Critical care: overview
Critical care is concerned with the diagnosis and management of serious acute conditions requiring organ support and/or invasive monitoring. The specialty consists of ITUs and high dependency units (HDUs) for both adults and children. There are also specialist ITU centres, e.g. cardiac, neurological, and liver centres. The intensive care team uses a MDT approach involving doctors, nurses, physiotherapists, dieticians, microbiologists, radiologists, and pharmacists, to name a few. There is much overlap with the specialty of anaesthesia, where intubation, ventilation, invasive monitoring, and anaesthetic, inotropic, and vasopressor drugs are commonly used. ITU doctors can be from anaesthetic, medical, surgical, or emergency medicine backgrounds. Main indications for admission are patients who have single or multiorgan failure, who require intubation and ventilation, inotropic support, and haemofiltration. The critical care team’s management focuses on supporting failing organ systems, while diagnosing pathology and implementing therapy.
Cases to see
• Severe sepsis and septic shock.
• Organ dysfunction.
• Complex surgical patients.
• Pneumonia.
• Exacerbation of COPD/asthma.
• Life-threatening asthma.
• Diabetic ketoacidosis.
• Pancreatitis.
• Head injury/low Glasgow coma scale (GCS) score.
• Intracranial haemorrhage.
• Cardiac arrest.
Investigations/procedures to see
• Airway management: oro/nasopharyngeal airway devices, endotracheal intubation, tracheostomy.
• Anaesthetic and sedation techniques.
• Lines: arterial, central venous catheters (e.g. internal jugular, femoral, subclavian) using ultrasound guidance.
• ABG interpretation.
• Inotropic/vasopressor drug infusions with cardiac output monitoring.
• Haemofiltration and/or haemodialysis.
• Naso/orogastric tube.
Things to do
Attend the morning and evening ward rounds with the ITU team. This is essential to observe the systematic assessment of critically ill patients and decision-making processes. This is also a good opportunity to elicit classical signs on patient examination and hone your presentation skills. Closely shadow whoever takes ITU referrals as they will be asked to review the sickest patients in the hospital (on wards and in ED) for admission and escalation of support. Find out who carries the cardiac arrest bleep and attend arrests with them.
See Table 9.1 for common ITU drugs.
Critical care: on the unit
Intensivists are responsible for the critical care area, supporting other teams and reviewing other critically ill patients on the wards and ED. Some patients who have return of spontaneous circulation post cardiorespiratory arrest will be admitted to the unit if they have continued severe organ dysfunction requiring support. As intensivists’ interests are not confined to a specific system or set of diseases, they see a wide range of medical and surgical cases. As well as seeing a range of different diseases, your time with the intensivists can help you understand how to best to manage acutely unwell patients. This will be the part of your job as a junior doctor that most find daunting and difficult to deal with. However, with practice and more experience, you will find yourself feeling more comfortable with critically ill patients and be able to assess and initiate appropriate management faster as your insight develops.
An Airway, Breathing, Circulation, Disability, and Exposure approach represents a systematic focused assessment, where the most urgent life-threatening problems are identified and addressed first.
Organ support
Cardiovascular support: can include IV fluids (± central venous monitoring), inotropes or vasopressors (± arterial monitoring), and cardiac output monitoring. Some ITUs utilize aortic balloon pumps to improve coronary perfusion in patients with cardiogenic shock.
Respiratory support: oxygen therapy and mechanical ventilation (invasive or non-invasive). Non-invasive ventilation (NIV) may include CPAP or bilevel positive airway pressure via a tight-fitting facemask, and invasive may include intubation or tracheostomy and mechanical ventilation to provide adequate oxygenation and carbon dioxide elimination.
Renal support: involves haemofiltration and haemodialysis (see  p. 211 for different renal replacement therapies). Haemofiltration is usually better tolerated in hypotensive patients (e.g. septic shock + acute kidney injury (AKI)).
p. 211 for different renal replacement therapies). Haemofiltration is usually better tolerated in hypotensive patients (e.g. septic shock + acute kidney injury (AKI)).
GI support: patients in ITU have higher metabolic requirements and may be unable to feed themselves adequately. They may have true or pseudo-ileus as a result of surgery or systemic illness respectively. NG tubes are commonly used in ITU. This is certainly a good opportunity to learn how to insert them (under appropriate supervision). Other means of support include prokinetics (erythromycin, metoclopramide), enteral feeding (via NG, nasojejunal, or longer-term percutaneous endoscopic gastrostomy, or percutaneous endoscopic jejunostomy), and total parenteral nutrition. The ITU dietician is a valuable source of information regarding nutritional requirements of critically ill patients.
Sepsis
Overwhelming infection can have substantial systemic effects (e.g. vasodilatation 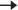 severe refractory hypotension) and
severe refractory hypotension) and 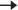 avoidable mortality, mainly due to delayed recognition and treatment. Know the difference between SIRS, sepsis, severe sepsis, and septic shock. Sepsis is managed in a similar manner almost regardless of the aetiology (e.g. UTI and LRTI): ‘Sepsis Six’ bundle; remember this as giving three things and taking three things ideally within 1 hour of presentation:
avoidable mortality, mainly due to delayed recognition and treatment. Know the difference between SIRS, sepsis, severe sepsis, and septic shock. Sepsis is managed in a similar manner almost regardless of the aetiology (e.g. UTI and LRTI): ‘Sepsis Six’ bundle; remember this as giving three things and taking three things ideally within 1 hour of presentation:
• Give:
• High-flow oxygen.
• IV fluids.
• Antibiotics (after blood cultures) and source control (e.g. laparotomy for abdominal sepsis).
• Take:
• Blood cultures.
• Lactate.
• Urine output measurement.
NB: it is worth familiarizing yourself with the Surviving Sepsis Campaign ( www.survivingsepsis.org/bundles/Pages/default.aspx).
www.survivingsepsis.org/bundles/Pages/default.aspx).
Acute severe asthma or status asthmaticus
ITU may be involved in very severe or life-threatening asthma (peak expiratory flow rate (PEFR) <33%) for intubation and ventilatory support. Use this opportunity to hear widespread wheeze on auscultation.
Features
Features of life-threatening asthma include silent chest, inability to speak full sentences, reduced consciousness, exhaustion, desaturation, and rising pCO2. Please read The British Thoracic Society/Scottish Intercollegiate Guidelines Network1 guidelines on management of acute severe asthma ( www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/).
www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/).
Management
This involves oxygen therapy, salbutamol and ipratropium bromide nebulizers, CXR, steroids, MgSO4 infusion, aminophylline/salbutamol infusion, and endotracheal intubation with ventilator strategies.
Head injury
Severe head injuries are most likely to be encountered at your local MTC where neurosurgical services may be available; ask a senior doctor to point out any features visible on CT scan (e.g. concave bleed in a subdural and convex in an extradural). Try to participate in the neurological examination; familiarize yourself with the GCS (see  ‘Glasgow coma scale’, p. 707); the most interesting signs are in the pupils (e.g. fixed unilateral/bilateral pupils indicative of coning), abnormal flexion and extension responses, and abnormal plantar responses. The primary head injury has already taken place and the focus is on preventing secondary brain injury. Management interventions are aimed at optimizing cerebral perfusion pressure and reducing intracranial pressure. Some of these interventions include:
‘Glasgow coma scale’, p. 707); the most interesting signs are in the pupils (e.g. fixed unilateral/bilateral pupils indicative of coning), abnormal flexion and extension responses, and abnormal plantar responses. The primary head injury has already taken place and the focus is on preventing secondary brain injury. Management interventions are aimed at optimizing cerebral perfusion pressure and reducing intracranial pressure. Some of these interventions include:
• Surgical intervention to decompress the brain, e.g. blood clot evacuation, burr hole, or craniotomy.
• Sedate, intubate, ventilate.
• Head-up position.
• PaO2 >13 kPa.
• PaCO2 4.5–5.0 kPa.
• Mean arterial pressure 80–90 mmHg.
• Avoid hypoglycaemia and hyperglycaemia.
• Avoid hyperthermia.
• Control seizure activity.
Severe intracranial haemorrhage
This may be traumatic (as previously described) or spontaneous (e.g. subarachnoid haemorrhage (SAH) from ruptured Berry aneurysm, or haemorrhagic stroke). Similar measures to maintain cerebral perfusion pressure are used as listed earlier.
Complex surgical patients
In complex procedures (elective or emergency) and/or in those patients with severe comorbidities, patients may be transferred pre-optimized to ITU/HDU before their surgery and returned to the unit for close monitoring, fluid and electrolyte replacement, ± vasopressors or inotropes and analgesia for a few days postoperatively.
Cardiac arrest
All calls are attended by an ITU/anaesthetist, who may decide to admit the patient in the event of successful return of spontaneous circulation (ROSC). Use this opportunity to ensure you are fully competent with basic life support (see  pp. 263–265). These events can occur anywhere in the hospital, from the ward to the main entrance. You can help by carrying the transfer bag and portable suction. As a doctor you will be expected to administer advanced life support (ALS) according to the Resuscitation Council (UK) guidelines. If you can learn this algorithm (always remember ABCDE), it will make managing these cases easier later on. Remember to bear in mind the reversible causes: 4 Hs and 4Ts.
pp. 263–265). These events can occur anywhere in the hospital, from the ward to the main entrance. You can help by carrying the transfer bag and portable suction. As a doctor you will be expected to administer advanced life support (ALS) according to the Resuscitation Council (UK) guidelines. If you can learn this algorithm (always remember ABCDE), it will make managing these cases easier later on. Remember to bear in mind the reversible causes: 4 Hs and 4Ts.
Honours
Define SIRS, sepsis, severe sepsis, and septic shock (Table 9.2).
Table 9.2 Definitions of SIRS, sepsis, severe sepsis, and septic shock
| Definition |
Explanation |
| SIRS (at least two criteria) |
36 > Temperature (oC) <36 or >38
HR (beats/min) >90
Respiratory rate (breaths/min) >20
White cell count (WCC) (× 109) <4 or >12 |
| Sepsis |
SIRS + infection |
| Severe sepsis |
Sepsis + organ dysfunction/organ hypoperfusion/hypotension |
| Septic shock |
Sepsis + intractable hypotension despite fluid resuscitation |
| Bacteraemia |
Positive blood cultures (i.e. presence of infection in blood) |
Clerking
As the patients are generally critically ill, their daily reviews are crucial in judging their progress. You will tend to find detailed proformas that need to be filled out each day for every patient. Every patient also has a parent team that reviews them daily and will determine which ward the patient is stepped down to after remaining stable. Typical contents from A to L include those in Table 9.3
Table 9.3 Clerking
| Definition |
Comments |
| Airway |
Alert, spontaneously ventilating, intubated, tracheostomy |
| Breathing |
Chest expansion, percussion, auscultation, coughing, sputum, respiratory rate, saturation levels, ABG results, ventilation settings, CXR |
| Circulation |
Capillary refill time, warm vs cool, JVP position, heart sounds, murmurs, HR, BP, mean arterial pressure, pitting oedema, ECG |
| Disability |
GCS, pupil size and reflexes, blood sugar, independently moving all limbs |
| Electrolytes |
U&E, magnesium, phosphate, calcium |
| Fluid balance |
Total input (IV fluids, feeds, IV drugs, blood products) versus output (urine, faeces, insensible losses, NG aspirate, vomiting) |
| Gastrointestinal |
Peripheral GI stigmata, abdomen size, palpation, percussion and auscultation |
| Haematology |
Hb, WCC, platelets, mean cell volume |
| Infection |
Temperature spikes, culture (blood, urine, faecal, sputum) results, duration of antibiotics |
| Joints |
Physiotherapy, mobility, independence of feeding, washing, etc. |
| Kilocalories |
Nutritional input: enteral vs parenteral feeding including rates of infusion |
| Lines |
Location and type of lines (IV, per rectal, urinary catheter, drains, NG tube) and their duration |
Investigations/procedures to see
Airway management
A range of adjunct devices can be used in the inadequately self-ventilating patient to open their airway in the event of airway obstruction.
Manoeuvres
Simple measures of head tilt (not in cervical spine injury), chin lift, jaw thrust, and suctioning the oropharynx should be used.
Airway adjuncts
If this has been unsuccessful, an airway adjunct such as the oropharyngeal airway (Guedel airway) and nasopharyngeal airways should be considered. Nasopharyngeal airways are often used in fitting patients with a clenched jaw and those with an active gag reflex (avoid in base of skull fracture). Learn how to size them. The correct sizing of an oropharyngeal airway is from the incisors to the angle of the jaw. Nasopharyngeal airways usually come in size 6 mm and 7 mm diameter and should fit comfortably inside the patient’s nostril.
Bag valve mask
(Trade name Ambu® bag.) Bag valve mask ventilation should be used in the apnoeic patient with high-flow oxygen attached using a two-man technique. There may be an opportunity to learn this technique in simulation or in a controlled supervised environment such as theatres.
Laryngeal mask airway (LMA)
This is a supraglottic airway device which sits above the opening to the trachea and has an inflatable mask which pushes it forward to seal around the glottis. Ventilation with high-flow oxygen is achieved by attaching an Ambu® bag. This is not a secured definitive airway, but does provide some protection from aspiration. Sufficient ventilation can be achieved with a LMA so that chest compressions do not have to be stopped to deliver breaths in cardiopulmonary resuscitation.
Endotracheal tube
Intubation provides a secured definitive airway, this is achieved by:
• passing the ETT through the vocal cords into the trachea using a laryngoscope
• the cuff being inflated to seal around the trachea
• secured with a tie outside the mouth
• confirmation of ETT placement with end-tidal CO2 monitoring
• attaches to a source of oxygen for ventilatory support.
Endotracheal intubation is the most effective at preventing aspiration and providing ventilation, even at higher inflation pressures.
Indications
• Cardiac arrest.
• Imminent respiratory failure.
• Reduced GCS.
• Unable to keep airway patent.
• High risk of gastric content aspiration.
• Transferring uncooperative patients to scans with life-threatening injuries.
Rapid sequence induction
A rapid sequence induction is used to intubate patients at high risk of aspiration of gastric contents. The patient is pre-oxygenated with high-flow oxygen. Fast-onset drugs such as thiopental and suxamethonium are used to achieve rapid intubating conditions to minimize the time between the patient being awake and being anaesthetized with an ETT in situ. Cricoid pressure (Sellick manoeuvre) is applied over the cricoid ring at induction of anaesthesia to prevent aspiration by temporarily compressing the oesophagus underneath.
Placement
You should know how to confirm ETT placement:
• The gold standard—capnography (for end-tidal CO2).
• Seeing the ETT pass through the vocal cords.
• Looking for equal chest expansion.
• Auscultating both lung fields and for gastric bubbles.
Complications
Things that can go wrong include:
• intubating the oesophagus
• aspiration
• placing into a right main bronchus (unilateral chest expansion and inadequate ventilation).
If in doubt that the ETT is in the trachea – remove it and resume bag valve mask ventilation. So, ‘if in doubt, take it out’.
Weaning
You may be asked when it is safe to extubate a patient in the ITU. Reasons include:
• awake, cooperative patient (off sedation)
• FiO2 <40%
• good cough—ability to clear secretions (which should be clear, minimal, and less viscous)
• CVS stability
• near-normal physiological ABG readings
• resolution of reversible cause (e.g. pneumonia).
Tracheostomies
These are placed in patients who are slow to wean from the ventilator (e.g. COPD, ARDS, prolonged ventilation).
Advantages include:
• patient comfort
• no sedation requirements
• improved oral hygiene
• easier ongoing suctioning of secretions (toileting).
Percutaneous tracheostomies can be done on the ITU, negating transfer risks. It is associated with fewer complications than a traditional surgical tracheostomy and does not require a surgeon or theatre team.
Vascular access
Peripheral venous cannulas
These are self-explanatory and you should aim to site all of your placements confidently but firstly under supervision using aseptic technique.
Central venous catheters
These are placed in central veins including:
• internal jugular vein (risk of carotid artery puncture inferomedially)
• femoral vein (may have  risk of line infection due to proximity of urethra and rectum)
risk of line infection due to proximity of urethra and rectum)
• subclavian vein (risk of pneumothorax).
Central venous catheters are inserted using an aseptic technique under ultrasound guidance. This reduces the risk of complications. A vascath is a specialized central line with two lumens, for withdrawal and replacement of blood for haemofiltration/dialysis.
Indications
• Fluid administration
• Inotropic/vasopressor drug infusions
• Central venous pressure (CVP) monitoring
• Haemofiltration
• Cardiac pacing
• Parenteral administration of certain medications (e.g. amiodarone, chemotherapy, total parenteral nutrition).
Complications
• Incorrect siting
• Arrhythmias
• Infection
• Bleeding
• Thrombosis
• Pneumothorax
• Air embolus.
Other specialist lines include Hickman, peripherally inserted central catheter (PICC), Tesio®, and portacath which are used for long-term venous access.
Arterial lines
These are used as an invasive, real-time measure of BP and mean arterial pressure guiding the use of vasopressor and inotropic support. Arterial lines can be connected to cardiac output monitoring devices. Arterial lines allow for multiple ABG sampling to monitor ventilation adequacy and acid–base balance.
Ventilation
Invasive support can be given in different modes:
Intermittent positive pressure ventilation (IPPV)
This is a form of controlled ventilation, where a set volume or pressure and a set ventilation rate are given regardless of the patient’s respiratory efforts (see Table 9.4).
Table 9.4 Volume- vs pressure-controlled ventilation
| Volume controlled |
Pressure controlled |
| Gives a set volume of gas (calculated by average tidal volume of 7–10 mL/kg) and a set ventilator rate |
When a ventilator mode gives a controlled set inspiratory pressure (minimum required to generate an adequate tidal volume) and a set ventilator rate |
| Used effectively in patients with poor compliance, e.g. asthmatic patient, where a volume can be administered despite the airway pressures, hence compensating for poor respiratory compliance. However, high airway pressures cause lung trauma |
Superior to volume-controlled ventilation as it reduces the risks of volutrauma, barotrauma, and biotrauma to the alveolar sacs. Generally lower peak pressures are generated in this mode |
Volume- vs pressure-controlled ventilation
Modes which give ventilator support when a patient takes a spontaneous breath can be triggered by changes in flow or negative pressure.
Synchronized intermittent mandatory ventilation (SIMV)
SIMV mode-controlled ventilations are given, but if the patient takes a spontaneous breath they can be supported with that breath. This is done using pressure support. When a spontaneous breath is detected a set ‘pressure support’ is given during that spontaneous breath to give a greater tidal volume and reduce the work of spontaneous breathing for the patient. This is useful when a patient is weaning from a ventilator. The mandatory ventilation can be slowly reduced as the number of spontaneous breaths increases. As the tidal volume of the patient’s breaths improves, pressure support is also reduced until the patient is breathing spontaneously without support. A disadvantage of SIMV mode is at certain points during the controlled ventilation cycle the patient’s spontaneous breath is not fully supported. There is still an  work of breathing as the patient’s breathing may not be synchronized with the controlled ventilation cycle.
work of breathing as the patient’s breathing may not be synchronized with the controlled ventilation cycle.
Bilevel
This is a form of ventilator support which allows the patient to spontaneously breathe at any point during controlled pressure ventilation. The controlled breaths are achieved by cycling between two different pressures. The difference between the two pressure settings determines the tidal volume. The patient can breathe at any point during the controlled ventilation cycle and receive a set pressure support for that spontaneous breath. Each spontaneous breath is supported and controlled pressure ventilation can be reduced, as can pressure support until the patient breathes unsupported.
Non-invasive ventilation
This is the administration of positive pressure ventilatory support to a patient without an ETT or tracheostomy. This is usually achieved via a tight-fitting nasal mask, face mask, or hood. This is a short-term strategy in the acutely unwell patient and is not a substitute for endotracheal intubation if required.
Continuous positive airway pressure (CPAP)
This is the application of continuous positive airway pressure throughout the respiratory cycle in a spontaneously breathing patient. It is used in managing obstructive sleep apnoea, atelectasis and pulmonary oedema due to cardiac failure.
Positive end-expiratory pressure (PEEP)
This is the application of positive airway pressure at the end of expiration only. It is used to prevent alveolar collapse and increase respiratory compliance.
BiPAP®
This is a trade name for a portable ventilator. BiPAP® is bilevel positive pressure ventilation. This can be applied with a tight-fitting facemask. As for invasive ventilation, this mode can be used in the spontaneous breathing patient to provide pressure support, by alternating between two different positive pressures, inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP). The IPAP and EPAP are adjusted depending on the level of respiratory support required. Bilevel positive airway pressure is frequently used in patients with severe COPD and patients in type 2 respiratory failure.
Acute respiratory distress syndrome (ARDS)
ARDS is one extreme of the acute lung injury spectrum 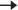 diffuse alveolar injury. It is characterized as the following:
diffuse alveolar injury. It is characterized as the following:
• Acute condition (within 1 week of insult) and potentially reversible.
• Radiographic evidence (CXR/CT) of bilateral pulmonary infiltrates (pulmonary oedema not due to cardiac failure or fluid overload).
• Respiratory failure from intrapulmonary shunting 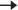 severe hypoxaemia (measured by ratio of partial pressure of oxygen in arterial blood [(PaO2) to fraction of oxygen in inspired air )FiO2)). Severity of hypoxaemia correlates to mortality risk according to the Berlin criteria (see Table 9.5).
severe hypoxaemia (measured by ratio of partial pressure of oxygen in arterial blood [(PaO2) to fraction of oxygen in inspired air )FiO2)). Severity of hypoxaemia correlates to mortality risk according to the Berlin criteria (see Table 9.5).
• Associated with pulmonary hypertension.
• Acute phase tends to resolve completely but residual pulmonary fibrosis occurs less commonly.
Table 9.5 Acute respiratory distress syndrome (ARDS)
| ARDS severity |
PaO2/FiO2 |
Mortality (%) |
| Mild |
200–300 |
27 |
| Moderate |
100–200 |
32 |
| Severe |
100 |
45 |
Risk factors
Pulmonary (direct) vs extrapulmonary (indirect):
• Sepsis/pneumonia
• Trauma and fractures
• Disseminated intravascular coagulation (DIC)
• Burns
• Transfusion
• Drug overdose
• Near drowning
• 20% idiopathic
• Pancreatitis
• Post-perfusion injury post CABG
• Fat embolism.
Management
• 5 Ps: perfusion, positioning, protective ventilation, protocol weaning, and preventing complications.
• pH >7.2, PaO2 >8 kPa, PaO2:FiO2 ≥20, PEEP ≤10 cmH2O, FiO2 ≤0.6.
• Recruitment manoeuvres.
• Inverse I:E ratio.
• Avoid volu-/baro-trauma.
• Prone position.
• High-frequency ventilation.
• Inhaled nitric oxide.
• Steroids.
• Surfactant replacement.
• Extracorporeal membrane oxygenation.
Renal replacement therapy
Haemofiltration and haemodialysis is used in ITU to manage:
• refractory life-threatening hyperkalaemia
• persisting fluid overload despite medical management
• resistant metabolic acidosis pH <7.1
• drug toxicity, e.g. aspirin and lithium.
ITU uses continuous haemofiltration/haemodialysis. This is a slow process, which is better tolerated in cardiovascular unstable patients.
Intermittent haemodialysis
This is used in outpatient departments where the patients are cardiovascularly stable and can tolerate large fluid shifts.
• Principle: haemodialysis works on the principle of diffusion of solutes and fluid across a semipermeable membrane (the haemofilter) from an area of high concentration to an area of low concentration. The rate of diffusion is dependent on the concentration gradient.
Haemofiltration
This works on the principle of convection (also known as solute drag), where a moving stream of fluid produces a positive pressure and effectively pushes solutes across the haemofilter. The rate of haemofiltration is dependent on the pressure gradient generated.
• Anticoagulants (heparin, citrate): used in the haemofiltration/dialysis circuit to prolong the life of the haemofilter.
• Complications: associated with renal replacement therapy are those related to large-bore insertion of central venous catheters and the haemofiltration process itself (e.g. cardiovascular instability, haemorrhage, electrolyte disturbances, metabolic disturbances, and air embolism).
 BP in conditions of systemic vasodilatation (distributive shock) such as sepsis
BP in conditions of systemic vasodilatation (distributive shock) such as sepsis SVR increases the afterload which the heart has to contract against
SVR increases the afterload which the heart has to contract against BP
BP p.
p.  severe refractory hypotension) and
severe refractory hypotension) and 