
The ocean’s smallest species have a huge impact on its chemistry and life.

At this very moment, there are 100 trillion bacteria on your seat. Don’t reach for the disinfectant spray—those multitudes bacteria are inside you. The human body houses ten times more active microbes than its own living cells. It’s a visceral reminder that for all of life’s progress, Earth remains a microbial world.1
Microbes are single-celled organisms too small to see with the naked eye. The group includes bacteria, and a bacteria-like group called the Archaea that tend to live in extreme habitats, as well as some more advanced single-celled species. The microbes were some of the first living things. They burst on the world stage more than 3 billion years ago, just after the cataclysms that shaped early Earth (see Chapter 1). Their modern descendants remain the most diverse life on Earth and retain an overwhelming power to control the world’s biosphere. They’ve explored more ways of living than any other type of creature, and today they crowd into every corner of every environment. From the jungle of your mouth to your palm’s dry desert to every drop of water on the planet, microbes rule the world beneath our very noses. The chemistry of the ocean itself was crafted by bacteria, and the planet’s smallest creatures play a jumbo-sized role in its maintenance.
Microbes are numerous but tiny. Lined up end-to-end, a thousand bacteria would barely span a period on this page. Amoebae and protozoa are larger; by the seventeenth century, naturalists could peer through early microscopes and see these tiny titans wobbling through samples of pond water.2 But their primitive lenses couldn’t pick out anything smaller, and for centuries bacteria remained hidden to science. Louis Pasteur, the great French biologist, was eventually able to prove their existence while also noting that microbes caused many diseases. The “germ theory” wasn’t as colorful an explanation for sickness as witchcraft or imbalanced humors, but it had the advantage of being correct. Before too long, even the most ardent skeptics yielded. Pasteur became a legend, and his theory now informs every branch of biological science.3
The famed Frenchman had been in his grave for a century before the technology would appear to adequately study the sea’s microbial life. The Englishman Charles Darwin perceived the importance of microbes as prey for larger organisms, but confessed he was stumped by the survival of the bacteria themselves. As he wrote in 1845, “I presume that the numerous lower pelagic animals persist on [microbes], which are known to abound in the open ocean: but on what, in the clear blue water, do these [microbes] subsist?”4
Starting about the 1970s, our understanding of oceanic microbes was radically overhauled. Researchers led by Lawrence Pomeroy and Farooq Azam used new methods of counting microbes in seawater.5 Their advanced techniques delivered a huge surprise, revealing not only new types of bacteria but population numbers beyond imagination. Projecting their results across the entire ocean, microbiologists faced a stunning realization: bacteria accounted for a big fraction of the ocean’s biomass. Whales, fish, lobsters and every other eye-grabbing animal were fleshy icebergs floating in a vast microbial sea.
Pomeroy and colleagues projected the oceanic bacteria population at 1029 organisms—a 1 followed by twenty-nine 0s, an inconceivable number representing more living creatures than there are stars in the universe.6 The number was a revelation, changing the picture of ocean ecology overnight. A column of open offshore water—an environment once thought to be unproductive— actually channels more metabolic energy through its bacterial residents than through any of its other creatures. This metabolic activity depends on diverse chemical exchanges. Some microbes consume tiny nuggets of dissolved carbon, while others dig minerals from the seawater. Multiplied by their sheer numbers, it’s a staggering amount of activity. And a staggering population size. Laid end-to-end, all the ocean’s bacteria would stretch thirty times around the Milky Way galaxy!7 Darwin’s “clear blue water” was anything but: it was a bubbling crockpot of life.
Microbes have the potential to control the chemistry of the ocean by virtue of their numbers and efficiency. There are about as many bacteria in a quart of seawater as there are people in India, about a billion. A billion bacteria weigh only about 0.1 milligram,8 but they can produce an outsized amount of energy if they are photosynthetic. Such is their metabolic rate that a colony of photosynthesizing bacteria the mass of 100 human beings could, under ideal conditions, produce as much energy as a nuclear power plant.9 When the conditions are right, bacterial populations harness this metabolic energy to explode into raging blooms that shift the ocean’s chemical balance.10
The most abundant photosynthetic organism on Earth was something of an ugly duckling; it waited a long time to be noticed and appreciated. It showed up first as a blot of unusual pigment in water samples from the North Sea, then as a mysterious lump in an electron microscope,11 and later as “noise” from the sophisticated instruments searching for marine microbes. The cells would eventually be named Prochlorococcus by oceanographer Penny Chisholm of the Massachusetts Institute of Technology and her colleagues. Prochlorococcus is among the smallest ocean organisms and is likely to be the most numerous worldwide, at least among the creatures we know well enough to put a name on.12 The world’s expansive seas are home to an estimated trillion-trillion individual cells of this species.13
This number is too big for our brains to easily process. Give this a try: if you took all the people on the planet and took all our cells (about 10 trillion per person, and there are 7 billion of us), and spread them out in the ocean, you would get about 70 billion trillion. It would take 15 planet Earths like this to equal the Prochlorococcus figure. So numerous are Prochlorococcus cells that their photosynthesis process and oxygen production support a good fraction of the rest of Earth’s life. Oceanographers aren’t much for grand pronouncements, but they estimate 10% of Earth’s atmospheric oxygen has come from Procholorococcus.14
Once these prodigious little engines were recognized, oceanographers started to find them everywhere. They live in so many places, except the polar oceans, that they have diverged, genetically, into what Chisholm calls a “federation” of cells adapted to live in very different parts of the ocean. And just a few decades back, we had no clue they existed.
Prochlorococcus hide in plain sight by dint of sheer size—or rather, the lack of it. Measuring a mere 600 nanometers (each nanometer is one billionth of a meter and one thousandth of a micron), two or three times smaller than most bacteria in culture bottles, they fade into the background even when you’re looking for microbes. Like electrons orbiting an atom, they seem to be everywhere while occupying no space at all. An average protein molecule is about 5 nanometers across,15 so Prochlorococcus cells can barely fit 100 protein molecules end-to-end inside them. Space inside the cell is so valuable that even DNA gets compacted, stripped down to just 1,700 crucial genes.16
There are smaller genomes in nature, but only a few. A tiny parasitic bacterium exists on primate genitals, boasting only 600 genes and complaining endlessly about “the view from down here.”17 But such parasites need hosts from which to sponge a living; their numbers never grow too large. Prochlorococcus makes food out of sunlight and is common enough in the wild ocean to be considered the true winner of the evolutionary race for abundance. How it does this with so few genes, and does it better than the larger genomes of other species, is not yet known.
Darwin’s dilemma was that all the bacteria he saw in the sea needed fuel: something that could feed a thousand trillion trillion cells. Prochlorococcus are photosynthetic, assembling food from sunlight and CO2. But the smallest, simplest bacteria don’t have that option. Dubbed heterotrophs, they’re unable to use sunlight to knit CO2 molecules together into bigger molecules, such as sugar (an ability that defines the plant-like autotrophs). Heterotrophic bacteria must feed on big organic molecules and break them down, usually burning oxygen in the process (as do larger heterotrophs like us mammals). They are amazingly efficient at this and almost totally indiscriminate in their tastes. They can eat anything below a certain size, starting with simple sugars. Large molecules—such as proteins and lipids—are chopped up like sushi rolls, separated into their component amino acids, and devoured. Many bacteria specialize in consuming complex oils, and some of these helped scrub the Gulf of Mexico following the Deep Horizon oil platform explosion of 2010.18 Bottom-feeding bacteria are even capable of absorbing snippets of foreign DNA through their cell membranes. They may strip it down, gulping down atoms of phosphorous and carbon. Or they may steal the DNA outright for its information content, crudely pasting new traits into their own genomes.19
This utter lack of choosiness makes heterotrophic bacteria the world’s greatest garbage crew. And it explains how so many bacterial cells can persist in the oceans. Every microscopic fecal pellet dropped by every planktonic crustacean is swiftly surrounded by colonizing bacteria. Sensing a new lode of precious materials, they kick their metabolisms into high gear. The pellet dissolves, breaking into smaller and smaller particles. The smallest bits, microscopic chains of carbon molecules by this point, are loosely classified as dissolved organic carbon (DOC). DOC forms strong bonds with dissolved nutrients and is a good food source, so it forms a high-efficiency slop that allows microbes to inhale calories at a prodigious rate.20 With 700 billion tons of dissolved matter in the ocean—more than every land animal and plant massed together21—DOC represents the world’s most eye-popping excess of food outside of a Las Vegas buffet.
Suppose you are a vegan at a typical American barbecue cookout. You might be hungry, but you’re surrounded by meat and dairy: food you can’t eat. Large marine animals face the same dilemma. The vast amount of food tied up in dissolved organic carbon—a big portion of the ocean’s whole biomass—can’t be consumed by the whales, sharks, fish, or tiny copepods. Bacteria small enough to feed on DOC are themselves too small to be eaten by most predators. Instead the industrious microbes provide every creature in the ocean with an enormous indirect benefit: by acting as the ocean’s recycler.
Because heterotrophic microbes will eat anything, biological “waste” is usually recycled. Bacteria consume the tiniest organic molecules, inhaling every last bit of energy and biomass, endlessly and rapidly replicating. When they die, their tiny bodies become DOC again. Many bacterial populations take 7 or fewer days to double.22 The amount of biomass cycled through this microscopic bank is astounding.
The total bacterial biomass in the oceans is about 11 billion tons.23 If every microbe reproduced every 7 days, they would produce about 11 million tons a minute. We humans take about 99 million tons of seafood from the ocean every year, a rate that we seem incapable of increasing. So, the global production of fish that humans use all year has the same organic content as that produced by bacteria in the sea in 90 minutes.
If this growth continued forever, the oceans would quickly fill with bacteria instead of water. But there are organisms that can quickly consume bacteria, culling their numbers like lions stalking in the Serengeti. These “lions” have manes of flagella, and though they lack claws or teeth, they are fierce predators. Every liter of seawater is a world of a billion microbes, and every drop is its own hunting ground.24 The predators are single-celled protists, like ameobae or Paramecium, and they can chew down the standing stock of bacteria as fast as it grows. That’s a very good thing for the rest of the denizens in the oceans, which are spared not only a glut of bacteria but also a dearth of diversity. Protists channel this food and energy upward, into the rest of the ocean’s food chain. The smallest creatures in the sea act as a universal currency for the largest and build a crucial foundation for the ocean’s amazing biomachine.
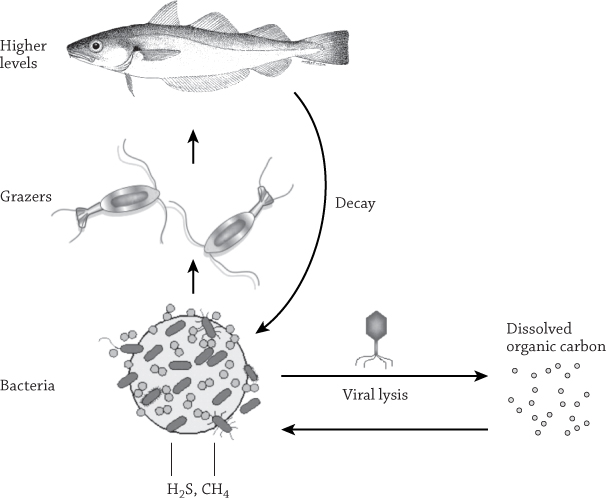
Schematic of the microbial loop/virus loop. Image from Chris Kellogg, U.S. Geological Survey.
It’s hard to overemphasize the scale of microbial production in the open water. Years after his famous discoveries about the hidden world of marine microbes, Lawrence Pomeroy wrote, “Earth’s ocean is a sea of microbes; without them it would be a very different place, less hospitable for all life. Indeed, without the activity of these organisms, the cycles of nature would very quickly come to a halt.”25 The tuna you see in the grocery store couldn’t exist without the microbial productivity sustaining the open oceans, funneling nutrients to larger life through predation. A tuna in the “clear blue water” looks large and powerful, but it’s sustained by trillions of invisible creatures.
Bacteria have a lot of metabolic tricks, often including the ability to become dormant when food is lacking or the balance of nutrients is wrong.26 Bacteria don’t die of starvation, they sleep through it. They do not die of old age, they simply divide and reconstitute their DNA to be young again. Bacteria rarely die in the passive sense; they’re killed.
Death often comes as described above: in the form of snarling protists, cells bigger than most bacteria and festooned with a fur of cilia or a set of whiplike flagellae. Patrolling through the water, they lack mouths to snatch and swallow their prey. Instead, each bacterium is collided with and held fast by sticky mucus. It sinks, slowly and still alive, into the protist’s body, where it is dismantled in a soup of digestive enzymes.
But it could be worse.
Death also comes from below, from tiny viruses much smaller than bacteria. Ocean viruses are hugely abundant, ten times more abundant that their bacterial prey.27 Barely meeting the definition of life as a self-replicating biological entity28 and only the size of large protein molecules, these viruses have little motile power on their own. They are complex protein bottles with a nasty surprise inside. The viral genome—its small thread of DNA-based information—is poised to take over a bacterial victim.
Like their counterparts that cause head colds in humans, these viruses function by attaching themselves to a cell and injecting their viral DNA into the host. Viral genes use the cell’s normal machinery to do what genes do everywhere: provide the instructions for making proteins. In this case, the proteins are viral proteins—proteins that kidnap the cell’s metabolism and use it to make more viruses. The cell doesn’t benefit and will no longer divide. Instead, it descends into living death as a slave factory for its viral controllers.
Quickly, the cell is forced to make the outer coatings of more viruses in vast numbers. Then the viral DNA is copied, coiled up, and packaged inside these simple sleeves like a plastic snake in a fake can of nuts. The hapless bacterium—now a virus factory—becomes a swollen, corrupted orb full to bursting with maggoty young. Eventually the ravaged cell can take no more, and it simply explodes. New viruses pour into the open water and disperse, already hunting for the next generation of prey.
Viral victims spew more than plagues into the ocean. The metabolic machinery of the former bacterium, battered and exhausted by the invaders, dissolves into the surrounding water like any other dead remains. Proteins, lipids, carbohydrates, and a host of rarer nutrients are then picked up by tiny heterotrophic scavengers. The remains become dissolved organic matter, feeding the microbial loop and connecting the discarded dead with the rest of the sea.29
This is the smallest predator-prey cycle in the world: viruses stalking bacteria, bursting them, and building up the dissolved food supply. And when scientists began looking into it and calculated how much biomass was involved, they were astounded: up to 30% of the ocean’s biomass was cycling daily (yes, daily) around this tiny, invisible predator-prey loop.30 Before the 1980s, both this system and its principal players were totally unknown.
Bacteria are incredibly diverse: perhaps a billion bacterial species inhabit Earth, with a good fraction of them in the ocean.31 But for all the myriad of species that ever lived, died, surged, and faded in the ocean, none ever achieved permanent dominance. Even a superabundant genus like Prochlorococcus is not very dominant ecologically; it exists alongside thousands of distant cousins. To this day, it’s rare to see a single species or colony crowd out the others. When we do, it is a signal of an ocean out of balance, and the explosion of microbes is disastrous: beaches are closed, seafood grows toxic, and even the air near such a toxic bloom becomes deadly (see Chapter 11).32
What hidden mechanism keeps the ocean from filling up with one super-bacterium? Why does explosive diversity seem to be an intrinsic microbial trait? One answer might lie in the evolutionary agility of the smallest predators of the bacteria, the viruses that search them out and burst them. Their extremely rapid life cycle and genetic manipulation has made them the world’s fastest-evolving predators.
A large population of bacteria, or any similar microbe, is a big prey base for viruses, and so the most successful microbes are also the biggest targets. Viruses can’t infiltrate their targets without special surface proteins that allow them to enter their victim’s cells. These proteins tend to be specific to one or a few types of microbes. A virus that evolves new surface proteins to target a large microbe population has a bright future. It has an enormous potential prey base and a huge potential growth rate. The cycle has all the elements of classic natural selection: mutation, predation, and tremendous reproductive rewards for the winners.33 And this cycle has a potentially huge effect on microbial diversity, too.
Oceanographers have given this cycle a name: “kill the winner.” Imagine an enormous bloom of microbes in the sea, clouding the water for miles with tiny bacterial bodies that are individually invisible. The bacteria in the bloom succeed by dint of some amazing adaptation to ocean conditions. But their very success makes them a target. Viruses mutate, and some of those mutations allow them to attack the huge hoard of bacterial prey, infiltrating the bloom. Fresh viruses exploded from a dead bacterium need only travel a few microns to the next bacterium to start the next killing cycle. Therefore, the theory goes, large microbial populations will inevitably come under intense viral attack, and the bloom will inevitably shrink. But only to a point. As countless host cells die, the bacterial population becomes so thin that the viral infection is no longer efficient. The viruses are still primed to kill, but they can’t move under their own power and have to bump into their prey. When the formally explosively abundant bacterium species becomes rare, its death rate drops, and its population stabilizes.
Kill the winner is a natural safety valve for success—a blind mechanism keeping any single microbe from taking over the environment. A microbe that is too successful will be pruned down to a smaller population size. We have seen it at work: on occasion, large single-celled microbes called coccolithophores bloom in the ocean. They grow so explosively that their blooms are literally visible from space. Using overhead satellites, microbiologists can observe the subsequent viral attack. From the edges, gaps and holes start to appear in the solid mass of teeming life. Within a week, the coccolithophores are decimated, their blooms shredded like high clouds on a windy day.34
The constant war of kill the winner generates an arms race between microbes and viruses, with adaptations and counteradaptations. Some infected coccolithophore cells initiate a cell-suicide process called apoptosis, killing themselves, throwing themselves on the proverbial grenade, to keep the virus from copying itself.35 The exploded cell dies but saves its daughter cells, copies cloned from itself in previous cell divisions. But evolution is happening in the virus too: more advanced versions of the virus contain a protein that inhibits apoptosis, keeping the host cell from killing itself. The most insidious may even take over this “suicide” machinery to further boost viral production.36 Still another escape strategy is exhibited by the single-celled coccolithophore Emiliania huxleyi. When under viral attack, these cells convert themselves to mobile escape pods with only half their DNA.37 Scenarios of assault, defense, and counterattack play out across the oceans and across the eons to create an amazing diversity of microscopic life.
Single-celled organisms and viruses are the fastest evolvers on our planet,38 and “kill the winner” is Earth’s single most high-stakes game. It’s been played in every drop of ocean, every day, for probably the past 3 billion years. Because it keeps any one microbial species from becoming too successful, too dominant, it may be partly responsible for the balance of diversity in the sea.
A few hints from the past suggest dire consequences when the microbial world is disrupted. One example was the mass extinction of 250 million years ago that wiped out 96% of all species on Earth: massive volcanism across the globe created a huge bolus of CO2 in the atmosphere. Massive global warming ensued, and the bathwater seas were awash in microbial blooms. The wholesale disruption of the ocean’s food chains, its ecological systems of checks and balances, caused an eruption of microbes that scoured the oceans of oxygen. The resurgence of normal ocean ecosystems was derailed for 5 million years.39
This might sound familiar: marine biologists today are concerned about rising CO2 levels and warming seas.40 Evidence from the past and parallel ecological studies of our current oceans point to a dangerous time in the ocean’s future if, once again, microbes come to rule the seas.