was not any of the fantastic predatory fish he’d seen—it was the lights. They bloomed against the darkness, twinkling and pulsing and filling the bathysphere’s tiny quartz porthole. All around him the lights spoke to one another in an unread language, telling a story of life and death, and the deception of desperate predators. Imagine these animals not as images from textbooks but as they would appear in their own world. Deprived of all light and cloaked in dark scales, they know one another only by the flickering of bioluminescence and the hint of a black silhouette.
As the first man to visit the deepest sea, Beebe felt an enormous responsibility. Having seen what no living person had ever seen, he was compelled to describe it. He understood that he had been to another world beyond terrestrial Earth. Writing 30 years before the first spacewalk, Beebe prophetically captured the abyss:
the only other place comparable to these marvelous nether regions must surely be naked space itself, out far beyond atmosphere, between the stars, where sunlight has no grip upon the dust and rubbish of planetary air, where the blackness of space, the shining planets, comets, suns, and stars must really be closely akin to the world of life as it appears to the eyes of an awed human being, in the open ocean, one half mile down.77