 www.meded.ucsd.edu/clinicalmed/breast.htm.
www.meded.ucsd.edu/clinicalmed/breast.htm.Clinical examination: overview
Nervous system: further examination
Clinical examination is the art of eliciting physical signs in order to diagnose conditions without recourse to laboratory or radiological investigations. Historically, physical examination was a physician’s main diagnostic tool. The advent of technology (e.g. CT scanning) should complement this modality rather than replace it.
A quick glance at a patient can often provide you with more information than a 10 min phone call or two pages of writing. Is the hypotensive patient sitting up drinking tea or unresponsive and in peri-arrest? Is this 80-year-old man sufficiently robust to survive an emergency AAA repair, or frail, malnourished, and better suited for conservative management? Other important skills such as fluid assessments and psychiatric evaluation can only be done well face to face.
A number of clinical signs are constant and reliable enough to convey a diagnosis, long before radiological or histological evidence is available. For example, if you see a patient in the ED with spider naevi, gynaecomastia, and palmar erythema, you can be reasonably confident he has chronic liver disease, even if his transaminases are unremarkable and it is days before he has a US showing a cirrhotic liver. In addition, clinical examination can provide answers that other diagnostic modalities can only hint at or infer. A CTPA showing a large PE may also raise the possibility of right heart strain (if paradoxical interventricular septal bowing or reflux into the inferior vena cava is present). However, this information is superseded by your clinical examination of the patient: what is their BP? Is their JVP dilated and raised? Is there a right heart heave? You are unlikely to make the decision to thrombolyse a patient on the basis of CT findings, but clinical signs might change your management.
A major part of clinical medicine is knowing what investigations to request in order to reach a diagnosis, while using them responsibly in a targeted fashion. If someone is admitted with breathlessness and your clinical examination finds a gallop rhythm or a heart murmur, you are much more likely to pursue a cardiological approach rather than a respiratory one.
During your first few months of clinical training, the greatest difficulty you will have is remembering which part of the examination comes next, and trying not to forget vital elements of it.
The next challenge will be trying to interpret clinical signs correctly and ultimately synthesize these findings into a coherent list of differential diagnoses (‘A systolic murmur could be caused by aortic stenosis or mitral regurgitation’).
You then need to use your clinical examination findings to formulate a management plan (e.g. ‘In light of these signs, I would like to perform an ECG, and a lying and standing BP’). In the setting of medical school exams, it is also helpful to be able to pre-empt examiners’ questions (‘What are the causes of mitral regurgitation?’)
This is a lot to ask! Nobody learns how to examine a patient in the space of a week. There is no substitute to repetition; you need to examine patients week in, week out for the duration of your clinical training so that you can perform the examination on autopilot and concentrate on finding the clinical signs. It is clear to examiners which candidates are not comfortable with clinical examinations. Also, you will only learn to recognize ‘normal’ by seeing as many patients as possible.
Do not invent things. You may think you should be hearing a systolic murmur because it would fit in with other findings, but if you do not hear one, then do not report one. It is a greater sin to invent something than to miss it. Be honest. ‘I could not see the JVP’ or ‘I was unable to palpate the carotid pulse’ are signs of an honest and trustworthy individual who does not confabulate.
Finally, keep an open mind. In the pursuit of passing finals, it is tempting to think in terms of classical patterns and syndromes. In reality, you will often be surprised by unrelated or unexpected findings which are just as important.
• Introduce yourself. Explain that as a medical student, the examination is for your own benefit and will not alter their current management but will help your training. If the patient is obviously distressed or tired, come back another time.
• Timing. Medical students should not try to see patients during meal times or any designated rest period. Similarly, try to work around any visitors they may have; seeing family and friends is very important for patient morale.
• ‘Popularity’. Be mindful of the fact that someone with rare but classic signs such as congenital heart disease or polycystic kidneys may have seen scores of students before you.
• Communication limitations. Establish early on if the patient has any communication difficulties (e.g. blind or hard of hearing).
• Ensure patient comfort. Making sure that they are not in pain or breathless is more important than slavish attention to lying the patient flat or asking them to sit at 45o.
• Appearance and personal hygiene. You will be close enough to the patient for them to notice and be affected by your personal hygiene. Always wash your hands (soap and water or alcohol gel) in front of the patient. Ensure that you are appropriately dressed in clean clothes and that if you have cycled 5 miles to the hospital that you do not smell as such. If you smoke, you should take measures to disguise the smell. If you openly smell of alcohol from the night before, give patients a miss that day.
• Dignity and privacy. You should draw the curtains around the bed for any patient interaction. Ensure you have either a blanket or dressing gown to keep the patient covered if you need to expose any part of them during the examination.
• Explain what you plan to do. A patient may agree to you performing a heart examination and then wonder why you spend so long looking at their hands and their neck. Similarly, it may seem obvious to you that you will palpate for the apex beat after inspecting the chest. All the patient will see is you diving towards her left breast. Pre-empt any such difficulties!
• Reassure. Explain that most of the signs you are examining for, and indeed are openly discussing, are not specifically relevant to them. Make sure that the patient is not left anxious or bemused by what you have been doing.
You will learn to pick up on a number of subtle clues to patients’ conditions just from inspecting their bed space and looking at the patient from several metres away.
• Are you in a high dependency bay? Is the patient on oxygen or a cardiac monitor? Are there infusion pumps running and if so what drug is being administered?
• Are you in a side room? If so, why? (Think MRSA, Clostridium difficile, and take appropriate infection control measures.)
• Is the patient on a special air mattress? (Think diabetes, pressure ulcers, immobility, vulnerable skin.)
• Are there walking aids nearby such as a stick, a frame, or even a wheelchair? This gives you a good idea of patient mobility
• Signs above the bed often describe dietary measures (diabetic, low potassium, soft consistency), nursing instructions (e.g. ‘red-tray’), or fluid restriction (cardiac or renal failure).
• Patient’s effects: magnifying glass or spectacles on the table (visually impaired), hearing aids (hard of hearing), sugar-free drinks (diabetes), sputum pot, inhalers (brown = steroids, blue = salbutamol), glyceryl trinitrate (GTN) spray (red). Cards and gifts may suggest strong family and social support.
• It is best to start off with obvious but important statements. Let the examiner know that you can spot an acutely ill patient. Confirm the ‘three Cs’; is the patient calm, comfortable, and conscious?
• General appearance. Do they look well or unwell? This is a simple but vital observation that will stand you in good stead in your career. Are they sitting in their chair doing the crossword or lying in bed looking grey?
• Are they overweight or malnourished? Well-kempt or evidence of neglect (prior to admission)? In pain or comfortable?
• Is there evidence of gross abnormality (e.g. amputation, severe peripheral oedema, distended abdomen, facial injuries), surgical scar visible despite clothing (e.g. sternotomy wound), medical devices (e.g. IV cannula, PICC line, central line or urinary catheter), paralysis, hair loss (cytotoxic chemotherapy).
• Patient’s affect. Does the patient respond and interact appropriately on your arrival? Could the patient have dementia or be depressed? Is their GCS score clearly compromised?
• Respiratory effort. Is the patient sitting up struggling for breath, using accessory muscles of respiration? Or are they comfortably asleep on their back on your arrival?
• After an overview, you can state more confidently whether the patient initially does or does not present as a medical emergency.
• That is a lot of things to look for in 10 sec, but you will be amazed what you begin to notice.
The following sections are intended as an introduction to clinical examination but are necessarily brief. You should consult monographs for more detail. The following are recommended:
• Thomas J, Monaghan T (2014). Oxford Handbook of Clinical Examination and Practical Skills, 2nd ed. Oxford University Press.
• Talley NJ, O’Connor S (2017). Clinical Examination, 8th ed. Elsevier.
• Alastair Innes J, Dover AR, Fairhurst K (2018). Macleod’s Clinical Examination, 14th ed. Churchill Livingstone.
• University of California. Practical Guide to Clinical Medicine.  www.meded.ucsd.edu/clinicalmed/breast.htm.
www.meded.ucsd.edu/clinicalmed/breast.htm.
• OSCE Skills:  www.osceskills.com.
www.osceskills.com.
• Geeky Medics:  www.geekymedics.com.
www.geekymedics.com.
Top tip
Ask your tutor or senior peers about previous mark schemes for your medical school, although most should contain mostly the same criteria. For a comprehensive list of example OSCE mark schemes, visit Mock Marking Schemes for OSCEs ( www.scionpublishing.com/resources/osce/OSCEs%20Marking.pdf).
www.scionpublishing.com/resources/osce/OSCEs%20Marking.pdf).
The key to a successful cardiovascular examination is being familiar with the heart sounds, and identifying abnormal or additional sounds. As well as spending a number of minutes listening to your own heart sounds, YouTube and recordings on websites are a good place to start.
• S1: ‘lub’. Closure of the mitral valve (MV) and tricuspid valve (TV). Think M1.
• S2: ‘dub’. Closure of the aortic valve (AV) and pulmonary valve (PV).
Both cause a ‘gallop’ rhythm:
• S3: ‘Kentucky’. Can be normal in young people. In older patients a sign of ventricular dysfunction or volume overload.
• S4: ‘Tennessee’. Sign of ventricular stiffness, e.g. LV hypertrophy.
• ‘Friction rub’: due to serosal inflammation, e.g. viral pericarditis or post MI.
Caused by turbulent blood flow. May be physiological (e.g. in pregnancy) or pathological. Often described numerically to denote intensity (grade 1 = only audible with accentuation, grade 6 = audible with stethoscope just removed from chest wall). Murmurs are also described by their timing in the cardiac cycle:
Systolic murmurs begin at or after the first heart sound and are usually caused by stenosed AV/PV or regurgitant MV/TV. Common examples include:
• ejection: aortic stenosis (AS)
• pan-systolic: mitral regurgitation (MR) (or rarely ventricular septal defect)
• others: MV prolapse, atrial septal defect, hypertrophic obstructive cardiomyopathy.
Diastolic murmurs being at or after the second heart sound and are caused by stenosed MV/tricuspid stenosis or regurgitant AV/PV. These are difficult to identify and rarer than lesions causing systolic murmurs:
• Early: usually aortic regurgitation (rarely pulmonary).
• Mid: mitral/tricuspid stenosis (both rare).
There are a number of techniques to distinguish murmurs which involve manoeuvres to alter the volume/speed of turbulent flow and therefore the intensity of the murmur. The most important are:
Right-sided murmurs are louder in inspiration due to  venous return and right heart filling. As a result, right sided murmurs are quieter in expiration while left-sided murmurs sound louder (and vice versa). Think Right Inspiration (RIght) and left expiration (LEft).
venous return and right heart filling. As a result, right sided murmurs are quieter in expiration while left-sided murmurs sound louder (and vice versa). Think Right Inspiration (RIght) and left expiration (LEft).
The murmur of MR radiates to axilla, AS radiates to the carotid.
Sitting forwards in expiration makes aortic murmurs louder as the left side of the heart is closer to the chest wall. Mitral murmurs are accentuated when patients are lying on their left side (lateral decubitus).
Valsalva manoeuvres: accentuates the murmurs of both hypertrophic obstructive cardiomyopathy and MV prolapse due to reduced LV volume and closer apposition of septum and MV. All other murmurs are quieter.
See Fig. 50.1.

Fig. 50.1 Precordial locations for cardiac palpation and auscultation of heart sounds. Closure of the mitral and tricuspid valves produces the S1 heart sound; closure of the pulmonic and aortic (semilunar) valves produces the S2 sound. Reproduced from Polanski, A, and Tatro, S., Luckmann's Core Principles and Practice of Medical-surgical Nursing, 1996, WB Saunders.
Aortic: 2nd intercostal space on right, right sternal edge.
Pulmonary: 2nd intercostal space on left, left sternal edge.
Mitral: 5th left intercostal space, left mid-clavicular line.
Tricuspid: 4th left intercostal space, left sternal edge.
Erb’s point: third left intercostal space/left lower sternal border (where S2 is best heard).
Another important element of the cardiovascular examination is to understand and identify the position and waveform of the internal jugular vein (IJV). The IJV is found medial to the sternocleidomastoid and can be distinguished from the carotid artery by being impalpable and compressible, having a bifid waveform and changing with respiration. Its position can be accentuated by gentle pressure in the RUQ (hepatojugular reflux or abdominojugular test). Remember, this is not an automatic reflex but a pressured reflUx. There are lengthy academic descriptions of the JVP waveform but in practical terms, the following are most important:
• Identify if it is elevated (heart failure or fluid overload, right heart strain, e.g. PE).
• Large ‘v wave’ (tricuspid regurgitation).
• Cannon or ‘a wave’ (AF and heart block).
Fluid overload is a cardinal sign of heart failure and you should become familiar with looking for signs of intra- and extravascular fluid status:
• Intravascular: HR, BP (postural), capillary refill, JVP, urine output.
• Extravascular: peripheral/pulmonary oedema, ascites, tissue turgor.
Fluid restriction or daily weights sign, GTN spray, infusions such as furosemide, cardiac monitor, oxygen.
Breathless at rest? Cardiac cachexia? Gross oedema visible from end of bed, urinary catheter? Consider patient’s age (if young, think of congenital cardiac disease). Evidence of systemic disease (ankylosing spondylitis, RA) or syndrome (Marfan’s, trisomy 21)? Listen for audible click of metallic valve.
• Hands: capillary refill (<2 sec), clubbing (congenital heart disease), long fingers (Marfan’s), tendon xanthelasma, warm and well perfused vs cold and shut down, peripheral cyanosis, peripheral stigmata of infective endocarditis (>three splinter haemorrhages, Osler’s nodes, Janeway lesions), tar (not nicotine) stains from smoking, palmar erythema (SLE, hyperthyroid, RA).
• Radial pulse: regular vs irregularly irregular (AF) vs regularly irregular (sinus arrhythmia). Rate, volume, and character (best assessed from a central artery such as the carotid and femoral). Radio-radial delay (coarctation/aortic dissection) and radio-femoral delay. Recent scar/cannulation in forearm (or groin) from angiogram.
• Brachial pulse: collapsing/water hammer (aortic regurgitation). Assess quality of pulse especially if the radial pulse is impalpable.
Ask for patient’s BP and calculate the pulse pressure (systolic BP − diastolic BP). Narrow pulse pressure (e.g. 100/80 mmHg) occurs in severe AS whereas wide pulse pressure (e.g. 180/70 mmHg) occurs in aortic regurgitation. A low BP may occur in cardiac failure or if compromised by fast AF.
Subconjunctival haemorrhage (infective endocarditis), conjunctival pallor in anaemia, jaundiced sclera, xanthomata (lipid), corneal arcus (pathological if <50 years old) vs corneal senilis (physiological).
Central cyanosis (congenital heart disease), high-arched palate (Marfan’s), poor dentition (risk factor for infective endocarditis).
Slow rising pulse (anacrotic in severe aortic stenosis), scar from endarterectomy, listen for bruit or radiation of AS murmur.
• Inspection: midline sternotomy scar, indwelling devices, e.g. permanent pacemaker or implantable cardioverter defibrillator, apical valvotomy scar (unusual).
• Palpation: apex beat (fifth intercostal space in midclavicular line), palpate for thrills (i.e. palpable murmurs) at apex and over sternum with hand lightly on chest wall. Feel for heaves (ventricular hypertrophy) with the heel of your hand.
• Auscultation: listen in the five areas, initially to assess S1 and S2 and then to identify added sounds such as S3/4 or murmur. Perform accentuating manoeuvres to distinguish murmurs while holding their radial pulse to distinguish systolic from diastolic. Take your time and listen carefully. Some even close their eyes to focus.
• Lungs: percuss at bases for effusions and listen for pulmonary oedema.
• Sacrum: assess for sacral oedema. Remember in bed-bound patients that the sacrum/hips are often the lowest part of the body.
• Abdomen: assess for AAA. If patient has signs of tricuspid regurgitation, palpate for a pulsatile liver. Look for ascites in heart failure.
• Lower limb: palpate for (and auscultate) femoral pulses, and also check for popliteal/dorsalis pedis pulses. Check feet for evidence of emboli/‘trash foot’/splinter haemorrhages, and lower limb oedema. Is there evidence of saphenous vein harvest (CABG)?
• Offer to check: dipstick urine (microscopic haematuria in infective endocarditis), lying and standing BP, resting ECG, pulse oximetry.
‘This is a 69-year-old man who presented with shortness of breath. He is comfortable at rest. Clinical signs include irregularly irregular pulse, displaced apex beat, a pansystolic murmur that radiates to the apex, bibasal crepitations and peripheral oedema. In summary these signs are consistent with mitral regurgitation, atrial fibrillation, and congestive cardiac failure. To investigate further I would like to perform an ECG, an echocardiogram and a plain chest radiograph.’
• Management of MR (medical and surgical).
• Pros and cons between tissue and metallic valves.
• Management of AF and risk score to guide anticoagulation.
It is useful to think of cardiac conditions in terms of patterns or syndromes so that you remember to look for additional signs during the course of your examination. (See Table 50.1.)
Table 50.1 Patterns of findings
| Common | ||
| Aortic stenosis (AS) | ESM radiating to carotids Quiet/normal S1, absent S2 Presence of S4 (late) Narrow pulse pressure | Slow rising pulse LV heave/hypertrophy Congestive cardiac failure (late) (Check for coexisting AR) |
| Mitral regurgitation (MR) | PSM radiating to axilla Quiet/absent S1 Presence of S3 Displaced apex beat | AF Congestive cardiac failure Hyperdynamic circulation |
| Rare | ||
| Aortic regurgitation (AR) | Early diastolic murmur Usually coexisting ESM Austin Flint murmur Wide pulse pressure | ‘Water hammer’ pulse Eponymous signs: Quincke, Corrigan, De Musset, Duroziez Cause: Marfan’s, ankylosing spondylitis |
| Mitral stenosis (MS) | Mitral facies Low diastolic murmur at apex Loud S1 | AF Main cause rheumatic heart disease which is rare now |
ESM, ejection-systolic murmur; PSM, pansystolic murmur.
• Tricuspid regurgitation: PSM at lower left sternal edge louder in inspiration (often absent). Raised JVP with large v waves. Pulsatile liver. Usually functional (dilated RV with normal valves).
• Prosthetic valves: midline sternotomy scar, metallic valves audible without stethoscope (think ticking clock).
Learn to recognize the different breath sounds associated with normal and pathological ventilation. Listen to your own breathing; start with the stethoscope over your lungs to identify vesicular breath sounds. Then move the stethoscope to your sternum to hear bronchial breath sounds. Breathe through your mouth to exclude any nasopharyngeal noise.
• Vesicular: this is the sound of normal air transit through the large airways, heard transmitted through normal lung tissue and the chest wall. The inspiratory phase is typically about two to three times longer than expiratory. The volume/intensity is reduced with poor respiratory effort,  muscle mass or obesity.
muscle mass or obesity.
• Bronchial: this is the sound of air moving through large airways without being filtered by normal (gas filled) lung tissue. It is normal to hear bronchial breath sounds when listening over the large airways but abnormal when listening over lung tissue. Bronchial breath sounds are heard over consolidated lung and in some cases adjacent to effusion or collapse.
• Wheeze: implies airways obstruction, usually expiratory but also present in inspiration. Obstruction of multiple smaller airways (e.g. asthma) causes polyphonic wheeze; beware that severe airways obstruction limits even the airflow required to make wheeze and a ‘silent chest’ is an emergency. Obstruction of single calibre airways (e.g. bronchial carcinoma) causes monophonic wheeze which does not clear with coughing.
• Inspiratory crackles (also referred to as crepitations/creps/rhonchi/rales): crackles are caused by the rapid re-expansion of collapsed airspaces during inspiration. The timing in the respiratory cycle is an important clue to the size of airspace involved. Early inspiratory crackles usually originate in smaller airways as in chronic bronchitis.
• Late inspiratory crackles are due to alveolar re-expansion and are further described by their nature:
• Fine: like Velcro as in pulmonary fibrosis.
• Coarse: bronchiectasis/pneumonia.
• Pleural rub: caused by inflammation of the pleural surfaces and seen in response to infection, infarction (PE) or systemic inflammatory disease (e.g. SLE). Described as the crunching of fresh snow. Distinguish from pericardial rub by asking patient to hold their breath (pleural rub disappears).
In addition to auscultation, percussion is another very important modality of respiratory examination. Practise tapping one (usually left) middle finger with your other (right) middle finger so that you can do it without looking or concentrating. It is common at early stages to miss and instead strike the patient; this is best avoided.
Percussion utilizes the difference in conduction between air- and liquid-filled tissues. Practise percussing your own chest wall to identify the normal sound over air-filled lung. This sound becomes dull/reduced over consolidated lung or pleural effusions while it becomes hyper-resonant over hyperinflated lung or pneumothorax.
Ask the patient to repeat a number while resting the ulnar aspects of both hands over the lung fields. Normally, the air-filled lung tissue greatly attenuates the conduction of sound waves. When the lung is consolidated, the thrill is more obviously palpable through the chest wall. TVF is reduced in effusion and pneumothorax.
This is the auditory correlate of TVF. Auscultate and ask the patient to say 99. Higher-frequency speech is rapidly transmitted through consolidated lung and sounds louder than over normal lung.
More helpful in comparing left to right than in absolute terms. Assess upper lobe expansion by resting hands over clavicles or observing clavicle movement with respiration from behind. Lower lobe expansion seen by movement of examiner’s hands on rib cage expansion.
• Unilateral reduction: pneumonia, collapse, effusion, pneumothorax.
• Bilateral: diffuse lung disease, e.g. fibrosis, emphysema.
Tracheal deviation is
• towards side of lesion: collapse, lung resection
• away from lesion: effusion, pneumothorax.
Significant volume expansion can cause mediastinal shift (e.g. tension pneumothorax). This is life-threatening. Clinical signs include circulatory collapse, tracheal deviation, displaced apex beat, absent breath sounds, and hyper-resonance (see Table 50.2). This is a clinical diagnosis so do not wait for the CXR!
Table 50.2 Summary of clinical findings
| Condition | Breath sounds | Percussion | TVF/VR | Expansion |
| Consolidation | Bronchial or  |
Dull |  |
 |
| Effusion |  |
Dull |  |
 |
| Emphysema |  , creps , creps |
Hyper-resonant |  |
Bilateral  |
| Collapse | Absent or  |
Dull |  |
|
| Pneumothorax | Absent | Hyper-resonant |  |
 |
| TVF, tactile vocal fremitus; VR, vocal resonance. | ||||
Chronic hypoxia and pulmonary hypertension can 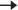 right heart failure (cor pulmonale) and a number of clinical signs which are useful clues to the severity/longevity of the patient’s condition:
right heart failure (cor pulmonale) and a number of clinical signs which are useful clues to the severity/longevity of the patient’s condition:
• Right ventricular heave (sternal edge).
Lung cancer can give rise to a number of specific signs. These can be caused by the following:
1. Airway obstruction from tumour (cough, monophonic wheeze, distal collapse/consolidation).
2. Local invasion by tumour (brachial plexus, SVC obstruction, Horner’s syndrome).
3. Metastatic spread (jaundice, palpable liver, bone pain).
4. Paraneoplasia (Eaton–Lambert, gynaecomastia).
5. Systemic: cachexia, lymphadenopathy, clubbing.
6. Previous surgical scars (thoracoscopy, lobectomy, pneumonectomy) which are usually on the back—so ensure you inspect both front and back.
Fluid restriction sign, inhalers (brown = steroid, blue = salbutamol, nebulizers), oxygen therapy (flow rate, nasal specula, or face mask), sputum pot (colour, consistency, viscosity, blood?). Is the patient isolated (TB?)
Breathless at rest?  Respiratory work (tachypnoea, pursed-mouth breathing, use of accessory muscles, ‘tripod’ position), added respiratory noises (cough, wheeze, stridor), cachexia, obesity (think sleep apnoea/ventilatory failure), peripheral oedema, age of patient (if young, think cystic fibrosis, alpha-1 antitrypsin deficiency, asthma, neuromuscular disorder).
Respiratory work (tachypnoea, pursed-mouth breathing, use of accessory muscles, ‘tripod’ position), added respiratory noises (cough, wheeze, stridor), cachexia, obesity (think sleep apnoea/ventilatory failure), peripheral oedema, age of patient (if young, think cystic fibrosis, alpha-1 antitrypsin deficiency, asthma, neuromuscular disorder).
• CO2retention flap: ask patient to stretch out arms/hands and observe for coarse myoclonic jerks. Salbutamol-induced fine tremor will also be evident.
• Hands: clubbing (cancer, suppurative lung disease), wasting of small muscles (brachial plexopathy in cancer), palmar erythema (SLE, RA), tar staining (smoking).
• Wrists: hypertrophic pulmonary osteoarthropathy (extreme form of clubbing). Peripheral cyanosis.
• Radial pulse: tachycardia/AF. Bounding pulse in CO2 retention. Count respiration rate at this point.
• Face: anaemia, central cyanosis, ptosis (Horner’s).
• JVP: raised in pulmonary hypertension.
• Trachea: (warn the patient). Deviated? Palpate sternotracheal distance (reduced in hyperinflated lungs).
• The chest: remember to examine both front and back, especially for hidden surgical scars!
• Inspection: pectus carinatum or excavatum, congenital anomaly (e.g. Klippel–Feil syndrome), thoracotomy scar, Hickman line for antibiotics.
• Palpation: assess for chest expansion in upper and lower zones.
• Percussion: compare like with like (i.e. left upper zone with right). Right axilla identifies right middle lobe. Cardiac dullness attenuated in asthma/COPD.
• Auscultate: front and back comparing left with right as you go. Ask patient to breathe through mouth to eliminate nasopharyngeal noise. Remember to auscultate over supraclavicular fossae and axillae. Ask patient to cough to see if crackles clear. TVF/vocal resonance. Listen in the pulmonary valve area for loud P2 (pulmonary hypertension).
• Lower limbs: peripheral oedema.
• Other: look for evidence of long-term steroid use (skin thinning, central obesity, finger pricks from diabetes).
• Offer to: check pulse oximetry, examine the sputum, and perform bedside spirometry.
‘This is an 71-year-old woman who presents with breathlessness and cough. She is tachypnoeic and tachycardic at rest. On examination I found tar-stained fingers, reduced sternotracheal distance, bilaterally reduced chest expansion, quiet breath sounds, and expiratory wheeze throughout with a hyper-resonant percussion note. She also has a loud P2, elevated JVP, and pitting oedema of the lower limbs. Finally, she has a number of bruises and thin skin. Taken together, I think this lady has evidence of a smoking history, emphysema treated with corticosteroids, and cor pulmonale. To investigate further, I would perform a plain chest radiograph and pulmonary function test’. (See Table 50.3.)
• What would you expect to find on pulmonary function testing?
• What are the characteristic radiological findings in emphysema?
• What are the complications of COPD?
• What are the risks of uncontrolled oxygen therapy?
• What are the other causes of small airways obstruction?
• What is the pathophysiology of emphysema?
• What are the criteria for long-term home oxygen therapy?
• What are the indications for surgery in COPD?
Table 50.3 Patterns of findings
| Obstructive airways disease | Hyperexpansion, quiet BS, hyper-resonance Prolonged expiratory phase Steroid use Salbutamol tremor COPD only: CO2 retention, smoking, pulmonary HTN, cor pulmonale |
| Lobar pneumonia |
Fever/tachycardia
Oxygen therapy
Focal consolidation: dull percussion, bronchial BS,
inspiratory creps and/or rub,  TVF/VR TVF/VR |
| Pulmonary fibrosis | Clubbing Fine bilateral late inspiratory crackles Pulmonary HTN/cor pulmonale Systemic disease: SLE, RA, ankylosing spondylitis Steroid use |
| Bronchiectasis/CF | Clubbing Sputum pot, haemoptysis Coarse bilateral late inspiratory crackles Hickman line (CF: cachexia, diabetes, Creon) |
| Pleural effusions | Dull lung base (s), reduced expansion, quiet/absent BS, reduced TVF/VR Bilateral: heart/renal failure Unilateral: cancer/pneumonia Evidence of pleurocentesis |
| Lung cancer | Clubbing Smoking Cachexia/wasting Lymph nodes Monophonic wheeze Focal consolidation/effusion Specific signs |
BS, breath sounds; HTN, hypertension.
• TB: ethnicity, cachexia, fever, lymphadenopathy, usually few lung signs. Old TB: pneumonectomy, ‘plombage’ on CXR, phrenic nerve crush scar above clavicle.
• Sarcoidosis: ethnicity, uveitis, lymphadenopathy, visceral involvement (liver, spleen, renal), erythema nodosum, usually few lung signs.
The abdominal examination takes in a number of different organ systems, and you need to be able to detect signs of liver disease, IBD, palpable kidneys or renal transplant, stomas, and splenomegaly.
Often the principal challenge is to detect organomegaly, and establish which organ it is you are palpating. In many cases, there is no organomegaly, and the diagnosis will be deduced from surgical scars or peripheral stigmata of systemic disease. The abdomen is usually divided into four or nine but remember that there is no such thing as nine quadrants.
Is classically described by the ‘five Fs’: fat, flatus, faeces, fluid, and fetus. Typically there will be generalized and often symmetrical distension. The causes can be identified on percussion (ascites dull, gaseous distension resonant), the presence of shifting dullness (ascites), auscultation (high-pitched bowel sounds in obstruction, absent in paralytic ileus).
Can be described as you would any other lump: location, size, shape, appearance, pain, fluctuance, consistency, percussion note, etc. This is a good place to start if you are completely lost and need some structure to gather your thoughts. However, it is more useful to learn the specific features of the intra-abdominal organs on examination so that you can rapidly decide what you are palpating.
RUQ, descends 1–2 cm in inspiration, upper border ~sixth intercostal space and expands distally. Usually smooth (if craggy, think metastatic disease). Tender if capsule stretched like in rapid expansion (acute hepatitis). Percussion note dull. Bruit present in hepatocellular carcinoma and acute alcoholic hepatitis. Remember that the liver is often impalpable in cirrhosis (small, scarred liver).
LUQ, expands inferomedially towards the RIF but often only detectable with patient in right lateral position. Start palpating from the umbilicus, diagonally upwards towards the left hypochondrium in order to detect splenomegaly. Inferior border is notched, though this is rarely discernible. Cannot palpate upper border which is above costal margin. Spleen moves with respiration. Dull percussion note. Splenic friction rub rarely present in acute splenomegaly or capsular inflammation (viral infection, infarction).
Enlarged kidneys are usually bilateral (e.g. ADPKD) but may be unilateral (renal cell carcinoma, hydronephrosis, ADPKD with nephrectomy—look for scar). Main distinction is between spleen and left kidney: kidney moves inferiorly with inspiration but not medially and has no notch. You should be able to palpate the upper aspect beneath the costal margin.
The term balloting is often used to describe the technique of propelling the kidney forwards with a hand on the patient’s loin, and feeling the anterior surface of the kidney move into your other hand on the hypochondrium. Percussion note is resonant due to overlying bowel gas (remember: kidneys are retroperitoneal).
Smooth mass in either iliac fossa beneath a hockey stick-shaped scar. You may feel only one pole of the kidney. It is extraperitoneal and dull to percussion. Often a bruit is present.
A very important skill in clinical practice but less often tested in exams. The bladder should be impalpable, and if you can detect it, the patient may have urinary retention. The bladder is suprapubic, extends superiorly in the midline, is smooth, and often tender in acute retention, and is dull to percussion when full of urine. If in doubt, perform a bladder scan and insert a urinary catheter.
Rarely palpable. Remember Courvoisier’s law: a palpable gall bladder in the presence of jaundice is rarely caused by gallstones. Where present, a gall bladder is palpated in the RUQ and is a rounded, focal swelling which can be detected on inspiration. Murphy’s sign is useful clinically: as the patient breathes in, the inflamed gall bladder (cholecystitis) descends onto the examiner’s hand causing the patient to catch their breath. Positive if LUQ is not tender.
Sometimes palpable in constipated patients. Distinctively, stool-filled bowel can be dented by the examiner’s hand.
Just to left of midline above the umbilicus. More commonly palpable in slim patients. Should be pulsatile but not expansile.
Signs of liver disease are a major feature of the GI examination. Be clear about the different clinical entities they represent.
(Stable, usually the result of long-standing hepatic insufficiency). Be able to explain the pathogenesis (e.g. gynaecomastia from oestrogen:androgen imbalance, spider naevi from reduced oestrogen metabolism). These are also signs of decompensated liver disease which are important to mention to the examiner:
Usually due to  vascular resistance in chronic liver disease (hepatic). Rarely acute, e.g. in Budd–Chiari syndrome (post-hepatic). Features include:
vascular resistance in chronic liver disease (hepatic). Rarely acute, e.g. in Budd–Chiari syndrome (post-hepatic). Features include:
• dilated abdominal wall veins
An acute decompensation probably caused by hyperammonaemia (can be measured in blood), usually on a background of chronic liver disease. Causes include GI bleed, infection (especially spontaneous bacterial peritonitis), shunt (e.g. transjugular intrahepatic portosystemic shunt (TIPSS) procedure), diuretics, acute liver insult (e.g. alcohol binge). Features include asterixis (‘liver flap’) and altered consciousness:
• Grade 1: ‘soft’ cognitive, sleep, or mood disturbance.
• Grade 2: disorientation especially in time, asterixis, lethargy.
• Grade 3: marked confusion and stupor.
• Withdrawal/delirium tremens (tremor, sweating, tachycardia).
• Wernicke–Korsakoff syndrome.
• Features of other substance use: tar-stained fingers, injection tracks.
• Head and other injuries from falls.
Look for: fluid restriction sign, low-salt diet, nil by mouth (recent GI bleed), infusion (fluids, furosemide, electrolytes, parenteral nutrition, PPI).
Observation of the patient: BMI and nutritional status (look for muscle atrophy, e.g. arms, thighs), skin and hair, tattoos or track marks (risk factor for hepatitis B or C), jaundice, conscious level (encephalopathy).
Ask patient to lift head off bed or cough (increase intra-abdominal pressure): often unmasks herniae, divarication of recti, abdominal masses.
• Asterixis: ask patient to hold out arms in front and look for coarse myoclonic jerks seen in hepatic encephalopathy.
• Hands: clubbing (cirrhosis), palmar erythema (chronic liver disease), Dupuytren’s contracture (chronic liver disease), koilonychia, leuconychia, Beau’s lines (chronic illness).
• Skin: jaundice, pruritus (obstructive jaundice, uraemia).
• Radial pulse: rate and character. Hyperdynamic circulation in liver disease.
Anaemia, scleral jaundice, xanthelasmata (PBC), glossitis, macroglossia (amyloid), aphthous ulcers (IBD).
Scleral jaundice, xanthelasma, Kayser–Fleischer rings of Wilson’s disease often mentioned but in practice only seen with slit lamp so do not say that you can see them with the naked eye.
Troisier’s sign/Virchow’s node: GI malignancy.
Look for spider naevi (in SVC distribution), gynaecomastia, Hickman line for parenteral nutrition, visible ribs in malnutrition.
Expose patient from xiphisternum to knees while preserving dignity with a bedsheet. Ask if the patient would like a chaperone. (See Fig. 50.2 for anatomy.)
• Inspection: look carefully for scars including posteriorly and in skin folds. Stomas? Remember ileal vs colon features (see  Chapter 34).
Chapter 34).
• Palpation: always ask about pain first! Usually start with a light-pressure exploratory circuit of the quadrants to identify obvious abnormalities. Then palpate specifically for:
• liver (start in RIF and move towards costal margin as patient inhales. If tense ascites, often only palpable laterally)
• spleen (starting at umbilicus moving diagonally to left hypochondrium) and feeling for notch. Ask patient to roll to their right
• kidneys: balloting or bimanually palpating within the renal angle
• exclude bladder/kidney transplant
• Percussion: percuss ~from sixth intercostal space downwards for liver, from umbilicus for spleen and remember percussion note over kidneys is resonant. Shifting dullness is important: identify a point between umbilicus and flank where resonant note becomes dull. Mark with two fingers. Ask the patient to roll away from your hand. Does the note over either finger change?
• Auscultate: liver/spleen rub, renal bruit (listen above umbilicus on both sides of midline, present in renal artery stenosis), bowel sounds (absent in ileus/postoperatively vs high-pitched tinkling/borborygmi in obstruction).

Fig. 50.2 Surface anatomy of the abdomen. Reproduced from Henry Vandyke Carter – Henry Gray (1918) Anatomy of the Human Body, Bartleby.com: Gray's Anatomy, Plate 1220. Image is in the public domain.
Peripheral oedema, skin changes (pyoderma gangrenosum), hair loss, general muscle mass.
Offer to: check hernial orifices, external genitalia in men and perform DRE and be able to explain what you are looking for (herniae, testicular atrophy, GI bleed or faecal impaction respectively)
‘This is a 42-year-old gentleman who presents with vomiting and confusion. He has a number of peripheral stigmata of chronic liver disease including palmar erythema, spider naevi, and gynaecomastia. He was deeply jaundiced and asterixis was present. On examination of the abdomen the liver was smoothly enlarged eight fingers beneath the costal margin and was tender to palpation. Shifting dullness was present. In summary, this man has evidence of acute decompensation on a background of chronic liver disease. To investigate him further I would take a full alcohol history, perform a liver/viral screen and request a liver ultrasound looking for cirrhosis and the direction of portal flow.’ (See Table 50.4)
Table 50.4 Patterns of findings
| Stable chronic liver disease | Peripheral stigmata of chronic liver disease No encephalopathy Underlying causes: alcohol, IV drug use, diabetes, rarer causes (e.g. PBC), Wilson’s disease Nutritional and fluid status Medications (spironolactone, diuretics) |
| Decompensated liver disease | Encephalopathy Jaundice Ascites Evidence of infection or GI bleed Evidence of chronic liver disease in background |
| Splenomegaly | Most common: chronic liver disease and portal hypertension Others: Myeloproliferative (pallor/purpura) Infectious (viral, malaria, endocarditis) Infiltrative (amyloid: macroglossia, cardiac failure) |
| Kidney transplant | Mass in iliac fossa beneath hockey stick-shaped scar Bruit (biopsy, stenosis) Cause of renal failure: diabetes, ADPKD, suprapubic catheter Previous dialysis (arteriovenous fistula, scars from lines or peritoneal dialysis catheter) Drug toxicity (steroids, tacrolimus tremor) |
| Crohn’s disease | Aphthous ulceration Abdominal abscess or stoma Anorectal disease: fissure/tags Nutrition (?Hickman line) Non-GI manifestations include malabsorption, clubbing, joint or eye involvement Drugs (steroids) |
| Ulcerative colitis | Usually limited to colon Stoma Toxic megacolon acutely Associated with pyoderma gangrenosum (legs) and PSC (jaundiced?) Drugs (steroids) |
• What are the main causes of cirrhosis in the UK?
• What are the causes of acute decompensation?
• How is hepatic encephalopathy managed?
• What other medical problems does alcohol misuse cause?
• What is the mechanism of gynaecomastia in liver disease?
• How is the severity of chronic liver disease graded?
• Name some rarer causes of cirrhosis.
• How is portal hypertension managed?
• Malabsorption: wasting, ulcers, glossitis, nutritional status, Hickman line, oedema, bruising.
Although we will focus solely on the neurological examination in this section, the reality of clinical neurology is that the diagnosis is often made primarily on the history with confirmation from the examination. For example, the potential causes to consider when examining a patient with weakness will be very different if the vignette brief is ‘Please examine this patient who has experienced sudden-onset left-sided weakness’ to one in which the brief is ‘Please examine this patient who has gradually worsening weakness in both legs’.
There is no one correct way to perform the neurological examination. As most medical students will agree, neurology can be one of the most daunting specialties and the complexity of the examination undoubtedly contributes to this.1 While examining a patient with a neurological disorder, try to localize the level of the lesion as you are going along. Students often display difficulty in eliciting reflexes. Make sure you practise on healthy colleagues so that you start to develop a sense of what is a normal reflex (and also develop confidence that you can actually find reflexes) and then try this on a range of patients on the ward. A good way to familiarize yourself with the appearance of abnormal signs is to watch neurologists examine patients online and look at images of abnormal findings ( https://meded.ucsd.edu/clinicalmed/neuro2.htm,
https://meded.ucsd.edu/clinicalmed/neuro2.htm,  www.neuroexam.com/neuroexam/). In the OSCE environment, you are most likely to be asked to examine only a defined region (e.g. cranial nerves, upper limbs, or lower limbs). It is appropriate at the end of a limited examination to say that you would like to go on to examine the remainder of the nervous system.
www.neuroexam.com/neuroexam/). In the OSCE environment, you are most likely to be asked to examine only a defined region (e.g. cranial nerves, upper limbs, or lower limbs). It is appropriate at the end of a limited examination to say that you would like to go on to examine the remainder of the nervous system.
As with any patient, make sure that you introduce yourself properly (while doing this listen to their acknowledgement to see if there is an obvious disorder of speech), enquire as to whether they have any pain, and ensure their comfort and dignity throughout the examination.
Look around the bed for signs of impaired mobility (wheelchairs, crutches, etc.), swallowing difficulties (nil by mouth signs etc.), visual difficulties (white stick, glasses, etc.), auditory difficulties (hearing aids etc.), or uneven gait (particular patterns of wear on the soles of shoes). Sufficiently expose the areas you have been asked to examine. Have a thorough general look at the patient and check for any asymmetry or obvious weakness (e.g. facial droop or decorticate posturing) and further clues (e.g. parkinsonian facies).
Reference
1. Pakpoor J, Handel AE, Disanto G, et al. (2014). National survey of UK medical students on the perception of neurology. BMC Med Educ 14:225.
Ask about any changes in sense of smell (can be formally tested by odorant detection tests).
Test acuity using a Snellen chart. (NB: does the patient need to wear glasses?) Test colour vision to check for an optic neuropathy. Use the red end of a Neurotip™ to check for gross defects in colour perception and perform a detailed examination with Ishihara charts if required.
Map out visual fields (Fig. 50.3). Ask the patient to focus on the bridge of your nose at all times. Ensure you are in direct confrontation with the patient with your finger held equidistant between you both. Bring your finger systematically in from the edge of the visual field in all four diagonal quadrants with the patient covering each eye in turn. Test for visual neglect by holding both hands out wide and asking the patient which hand is moving while wiggling each set of fingers individually.

Fig. 50.3 Patterns of visual field loss. Reproduced with permission from Piers Page and Greg Skinner, Emergencies in Clinical Medicine, 2008, Oxford University Press.
Test reflexes: pupillary reflex can be tested by shining a light into each eye in turn while looking either at the eye into which the torch is being shone (direct) or the other eye (indirect). Test for a relative afferent pupillary defect (RAPD) by ‘swinging’ the torch from eye to eye. A positive RAPD is relative dilation of one pupil when the torch is moved from the contralateral eye.
Test the accommodation reflex by asking the patient to look at your finger and moving it in towards the patient’s nose. A normal response is bilateral pupillary constriction.
Perform ophthalmoscopy: first of all look through the ophthalmoscope into each of the patient’s eyes in turn from ~30 cm and check that the ‘red reflex’ is present (a normal sign—the absence of this can indicate cataracts or retinal pathology). Look carefully at the optic disc, checking for any swelling or cupping, and trace vessels out into the peripheral retina. Finally assess the macula. Compare both eyes.
Ask the patient whether they have double vision. Ask the patient to follow your finger with their eyes, keeping their head still, as you trace a large ‘H’ in front of them. Check that there is smooth, symmetrical tracking of your finger with both eyes. Notice if nystagmus occurs in any particular gaze position (a few beats of nystagmus at extreme peripheral gaze can be normal) and whether any eye fails to move in a particular position. Check for defects of saccadic eye movement by asking the patient to look rapidly from one hand to another while you hold your hands at both extremes of the lateral visual fields. Normal eye movements are shown in Fig. 50.4.

Fig. 50.4 Extraocular movements. Reproduced with permission from Brodal, Per, The Central Nervous System (5 ed.), 2016, Oxford University Press.
Test light touch in each of the three areas of trigeminal innervation (midpupillary line: lower jaw (mandibular branch, V3), cheek bone (maxillary branch, V2), and forehead (ophthalmic branch, V1)). Test power of jaw closure (feel contraction of temporalis and masseter) and jaw opening (pterygoids). Offer to test corneal reflex (touch the cornea with gauze, both eyes should blink but painful) and jaw jerk.
Ask the patient to smile, blow out their cheeks, and raise their eyebrows (NB: unilateral upper motor neuron facial weakness will spare the forehead but will not be spared in lower motor neuron).
Whisper a number in each of the patient’s ears in turn and ask them to repeat that number (NB: does the patient use a hearing aid?). Perform the Rinne test. Strike a tuning fork, hold it initially in front of the patient’s ear and then place the end opposite on the mastoid process. The Rinne test is positive if normal (i.e. air conduction louder than bone conduction). If the patient perceives the bone conduction loudest, that indicates either a conductive hearing loss or an ipsilateral sensorineural hearing loss (these can be distinguished by the following test). Perform the Weber test by striking a tuning fork and placing it in the centre of the patient’s brow. Normally the patient should hear it equally in both ears. In conductive hearing loss, it is louder in the affected ear, whereas the reverse is true in sensorineural hearing loss.
Examine for asymmetry of palate (IX) at rest and with the patient saying ‘ah’. Offer to test gag reflex (sensory arm (IX) and motor arm (X)). Examine for wasting or fasciculations (if bilateral, think of motor neuron disease) of the tongue (XII). Ask the patient to protrude their tongue, press it laterally into the cheek against resistance, and wiggle it from side to side (XII). In unilateral palatal weakness, the uvula points away and the tongue towards the weak side.
Ask the patient to turn their head against your hand on both sides. This tests the contralateral sternocleidomastoid (i.e. a patient weak when turning their head left may have a right XI nerve palsy). Ask the patient to shrug their shoulders against resistance to test the trapezius.


Fig. 50.5 Cutaneous distributions of dermatomes and corresponding peripheral nerves. Reproduced with permission from A. Arturo Leis and Michael P. Schenk, Atlas of Nerve Conduction Studies and Electromyography (2 ed.), 2012, Oxford University Press.
Ask the patient (if able) to stand up and walk (make sure you follow them closely to ensure their safety). Assess their gait as they walk for a few steps before asking them to turn around and walk back to you heel-to-toe. Observe for specific patterns of gait disturbance and whether this is symmetrical or asymmetrical. Ask them to stand on their tiptoes and heels to assess flexor and extensor compartments of the legs. Assess limb girdle power by asking them to stand from a sitting position without using their arms. Test for Romberg’s sign by asking them to stand facing you, get their balance, and then close their eyes. Be ready to support them if the test is positive! You are responsible for ensuring that your patient does not hit the ground.
Look (and feel) for muscle wasting, particularly in the thenar eminence, hypothenar eminence, and between metacarpals in the hands and in the quadriceps (ask the patient to tense their leg to give yourself a better chance of seeing this). Look for evidence of fasciculations (particularly in large muscle groups). Look for contractures, tremors, and scars. Look for evidence of diminished sensation, such as burns or damaged skin (rarely neuropathic arthropathy) in the extremities. Look for any signs of risk factors or associated systemic disease that might predispose to neurological conditions as appropriate (e.g. tar-stained fingers, a vasculitic rash, or an irregularly irregular pulse).
Test for abnormal tone by moving each joint in the limb through all possible motions. This should be done both relatively slowly (feeling for parkinsonian rigidity) and quickly (feeling for a spastic catch from an upper motor lesion). In the lower limbs, gently roll the legs from side to side. In the case of normal tone, the foot should lag slightly behind the rotation of the leg. Specifically feel for cogwheel rigidity (associated with parkinsonism) in the wrist by rotating the hand in circles. Test for clonus in each lower limb in turn by asking the patient to relax their legs, gently rotating the heel and then jerking the foot briskly into plantarflexion. Up to 4 beats of non-sustained clonus may be normal. Remember that tone can be pathologically  or
or  . Hypertonia is of two forms: upper motor neuron lesions of the pyramidal tract cause spasticity, which is a velocity-dependent
. Hypertonia is of two forms: upper motor neuron lesions of the pyramidal tract cause spasticity, which is a velocity-dependent  in tone, catching when moved suddenly, whereas extrapyramidal lesions cause rigidity, which is velocity independent. In the arms, the best way to detect spasticity is with the pronator catch—flick the hand from pronation to supination, looking for a brief catch. In the legs, spasticity is best detected by quickly lifting the knee off the bed; watch the foot, which will lift off the bed if there is spasticity. Also look for clonus at the ankles by rapidly dorsiflexing.
in tone, catching when moved suddenly, whereas extrapyramidal lesions cause rigidity, which is velocity independent. In the arms, the best way to detect spasticity is with the pronator catch—flick the hand from pronation to supination, looking for a brief catch. In the legs, spasticity is best detected by quickly lifting the knee off the bed; watch the foot, which will lift off the bed if there is spasticity. Also look for clonus at the ankles by rapidly dorsiflexing.
Test each of the reflexes in Table 50.5.
Table 50.5 Reflexes and their origins/locations
| Reflex | Muscle, root, and nerve | Location |
| Upper limb | ||
| Biceps | Biceps, C5–6, musculocutaneous, | Anterior elbow over biceps tendon |
| Supinator | Brachioradialis, C5–6, radial | Radial side of wrist ~8 cm proximal to thumb |
| Triceps | Triceps, C6–7, radial | Posterior elbow over triceps tendon |
| Lower limb | ||
| Knee | Quadriceps, L2–4, femoral | 72 cm inferior to patellar |
| Ankle | Gastrocnemius/soleus, S1–2, tibial | Achilles tendon ~3–4 cm above heel |
| Plantar (Babinski) | Extensor hallucis longus, L4–5/S1–2, tibial | Base of foot (see text) |
Deep tendon reflexes should be elicited with a brisk motion, using the weight of the tendon hammer to generate the power of the strike ( www.neuroexam.med.utoronto.ca/motor_6.htm). Compare reflexes at the same level between each limb, ensuring that you look at the muscle that will contract with the reflex, rather than just the movement of limb. If the reflex appears absent, ask the patient to clench their teeth just before you test the reflex (reinforcement/Jendrassik manoeuvre). Grade the reflexes as 0+ absent; ± present with reinforcement; 1+ reduced 2+ normal; 3+ increased without clonus; 4+ increased with clonus. Testing for presence of the Babinski sign should be conducted by firmly scratching with a thumb or blunt item (not the sharp end of a tendon hammer) from the heel, along the lateral side of the foot and then medially across the base of the toes. An abnormal response (extensor plantar response = upper motor neuron sign) is extension of the hallux (vs flexion normally) ± fanning of the other toes.
www.neuroexam.med.utoronto.ca/motor_6.htm). Compare reflexes at the same level between each limb, ensuring that you look at the muscle that will contract with the reflex, rather than just the movement of limb. If the reflex appears absent, ask the patient to clench their teeth just before you test the reflex (reinforcement/Jendrassik manoeuvre). Grade the reflexes as 0+ absent; ± present with reinforcement; 1+ reduced 2+ normal; 3+ increased without clonus; 4+ increased with clonus. Testing for presence of the Babinski sign should be conducted by firmly scratching with a thumb or blunt item (not the sharp end of a tendon hammer) from the heel, along the lateral side of the foot and then medially across the base of the toes. An abnormal response (extensor plantar response = upper motor neuron sign) is extension of the hallux (vs flexion normally) ± fanning of the other toes.
Check for weakness by opposing each movement in the patient with your own. For example, for finger abduction, put your hand alongside the patient’s and directly compare the strength of your own finger abduction with that of your patient (i.e. like for like). Isolate the muscle being tested and immobilize the proximal parts. Score the patient’s power in each movement while stabilizing the joint using the MRC grading system.1 Make sure that you do use sufficient strength yourself that the patient has to exert him- or herself to overcome you.
For each movement, try to think about the peripheral nerve and nerve root territory you are testing as you test that movement. Minimize your instructions to either push or pull to avoid confusion to the patient and to yourself. You will look more slick. (See Table 50.6 and Table 50.7.)
Table 50.6 Scoring power
| MRC grade | Definition |
| 0 | No visible contraction |
| 1 | Visible trace of contraction |
| 2 | Movement with gravity removed (e.g. can flex knee with leg horizontal on bed) |
| 3 | Movement against gravity (but collapses with resistance) |
| 4 (±) | Movement against resistance (4− low; 4 medium; 4+ high) |
| 5 | Normal power |
Table 50.7 Limb movements
| Action | Muscle | Roots | Nerve |
| Shrug shoulder | Trapezius | C3, C4 | Spinal accessory |
| Abduct shoulder | Supraspinatus, deltoid | C5, C6 | Suprascapular and axillary |
| Flex forearm | Biceps | C5, C6 | Musculocutaneous |
| Extend forearm | Triceps | C6, C7 | Radial |
| Extend wrist | Extensor carpi | C5, C6 | Radial |
| Extend fingers | Finger extensors | C7, C8 | Posterior interosseous |
| Flex fingers | FDP and FDS | C8, T1 | Median and ulnar |
| Abduct thumb | Abductor pollicis brevis | C8, T1 | Median |
| Thumb to 5th finger | Opponens pollicis brevis | C8, T1 | Median |
| Abduct 5th finger | Abductor digiti minimi | C8, T1 | Ulnar |
| Abduct index finger | First dorsal interosseous | C8, T1 | Ulnar |
| Flex hip | Iliopsoas | L1, L2 | Femoral |
| Adduct hip | Hip adductors | L2, L3 | Obturator |
| Extend hip | Hip extensors | L5, S1 | Inferior gluteal |
| Extend knee | Quadriceps | L2, L3 | Femoral |
| Flex knee | Hamstrings | L5, S1 | Sciatic |
| Dorsiflex foot | Tibialis anterior | L5, S1 | Deep peroneal |
| Plantarflex foot | Gastrocnemius | S1, S2 | Tibial |
| Invert foot | Tibialis posterior | L4, L5 | Tibial |
| Dorsiflex hallux | Extensor hallucis longus | L5, S1 | Deep peroneal |
| Evert foot | Peroneus longus and brevis | L5, S1 | Superficial peroneal |
| FDP/FDS, flexor digitorum profundus/superficialis. | |||
Hold your index finger ~ one arm’s length in front of the patient. Ask the patient alternately to touch their nose and the tip of your finger. Look for any past pointing, intention tremor. or bradykinesia. Test for dysdiadochokinesia by asking the patient to rapidly pronate and supinate one hand in the palm of the other (demonstrate this to the patient). Test for cerebellar rebound by asking the patient to stretch their arms straight out in front of them and closing their eyes. Tell the patient to keep their arms where they are and then briskly push the arms downwards. A positive test is when the arms rebound above their initial position. Test coordination in the lower limbs by asking the patient to run the heel of one foot down the opposite leg and then lift the foot up to touch their leg again (heel–shin test). Repeat as quickly as possible.
Test each of the modalities in Table 50.8.
Table 50.8 Testing modalities of sensation
| Modality | Equipment | Spinal pathway tested |
| Light touch | Cotton wool ball | Anterolateral |
| Pinprick | Neurotip™ | Anterolateral |
| Proprioception | None (flex and extend joint) | Dorsal columns |
| Vibration | Tuning fork | Dorsal columns |
Test for light touch, pin-prick, vibration, and joint position (proprioception) sense. Ensure that the patient’s eyes are closed when testing all modalities. When testing proprioception, check that you are not supplying inadvertent cues by applying pressure to the skin of the sensitive finger pads. Test each joint at least three times. In general, test sensation from distal to proximal, since this is most likely to identify an abnormality quickly in the majority of cases. If an abnormality is detected, then systematically map out the extent of the sensory deficit. Adjust your method to suit: if you are looking for a mononeuropathy, test around the distribution of the nerve concerned; for a polyneuropathy, test distal to proximal (e.g. hallux, malleolus, leg, etc.); and for a root problem, test dermatomes. (See Fig. 50.5.)
Reference
1. Hahn AF, Bolton CF, Pillay N, et al. (1996). Plasma exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham controlled, cross-over study. Brain 119:1055–66.
Clinically, every neurological patient should have at least a basic assessment of cognition, speech, and their functional status (i.e. ability to carry out activities important for daily life). In addition, if particular signs are detected on the general examination, the examiner should attempt to elicit some specific signs supportive of particular diagnoses.
This is a discipline in its own right. There are multiple screening tools that can be used, such as the MMSE and MoCA ( www.mocatest.org/).1, 2 Cognitive defects may also be picked up during the general examination if the patient is finding it difficult to follow commands etc. As part of this, you should check for pathological primitive reflexes, including the snout, rooting and palmomental reflexes.
www.mocatest.org/).1, 2 Cognitive defects may also be picked up during the general examination if the patient is finding it difficult to follow commands etc. As part of this, you should check for pathological primitive reflexes, including the snout, rooting and palmomental reflexes.
Test for receptive dysphasia by asking the patient to perform a three-step action (e.g. take this paper with your left hand, fold it in half, and place it on the table). Check for executive dysphasia by asking the patient to name common items (e.g. pen, watch, book, etc.). Test for defects in speech production at different levels within the palate by asking the patient to repeat particular phonemes (e.g. ‘ga’, ‘ka’, ‘ma’;  http://neuroexam.med.utoronto.ca/cranial_9_10.htm). Examine palate and tongue coordination by asking the patient to repeat the phrases ‘baby hippopotamus’ and ‘British constitution’. Ask the patient to tell you about their day and assess their speech as they converse with you.
http://neuroexam.med.utoronto.ca/cranial_9_10.htm). Examine palate and tongue coordination by asking the patient to repeat the phrases ‘baby hippopotamus’ and ‘British constitution’. Ask the patient to tell you about their day and assess their speech as they converse with you.
In addition to the general neurological examination, examine for the following signs:
• Resting tremor—ask the patient to put their hands on a pillow while counting backwards from 20.
• Cogwheel/lead pipe rigidity.
• Bradykinesia—ask the patient to open and close both hands quickly.
• Parkinsonian gait—flexed posture with short, festinating steps and turning en bloc.
• Postural instability—warn the patient what you are going to do, stand behind them and tug back quickly on their shoulders; Parkinson’s patients typically fall like a ‘beanpole’ or take a few steps back (retropulsion).
• Micrographia—ask the patient to write several sentences.
• Parkinsonian voice—speak to the patient and assess whether they have a quiet, often monotonous voice.
• For extra marks, check for signs of atypical parkinsonism syndromes: vertical gaze palsy (PSP), cerebellar signs/postural drop (MSA), and spatial neglect (CBD).
Most relevant signs are contained within the general examination, but in particular check for ‘DANISH’: dysdiadochokinesia, ataxia (head, trunk or limb), nystagmus, intention tremor, slurred or staccato speech and hypotonia. Past pointing and cerebellar rebound are also recognized features.
Arguably the most important assessment once the diagnosis is made. This can be done in many ways but can include timed walks, stair tests, and assessing the ability to dress, wash, make a cup of tea, etc. Are the current aids the patient has appropriate to their needs?
If you find signs of a serious acute/subacute illness on general neurological examination, specific further examination may be necessary. If there are signs consistent with an acute/subacute progressive motor neuropathy, it is vital to check for respiratory compromise by measuring (and monitoring) forced vital capacity. If there are signs of an acute spinal cord syndrome, it is necessary to check for anal sphincter compromise. Concisely report your assessment as a list of positive findings and important negatives. Try to bring your findings together as a differential diagnosis.
• What are the causes, investigation, and treatment of strokes?
• Which MDT members will you involve in managing stroke patients?
• How can you differentiate between upper vs lower motor neuron lesions?
• What are the nerve roots for myotomes, dermatomes, and reflexes?
• What are the associations between area of blindness and area of pathology?
• What is RAPD and name types of pupillary responses?
Patterns of neurological deficit are associated with specific lesions (see Fig. 50.6).

Fig. 50.6 Common patterns of neurological deficits. Reproduced from Aaron L. Berkowitz, Clinical Neurology and Neuroanatomy: A Localization-Based Approach, 2017, McGraw Hill.
Much of neurological examination is directed at identifying the location of a potential lesion within the neuraxis. Throughout your examination you should consider whether the signs you identify fit with particular patterns. The main division within an examination of the peripheral nervous system will be between an upper and lower motor neuron lesion. Table 50.9 should help you think about your examination findings systematically:
Table 50.9 Patterns of findings
| Lower motor neuron (LMN) | Upper motor neuron (UMN) | Sensory neuropathy | Mixed | |
| Inspection | Fasciculations, wasting | Contractures | Burns, injuries | Fasciculations, wasting |
| Tone |  |
 ± clonus ± clonus |
 |
 / /  |
| Power |  |
 |
 (if pure) (if pure) |
 |
| Reflexes |  |
 |
 |
 |
| Coordinationa |  |
 |
 / /  (sensory ataxia—improves with patient observing movement) (sensory ataxia—improves with patient observing movement) |
 / /  |
| Sensation |  ( (  if sensory nerves involved too) if sensory nerves involved too) |
 ( ( if spinal) if spinal) |
 |
 |
| Common patterns and causesb | Glove and stocking (e.g. MMN, GBS, CIDP, Charcot–Marie–Tooth) Single/multiple peripheral nerve, spinal root or plexus lesions |
Symmetrical with sensory level (spinal—e.g. disc prolapse, tumour) Unilateral (cortical—stroke) |
Glove and stocking (e.g. diabetes mellitus, folate/vitamin B12 deficiency, toxin, para-neoplastic, drugs etc.) | UMN and LMN signs (motor neuron disease, cervical myelopathy, vitamin B12 deficiency) |
CIDP, chronic inflammatory demyelinating polyneuropathy; GBS, Guillain–Barré syndrome; MMN, multifocal motor neuropathy.
a Coordination can be difficult to assess in the presence of profound weakness.
b Become familiar with the distribution of nerves and nerve roots so as to localize lesions ( e.g. Oxford Handbook of Neurology, Chapter 2).
e.g. Oxford Handbook of Neurology, Chapter 2).
References
1. Folstein MF, Folstein SE, McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–98.
2. Nasreddine ZS, Phillips NA, Bédirian V, et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–9.
Pregnancy is a very important time in a woman’s life, so extra care and sensitivity is important when performing an examination of the pregnant abdomen. Building a good rapport early by simply congratulating them or asking them if they have thought of a name will help put your patient at ease!
As with any clinical examination, the following steps much be taken:
• Obtain informed consent—talk through what you are going to do.
• Expose: ask the patient to lie as flat as is comfortable for her and expose the abdomen ideally from pubic bone to xiphisternum.
• Pattern of examination is as follows:
• Symphysis–fundal height (SFH)
• Hands: swelling, pallor, pulse, BP, capillary refill time.
• Face: jaundice, anaemia (conjunctiva), periorbital oedema.
• Expose from the xiphisternum to the pubic symphysis.
• Abdominal distension consistent with appropriate gestation.
• Surgical scars, e.g. appendicectomy, laparoscopy, previous caesarean section through Pfannenstiel incision (low transverse scar across the abdomen above the pubis).
• Linea nigra: dark line from the xiphisternum to the pubic symphysis due to  pigmentation.
pigmentation.
• Striae gravidarum: stretch marks.
• Ask about pain before starting.
• In a single pregnancy there should be two fetal poles (unless the head is deeply engaged, i.e. entered the pelvis).
Relationship between the longitudinal axis of fetus and mother. Feel either side of the uterus abdominally, and then the back and limbs of the fetus. The lie can either be:
1. longitudinal (resulting in either cephalic or breech presentation)
2. oblique (unstable, should ideally become either transverse or longitudinal)
3. transverse lying across the abdomen from left to right or vice-versa (resulting in shoulder presentation).
Feel the maternal abdomen to feel which side is the back (i.e. which side of the abdomen feels more full and similar to a smoother structure, e.g. back). This is compared to the other side, where limbs can be felt, including movements.
Identify the anatomical part of the fetus that is closest to the pelvic inlet (cephalic, breech, or malpresentation, e.g. limb) and felt by placing thumb and fingers on either side of the presenting part.
‘How many 5/5th palpable?’
• Divisions for the fetal head into fifths ~1 finger width = 1/5th.
• If all of the fetal head is felt, that is ‘5/5ths palpable per abdomen’.
• If none of the fetal head is felt, that is ‘0/5th palpable per abdomen’.
• The head is engaged when the widest part (the biparietal diameter) has passed through the pelvic brim.
• Palpate using ulnar border of left hand moving from sternum downwards.
• It is easier to feel for the fundus firmly first (see Fig. 50.7).
• Locate upper border of pubic symphysis.
• Measure the distance in cm using a tape measure that should correlate with gestational age (± 2 cm).
• Plot as an ‘x’ on the customized growth chart.
• The SFH in cm should be the same as the gestation ±2 cm from 20 weeks onwards.

Fig. 50.7 Location of fundus as pregnancy progresses. Reproduced with permission from Raine, Tim, et al, Oxford Handbook for the Foundation Programme (4 ed.), 2014, Oxford University Press.
Once you have identified the lie of the fetus and where the back is, listen over the anterior shoulder of the fetus. Use some gel and a Sonicaid to listen for 1 min for the baseline heart rate which should usually be between 110 and 160 bpm.
• Wipe the gel away from both the patient’s abdomen and the Sonicaid probe.
• Wash hands and thank the patient.
• Offer her help to sit back up.
There are three parts to a gynaecology examination:
The basic examination format is the same but as this is an intimate exam, it is vital that you take a professional approach and make your patient feel comfortable and at ease. It is also important to address any personal barriers you may have to performing the examination.
• Obtain informed consent for the examination.
• Get chaperone and equipment ready (e.g. speculum, gel ± swabs).
• Ensure the light at the examination table is working well, otherwise get a torch—do not under any circumstances use the torch application on your mobile device, even if you assure the patient that only the torch is in use.
• Ask the patient if she would like to go to the toilet to empty her bladder (making the examination more comfortable as there is less pressure on the bladder when the speculum opens or when you are performing a digital examination).
• Inform the patient that she can remove appropriate clothing behind a curtain and lie down on the examination couch with a sheet to cover herself.
• Check for whether she is ready for the examination, and enter the room/behind the curtain with the chaperone.
Explain to the patient that the position she should be lying in is supine, with knees bent, heels brought up towards bottom, and then letting legs fall to either side of the bed. It is important to look out for other signs, which may be relevant to any gynaecological pathology. For example:
• Endocrine—goitre, skin changes such as acanthosis nigricans (velvety, hyperpigmented patches of skin which usually develop around the groin, neck and armpit, associated with obesity, insulin resistance suggestive of polycystic ovary syndrome (PCOS)).
• Secondary sexual characteristics—hirsutism, acne (suggestive of PCOS).
Make sure you ask about existing pain before starting. Like any examination, follow the normal routine: inspection, palpation, percussion, and auscultation. Important things to note include body hair distribution, scars, striae, tenderness, distention, masses, organomegaly, and inguinal lymphadenopathy.
Explain to the patient that the position she should be lying in is supine (flat), with knees bent, heels brought up towards bottom, and then letting knees fall to either side of the bed. Be aware of older women or women with restricted hip mobility. Draw back the cover sheet towards the suprapubic area to inspect the vulval area for:
To ensure full visualization of the labia minora, clitoris, urethral orifice, and vaginal introitus (opening), use your gloved left hand and part the labia majora with your thumb and index finger. Ask the patient to cough to look for a prolapse or stress urinary incontinence.
1. Think about the size of the speculum needed and use lubrication.
2. Explain to the patient what you are going to do before proceeding.
3. Expose the introitus by spreading the labia from below using the left index and middle finger.
4. Gently insert the speculum at a 45° angle and pointing slightly downward using your right hand until at least half of it is inserted.
5. Gently rotate the speculum to a horizontal position and gently open the blades until the cervix is in view (the blades may not need to be fully opened).
6. Secure the speculum by turning the thumb nut.
7. Visualize the cervix and vaginal walls for any abnormalities, e.g. ectropion, cysts, polyps, and contraceptive coil threads (if you can’t see the cervix, carefully draw the speculum back, reposition it, and try again).
8. Comment on whether the cervical os is open or closed (parous or nulliparous).
9. Perform any necessary tests, e.g. high vaginal swab, endocervical swab for culture and sensitivity, or cervical smear.
10. Withdraw the speculum slightly to clear the cervix and gently loosen the speculum to close the blades.
11. Continue to withdraw while rotating the speculum to 45°, avoiding contact with the vaginal walls.
Explain to the patient what you are going to do before proceeding. Using your gloved right hand, abduct your thumb and insert a lubricated index and middle finger into the vagina while flexing the remaining fingers into your palm. Initially, enter with your palm facing sideways and then rotate so it is facing upwards. Now, with your left hand press down on the lower aspect of the abdomen, so the uterus can be palpated bimanually. (See Fig. 50.8.)
While placing two fingers into the vagina, examine the following in order:
• Cervix: consistency, tender, mobile, cervical excitation.
• Uterus: place your two fingers behind the cervix and press upwards to ballot the uterus between your fingers inside and hand on the suprapubic area.
• Tilt: check whether the uterus is anteverted or retroverted, the size of the uterus, whether it is mobile, smooth, and tender to ballot.
• Adnexae: these are both lateral fornices. Place the two fingers on the right side of the vaginal wall and using the left hand on the abdomen, try and ballot to check for masses or tenderness. Do the same with the left side.
• Posterior vaginal wall: feel for nodules and tenderness.
• Gently withdraw fingers and check glove for any blood or discharge.

Fig. 50.8 Examination of the female reproductive system. Reproduced with permission from Raine, Tim, et al, Oxford Handbook for the Foundation Programme (4 ed.), 2014, Oxford University Press.
• Cover the patient back up with a sheet once you have completed your examination.
• Offer her help to sit back up.
• Offer the patient tissues—never wipe excess gel yourself.
• Close the curtain and allow the patient to dress in privacy.
• Inform her you will discuss the findings when she is dressed and back in the consulting room.
• Dispose of equipment correctly.
• Wash hands and summarize findings.
During your ophthalmology placement, ensure you acquire the following basic examination skills: history taking, visual acuity, visual field and colour vision testing, assessment of pupil reflexes, ocular motility, and fundus examination using the direct ophthalmoscope.
Ophthalmic history taking may centre on a PC of visual loss in an OSCE setting. An efficient, focused history is required in these circumstances to determine the timing of onset, laterality, and whether associated with any other symptoms (headache, jaw claudication, flashes, floaters, etc. if acute visual loss; photophobia, epiphora, pain, itching, or reduced vision if red eye). Ask about precipitating/relieving factors, and timing to resolution if relevant.
Recent surgery, use of contact lenses, recurrent pathology (iritis, recurrent corneal erosion syndrome), known chronic ophthalmic disease (diabetic retinopathy/glaucoma), and current treatments (drops) are important to elicit.
The eyes are often affected by systemic disease. Maintain a high index of suspicion in immunosuppressed patients. Ask about vascular risk factors in the setting of acute vascular events (retinal artery occlusion). Topical drugs undergo significant systemic absorption: asthma/COPD, diabetics on hypoglycaemic drugs, and those with ischaemic heart disease are at risk of adverse events with topical beta blockers. Likely longevity is important when determining the management of glaucoma and risk of blindness.
Certain disorders demonstrate Mendelian patterns of inheritance (retinitis pigmentosa, retinal dystrophies, etc.) FHx of glaucoma (first-degree relative) confers  risk of glaucoma in any patient. Viral conjunctivitis may spread rampantly through households; ask about contacts.
risk of glaucoma in any patient. Viral conjunctivitis may spread rampantly through households; ask about contacts.
Establish what their current drop regimen is, whether they are able to physically use their drops, who instils them, and ask about compliance (‘Do you ever forget to use them?’). Ask about systemic drug use. Systemic beta blockers may reduce IOP. Chloroquine, fingolimod (MS), amiodarone (AF), and topiramate (epilepsy) may cause visual loss. Warfarin and use of alpha antagonists (tamsulosin) may render ophthalmic surgery more difficult, due to an  risk of bleeding or poor pupil dilation.
risk of bleeding or poor pupil dilation.
The DVLA has strict visual criteria for driving: vision must be 6/10 or better in the binocular state with a healthy binocular visual field. Patients with age-related macular degeneration, glaucoma, or diabetic retinopathy, for instance, may risk losing their driving licences (and independence).
Smoking is a vascular risk factor and may contribute to poor outcome (thyroid eye disease). Alcohol and smoking may cause a toxic optic neuropathy. Phosphodiesterase inhibitors for erectile dysfunction may cause photopsia (flashing lights). Digoxin and ethambutol toxicity may result in colour vision disturbances. Recreational drug use may be relevant (cocaine, amphetamines) as IV drug use  the risk of endogenous endophthalmitis.
the risk of endogenous endophthalmitis.
A basic ophthalmic examination involves visual acuity testing, confrontation visual fields, pupil responses, colour vision, ocular motility, inspection of the ocular surface with light source, red reflex, and fundoscopy. In many circumstances as a doctor (such as in general practice) this will need to be performed without use of a slit lamp.
This is often documented poorly, yet it is medico-legally indefensible to omit it in patients with visual complaints. You should learn how to accurately measure distance and near acuity, and perform this throughout your career on patients in your care.
Test each eye in turn with the appropriate spectacle prescription (best corrected visual acuity). Distance vision is measured with a Snellen chart, typically at 6 metres (see Fig. 50.9). Occlude one eye in turn and ask the patient to read to the smallest line visible. Record the number under the visible line as the denominator (e.g. 6/12: notating that patient can see from 6 metres what a person with normal vision can see from 12 metres). A pinhole corrects many forms of refractive error, and if patient cannot see 6/6, ask patient to look through pinhole and record this acuity. This is useful if a patient has forgotten their glasses.
Jaeger cards are often used, with letter sized denoted as N48–N4.5 (smaller number denotes smaller letter). Test each eye in turn, with reading glasses if appropriate and good lighting. Myopic patients have good unaided near vision but poor unaided distance vision.
This is critical for identifying neurological visual field defects (from cerebrovascular disease, tumours, etc.). Sit opposite your patient at the same height. Ask them to cover one eye and the examiner covers the opposite one. Ask them to look at your pupil with the unoccluded eye and present fingers in each of the four visual fields at mid distance, asking the patient to count how many you are holding up (make sure they maintain fixation on your eye). This objectively confirms the integrity of each visual field quadrant. Repeat for the other eye.

Fig. 50.9 Snellen chart. Reproduced from ‘Snellen chart’ by Jeff Dahl – Own work by uploader. Licensed under CC BY-SA 3.0 https://commons.wikimedia.org.
Note the pupil size in both eyes in ambient light conditions. Direct the light in one eye and observe the direct pupil reflex (same pupil) then the consensual pupil reflex (contralateral pupil), and repeat for the other eye. The Marcus–Gunn swinging flashlight test examines for a RAPD, a sensitive test of unilateral optic nerve or retinal disease. You should ask an ophthalmologist to demonstrate this as it is often done poorly, yet is a very important objective clinical sign that should be performed on any patient with visual loss. The light is directed in the affected eye, before swinging to the unaffected eye, then back to the affected eye (if this pupil dilates in response to light, a RAPD exists). Test pupil response to accommodation (ask patient to look at a near target): both pupils should constrict as part of the accommodation response. (See Fig. 50.10.)

Fig. 50.10 The pupillary light reflex is a simple reflex that is of great clinical utility. (a) Information regarding a change in luminance travels from the retina to the midbrain through the optic nerve. Within the midbrain, luminance information is processed by the pretectal nucleus and then passed on to the Edinger–Westphal nucleus, which contains preganglionic parasympathetic neurons that travel in the oculomotor nerve and ultimately control the pupillary constrictor muscle. Importantly, information crosses the midline within the midbrain so that a luminance change in one eye affects the pupils of both eyes. (b) The effects of three common lesions on the direct and consensual pupillary reflexes evoked by light flashed into each eye are illustrated. Reproduced with permission from Peggy Mason, Medical Neurobiology (2 ed.), 2017, Oxford University Press.
Holmes–Adie pupil causes a unilateral tonically dilated pupil, often in young females. Near acuity is reduced due to impaired accommodation. Oculomotor nerve palsy may cause ptosis, reduced ocular motility (due to paralysis of four out of six extraocular muscles), and a non-reactive pupil. This may indicate a life-threatening intracranial pathology. Horner’s syndrome results in unilateral subtle ptosis, miosis, apparent enophthalmos, and variably, anhidrosis due to interruption of sympathetic innervation to the eye. Pancoast’s tumour (apical lung cancer) and carotid artery dissection are two pathologies that must be excluded in this patient group.
This is often examined with the Ishihara colour plates (although these are designed to test congenital red–green colour blindness). Each eye is tested in isolation and number plates are read consecutively, recorded as number of plates read over number presented (i.e. 14/17).
This is a key part of the neurological examination, and may be employed by examiners to distinguish candidates at finals. The eye clinic is the only place in the hospital with opportunities to perform dilated fundoscopy, and to receive feedback from an ophthalmologist.
It may be your only opportunity to learn or refine your technique before you qualify. You should aim to be able to examine an optic disc through an undilated pupil before the end of the placement. Practise, practise, practise is the only way to master this skill.
Three key tips: (1) get as close as you can with your ophthalmoscope, ~1 cm from the eye! (2) Ask patient to fixate at specific target to prevent ocular movement. (3) Dim room lights to maximize pupil size if not pharmacologically dilated.
1. Introduction, consent, and instructions: ‘May I examine the back of your eye? I am going to come quite close. May I put my hand on your head? Please look at the sign on the wall.’
2. Switch ophthalmoscope on, adjust to medium-sized white light beam, keep the lens dial on 0 unless you need to dial in your prescription (on Welch Allyn, red numbers = myopic prescription, green = hyperopic).
3. Find the red reflex from 30 cm, and follow the red reflex in (this keeps light beam on the pupil), put your hand on patient’s head with thumb on brow (to provide proprioceptive information), and get as close as physically possible to the ocular surface to maximize view. Approach 15° temporally to find the optic disc.
4. Find a retinal vessel and follow it. If two smaller vessels join to a larger one in the direction of movement, you are heading towards the disc, keep going (all retinal vessels originate at the optic disc).
5. When visualizing the disc, comment on margin (excludes disc swelling), colour (e.g. pallor), significant optic disc cup (glaucoma), and presence of new vessels (proliferative diabetic retinopathy).
6. Ask patient to look up, find red reflex and inspect retina, and do the same while asking patient to look right, down and then left (demonstrates systematic retinal examination).
7. Finally, ask patient to look at the light (this will bring the light beam onto the macula/fovea) and inspect for haemorrhages, exudates (diabetes), or scarring.
In the binocular state, with the head kept still, ask the patient to track a target in the nine principal positions of gaze, and to report any double vision (diplopia). Ensure full abduction (abducens nerve—sixth cranial nerve), ability to look down and in (trochlear nerve—fourth cranial nerve), and adduction (oculomotor nerve—third cranial nerve). Complete third nerve palsy results in complete ptosis (paralysis of levator palpebrae superioris), fixed and dilated pupil, and eye looking down and out underneath the closed eyelid. Partial third nerve palsy may spare the autonomic features (pupil sparing) and suggests a microvascular cause such as diabetes/hypertension/vasculitis.
Orthopaedic examination is an art, which can be learnt and mastered with practice. The focus should be on eliciting pertinent signs and the manner in which they affect the patient, which will aid in formulating a diagnosis and subsequently tailoring treatment plans. Patients should be respected, made to feel at ease, and their dignity should be preserved. The examiner should be aware that if the examination causes discomfort, the patient should be asked if they would like to proceed with the rest of the examination. Handwashing, introducing yourself, and explaining your role demonstrates professionalism and is likely to instil confidence in your patients. Communication with patients is vital so give them clear instructions on what you wish them to do and expose the region to be examined.
A tape measure, goniometer (instrument to measure angles), and a tendon hammer are essential for orthopaedic examinations. Aids to functional assessment in a hand examination include a pen, comb, key, and coin.
The examination starts from the moment that we encounter the patient. A plethora of information can be obtained by their general appearance, posture, and gait. Do they have a short limb, ‘knock-knees’, are they in pain, or not using a limb? Looking around the bedside for braces, walking aids, orthoses, and slings may help not only in establishing a diagnosis but also in understanding how the condition may disable the patient. Exposure of the joint to be examined must be done with clear instructions while preserving the patient’s dignity. Both limbs should be exposed and examination should start with the unaffected limb for comparison.
The commonest examination technique is derived from Apley’s mantra of:
Having a systematic approach provides a repeatable and transferrable examination, which will pick up on important findings related to the diagnosis and the patient’s disability.
• Shape and posture: patient thin or obese? Spine straight/curved? Shoulders level? Abnormal limb posture? Flexed posture (assess for a fixed deformity on movement—look for deformity of three planes, compare with unaffected side.
• Wasting of muscle groups? (disuse, denervation). Swelling—diffuse (joint-synovial fluid, blood, pus). Localized (ganglion, meniscal cyst).
• Skin: colour—pallor (ischaemia), blueness (cyanosis), redness (inflammation, infection, pain syndrome), purple (bruise/ecchymosis). Markings (wounds, scars, ulcers, sinuses). Quality—shiny (oedema, trophic change), abnormal creases (underlying fibrosis).
This is an exploration of anatomy and therefore should be communicated with your examiner (e.g. ‘I am palpating the medial joint line of the knee between the medial tibial plateau and the medial femoral condyle while observing the patient’s face, this does not appear to elicit pain’). Find landmarks and use them! Look at the patient’s face to appreciate areas of tenderness.
• Skin: warm/cold (use the palm of your hand as more nerve endings/sensitive), moist/dry.
• Soft tissues: lump (size, shape, surface, consistency, compressibility, fluctuation, mobility, illumination, percussion, auscultation), pulses.
• Joint: palpable effusion, synovial tenderness (inflammation/infection).
• Tenderness: correlating your anatomical landmarks with areas of tenderness gives you an idea of what the underlying structure/problem is.
Demonstration of movement makes communication clearer. Measurements should be carried out with a goniometer and both limbs compared. This can be compared with reference ranges for each joint.
• Range of movement (ROM): in degrees. Starting at 0, anatomic position of joint (hyperextension is denoted by –ve degrees referenced from anatomical position). End range finishes when joint stops moving (e.g. ROM of −10° to 140° is a hyperextension of 10° and 140° flexion from the anatomical position).
• Active: patient moves without assistance. Functional degree of movement. Painful? Muscle power (MRC grade). Feel for crepitus (coarse and diffuse—joint, fine and specific—tenosynovial).
• Passive: examiner moves joint. Limited by pain and joint stiffness.
• NB: sometimes active movement cannot be performed due to limitations in understanding (young children/some neurological disorders).
• Arthrodesis: lack of active and passive ROM.
• Degenerative joint/adhesive capsulitis: limited active/passive ROM.
• Tendon/muscle/nerve injury: no active, only passive ROM.
• Ligament laxity: excessive ROM (hypermobility).
These are discussed elsewhere and are used to elicit suspected abnormalities.
Length of limbs and muscle bulk can be measured using a measuring tape. It is vital when comparing limbs that the same fixed landmarks are used.
Involves two main phases: swing (40%) and stance (60%) (See Fig. 50.11 and Table 50.10.)

Fig. 50.11 Gait. Reproduced from Stöckel T. et al, The mental representation of the human gait in young and older dults. Front Psychol. 2015 Jul 14;6:943, under the CC Attribution 4.0 International license.
Table 50.10 Description of gait analysis
| Gait | Description |
| Antalgic | Shortened stance phase (painful) |
| Spastic | Stiffed-legged (flexed hips and knees, equinus feet) ‘scissoring’ of legs |
| Foot drop | Equinus: in swing—peroneal nerve injury |
| High stepping | Foot drop, balance, proprioception |
| Waddling (Trendelenburg) | Trunk thrown from side to side—abductor weakness |
| Ataxic | Broad based, loss of balance |
Further examination of the joint above and below is essential to exclude referred pain (e.g. a patient with hip osteoarthritis presenting with pain referred to the knee). A complete neurovascular examination should be done to ascertain whether there is a true neurological deficit or if neurological symptoms are mimicking MSk symptoms. The MRC grading system is used to assess muscle strength.
Both upper limbs should be exposed to above the elbow. Place a pillow on the lap. Inspect hands with palms up, make a fist, turn to palms down and make another fist in this position. Ask to flex elbows and inspect olecranon fossa. Usual format of inspection for scars (surgery/trauma), sinuses.
• Look: skin colour, dry/moist, hairy/smooth, puckering and ridging of skin (Dupuytren's disease).
• Swellings: subcutaneous (dermoid cyst), tendon OA, ganglion at wrist.
• Muscle wasting: thenar eminence wasting (median nerve dysfunction).
• Nails: atrophy, ‘pitting’ (psoriatic arthropathy), ‘grooved’ nail, ganglion cyst at nail bed, subungual exostosis.
• Swellings: dorsal ganglion over wrist, RA nodules/gouty tophi (extensor surface).
• Joint swelling: metacarpophalangeal joint (MCPJ) plus proximal interphalangeal joint (PIPJ)—RA. Distal interphalangeal joint (DIPJ)—OA, PIPJ (Bouchard’s nodes), DIPJ (Heberden’s nodes), carpometacarpal bossing (OA mass).
• Temperature, texture of skin, and pulses felt.
• Swelling/thickening, subcutaneous tissue, tendon sheath, joint (each palpated for an effusion/tenderness), or one of the bones.
If a nodule is felt, flexion/extension of finger will ascertain if it is attached to underlying tendon. Does tendon glide freely or snap in extension (trigger finger)? Can a cord be felt or Garrod's pads on dorsal aspect of PIPJ (Dupuytren's)?
Perform active movements then check additional passive movement. Active movements: clue from initial screening with palms up and making a fist may show ‘lagging’ finger. Thumb and each finger examined in turn.
Fingers:
• MCPJ = 0–40° of hyperextension to 90° flexion.
MCP joints in extension relaxes collateral ligaments and allows maximal movement.
To assess thumb movements with patient’s palm up, hold hand flat on table. Ask to:
• ‘stretch to the side’—extension
• ‘point to the ceiling’—abduction (weak in median neuropathy)
• ‘touch your thumb to little finger’—opposition
• thumb behind plane of hand is retroposition.
• Muscles: gross grip strength should be assessed by asking the patient to squeeze examiner’s index and middle fingers:
• Flexor digitorum profundus (FDP): PIPJ held and immobilized in extension and patient asked to bend tip of finger.
• Flexor digitorum superficialis (FDS): grasp other fingers in full extension to inactivate FDP. FDP shares a common muscle belly. Isolate the MCPJ in extension of the finger that is being examined. Ask the patient to flex the finger. NB: little finger sometimes has no independent FDS. Index finger often has completely independent FDP therefore cannot separate mass action, FDS is tested with DIP in full extension and FDS flexed, patient asked to pinch against resistance—if unable then ?FDS rupture.
• Flexor pollicis longus (FPL): immobilize thumb MCPJ, ask to flex interphalangeal joint (IPJ).
• Long finger extensors: ask to extend at MCPJs.
• Lumbricals: flex MCPJ and extend IPJ.
• Interossei: Palmar ADduct and Dorsal ABduct (‘PAD DAB’).
This is the most significant part of the examination as it gives the examiner an idea of the disability caused. For the purposes of most examinations, props such as a key, coin, and pen are sufficient for a functional assessment. A series of tasks can be given to the patient to assess function and grip:
• Sideways pinch: ‘Holding a key’.
• Chuck pinch: ‘Holding a pen’.
• Hook grip: ‘Holding a bag handle’.
• Hueston tabletop test: demonstrates contractures of the PIPJ and MCPJ in Dupuytren's disease. Ask patient to place palm of the hand flat on the table top—if contractures exist, this cannot be performed.
• Boutonnières deformity: damage to central slip causes proximal phalanx to button-hole through and cause a PIPJ flexion deformity. As central slip tear extends, the lateral bands displace palmar, further worsening the PIPJ flexion and causes secondary DIPJ extension.
• Mallet finger: flexion deformity of DIPJ. Injury to lateral slips leads to inability to extend DIPJ.
• Swan neck deformity: volar plate damage—PIPJ hyperextends and due to muscle imbalance 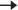 secondary DIPJ flexion.
secondary DIPJ flexion.
See Fig. 50.12.

Fig. 50.12 Hand deformities in rheumatoid arthritis. Reproduced from www.arthritisresearchuk.org, Looking after your joints when you have arthritis Photographs reproduced from Marc C Hochberg et al, Practical Rheumatology, 3rd edition, 2011, Elsevier.
Further examination of the wrist is essential to exclude referred pain. A complete neurovascular examination taking into account the possibility of carpal tunnel/cubital tunnel syndromes and Allen’s test for vascular supply to the hand.
Both upper limbs should be exposed. Observe from front, back, lateral, and medial aspects of the elbow. Compare both sides. Usual format of inspection for scars (surgery/trauma), sinuses.
• Front: arms by side, elbows extended with palms forward. Normal carrying angle is 5–15° valgus. Look for cubitus varus or valgus. Ask to lift arms to shoulder height with elbows extended—look for ‘gunstock deformity’—varus deformity often results from previous childhood supracondylar fracture or a growth arrest on the medial side of distal humerus. NB: carrying angle can only be assessed once elbow is straight.
• Behind: prominent olecranon bursa, swelling, rheumatoid nodules.
Anterior and lateral aspects of the elbow are best examined from the front, and medial and posterior from behind.
• Front: from lateral to medial, palpate the brachioradialis muscle, biceps tendon with lacertus fibrosus, brachial artery, and the median nerve.
• Abnormal hard swelling: myositis ossificans after elbow dislocation.
• Biceps rupture: ‘hook test’ use finger to hook under tendon insertion—absent with more proximal muscle bulge.
• Lateral: palpate lateral supracondylar ridge, lateral epicondyle to common extensor origin (tender—lateral epicondylitis), and lateral collateral ligament. Extensor carpi radialis brevis (ECRB) and extensor carpi radialis longus (ECRL) are assessed with resisted wrist extension in neutral and radial deviation respectively. ERCB tenderness—tennis elbow.
• Capitellar joint line: tenderness 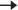 articular injury/osteochondritis dissecans.
articular injury/osteochondritis dissecans.
• Radiocapitellar joint line: normal sulcus. Synovial hypertrophy—boggy. Fluid—fluctuant.
• Radial head: palpate while supinating and pronating at wrist. Feel for subluxation (congenital), crepitus (degenerative change) worsens with gripping of examiner’s fingers during rotation.
• Posterior: feel for joint warmth. Tip of olecranon, medial and lateral epicondyles form a straight line with the elbow in extension and an isosceles triangle in 90° of flexion. If not normal—?previous injury. Palpate triceps insertion against resisted extension. Tenderness—partial tear. Inability to extend against gravity—complete tear. Palpate olecranon fossa in 30° flexion—loose body. Feel for rheumatoid nodules or olecranon bursa.
• Medial: palpate medial epicondyle to common flexor origin (tender—medial epicondylitis), belly of pronator teres (pronator syndrome). Feel for ulna nerve behind medial epicondyle, subluxes in flexion in 10% of people.
Perform active movements then check additional passive movement.
• Observe front—flexion and extension: in sagittal plane with forearm supinated and fully extended. ROM −10° to 140°. Any more extension—?hyperlaxity. Reduced extension first sign of intraarticular injury.
• Forearm rotation: elbow flexed to 90°, arms adducted, start with thumbs pointing up. Supination 85°, pronation 85°.
• Rotational deformity: shoulder fully extended, elbow bent to 90° behind back, patient bends over. Observe from behind. Look for forearm asymmetry. If forearm not parallel to floor then internal rotational deformity of distal humerus (likely malunion of supracondylar fracture).
• Muscles: strength in flexion/extension and supination/pronation can be resisted to test muscle groups.
• Lateral epicondylitis (tennis elbow): passive volar flexion of the wrist, elbow extension, and pronation. Tender lateral epicondyle.
• Medial epicondylitis (Golfer’s elbow): passive extension of the wrist and clenching of fist worsen symptoms. Tender medial epicondyle.
• Impingement: posterior (olecranon osteophytes/loose bodies)—just short of full extension—gentle rapid passive extension reproduces posterior pain. Anterior (coronoid osteophytes/radial fossa osteophytes)—just short of full flexion—gentle rapid passive flexion reproduces posterior pain.
• Varus instability: lateral collateral ligament (radial collateral plus lateral ulna collateral (LUC)).
• Assess: elbow flexed to 30° (unlocks olecranon and relaxes anterior capsule). Shoulder in full internal rotation—locks shoulder. Apply varus stress (under image intensifier)—radio-capitellar space increases.
• Valgus instability: ulna collateral ligament.
• Assess: elbow flexed to 30° (unlocks olecranon and relaxes anterior capsule). Shoulder in full external rotation—locks shoulder. Apply valgus stress (opening of joint/tenderness suggests injury).
• Rotational instability: lateral ulna collateral ligament—‘pivot shift’ similar to that in knee examination. Under image intensifier and GA, patient supine, elbow and shoulder at 90°. Fully supinate while holding hand and forearm. Slowly extend elbow applying valgus and axial compression. Elbow subluxation—dimple over radiocapitellar joint and prominence posterolaterally over radial head. At 45° ulna reduces with a ‘clunk’.
• Apprehension sign: ask to rise from chair using arms to push up. May be reluctant due to instability. This creates axial load, valgus and forearm supination as above.
Patient undresses to waist, scapulae should be visible. Observe from front, back, side, and axilla. Compare both sides. Look for slings or braces around the patient. Usual format of inspection for scars (surgery/trauma), sinuses.
• Front: prominence of acromioclavicular joint (ACJ) or sternoclavicular joint (SCJ)—degenerative/traumatic. Deformity of clavicle—previous trauma. Long head of biceps rupture—‘Popeye’ sign. Pectoralis major wasting or rupture (loss of anterior axillary fold).
• Behind: prominent shoulder blade—malunion, osteochondroma, positional (static winging). Wasting of deltoid (‘squared-off’ appearance of shoulder)—axillary nerve injury/chronic shoulder pain. Hollowing of supraspinous and infraspinous fossa—supraspinatus and infraspinatus full-thickness cuff tears/suprascapular nerve lesion.
Palpate SCJ, clavicle, ACJ, acromion, subacromial margins, and margins of glenoid, bicipital groove.
Perform active movements then check additional passive movement. Movements are a combination of glenohumeral joint (GHJ), scapulothoracic joint (STJ), SCJ, and ACJ.
• Flexion and extension: in sagittal plane. Flexion ROM 0–160°/170° (acromion limits movement). Extension ROM 0–40°.
• Adduction: not normally recorded.
• External rotation: arms by side and elbows at 90° of flexion. ROM 0–80°.
• Internal rotation: ROM limited by trunk getting in the way. Hence functional—how far behind one can reach with the tip of thumb (vertebral level T8, T5, etc.)—tests adduction and internal rotation combined.
• Abduction: referenced in coronal plane but does not correspond to plane of GHJ as scapula faces forwards by 30°. ROM 0–160°/180° in scapula plane. Stand behind and observe scapulae.
• Scapular rhythm: GHJ movement: STJ movement 2:1. More GHJ movement in lower range (90°), STJ in later range (60°). Isolate GHJ movement by fixing tip of scapula and acromion (abduction restricted to 90°). While assessing scapulothoracic rhythm, look for winging of scapula (from either injury to serratus anterior or to the long thoracic nerve).
• OA: full movement at STJ, little at GHJ.
• Painful arc: pain in midrange of abduction (70–120°)—cuff tear/supraspinatus tendinitis.
• ACJ arthritis: pain at end range of abduction.
• Infraspinatus: most powerful external rotation—elbow flexed to 90° power of external rotation assessed.
• Hornblower’s sign: complete tear, flex elbow and abduct shoulder to reach mouth (like sounding a hunting horn).
• Infraspinatus and teres minor:
• Drop sign: arm passively maximally externally rotated then released if weakness falls ‘drops’ into internal rotation.
• Lag test: examine from behind. Elbow at 90°, elevate 20° in scapula plane. Passively put in full external rotation. Patient asked to maintain position but drops into neutral rotation.
• Supraspinatus: abductor. Most patients with a tear can still initiate abduction using the deltoid. Shoulder elevated in plane of scapula (30°) forward from coronal plane. At 60° maximum tension of muscle fibres, internal rotation (reduced deltoid contribution), thumb down. Test resistance—Jobe test.
• Subscapularis: powerful internal rotator.
• Lift off test (most reliable): hand behind back and push examiner’s hand away from small of back (reduced pectoralis contribution). Pain in shoulder may limit test. ‘Lag’ test pull hand away from back, ask patient to keep away from back—‘lags’ back—complete subscapularis rupture.
• Napoleon/belly press test: hand on abdomen, patient pushes elbow forward (reduced pectoralis contribution).
• Deltoid: tested in 90° abduction or by side of patient. Ask to forward flex (anterior), abduct (lateral), and extend (posterior) heads of deltoid.
• Pectoralis major: hands on waist and squeeze inwards—palpate muscle.
• Latissimus dorsi: downwards and backwards pressure of arm against resistance—palpate muscle.
• Rhomboids: hands on hips pushing elbows backwards against resistance—palpate muscle.
• Serratus anterior: patient pushes against wall with outstretched arm, fingers and palm pointing down—scapula winging observed (long thoracic nerve of ‘Bell’ palsy).
• Trapezius: patient shrugs shoulder against resistance.
• Neer’s sign: passively elevate the internally rotated shoulder in scapula plane while stabilizing the scapula. Pain 70–120°—downward pressure on scapula exacerbates.
• Neer’s test: inject local anaesthetic into subacromial space and repeat manoeuver—positive if pain abolished.
• Hawkins and Kennedy sign/test: arm elevated to the horizontal and adducted by 10°. Subsequent internal rotation of the arm provokes pain. Can be repeated after local anaesthetic infiltration.
• Scarf test: elevate arm to shoulder height and adduct arm across chest. Positive if pain occurs—ACJ arthritis.
• O’Brien’s test: arm elevated to horizontal, adducted by 15° fully internally rotated. Patient asked to resist downward pressure on wrist, which causes pain. Less sensitive for ACJ OA, more sensitive for rotator cuff tears or SLAP (superior labrum, anterior to posterior) lesions.
Long head of biceps (LHB), biceps pulley (in intertubercular groove), and insertion into supraglenoid tubercle (SLAP tears) cause pain.
• Popeye sign: bulky of biceps visible more distal in arm—LHB rupture.
• Speed’s test: elbow fully extended, forearm supinated. Anterior shoulder pain on resisted elbow flexion—positive.
• Yergason test: taking the patient’s hand as if to perform a handshake, elbow at side, 90° flexion. Resisted supination, not pronation, indicates biceps pathology.
• Crank test: shoulder abducted and elbow flexed to 90°. Resisted elbow flexion recreates pain.
• Sulcus sign: inferior laxity on passive downward traction on the humerus in neutral rotation. If present in external rotation—rotator interval incompetence (coracohumeral and superior glenohumeral ligaments)
• Load and shift tests: stand behind patient. Stabilize scapula using thumb behind acromion and index finger on coracoid. Grasp proximal humerus close to the humeral head between thumb, and index and middle fingers. Passively move humeral head anterior and posterior to respective glenoid rims 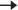 pain/clicks—indicate rim damage/labral tears.
pain/clicks—indicate rim damage/labral tears.
• Apprehension tests: patient seated or lying with shoulder at edge of couch.
• Anterior apprehension test: shoulder abducted to 90° and elbow flexed to 90° with/without anterior pressure to back of humeral head and gentle external rotation  patient fears dislocation may occur, involuntary pectoralis major contraction = positive test.
patient fears dislocation may occur, involuntary pectoralis major contraction = positive test.
• Posterior apprehension test: horizontal adduction of internally rotated arm while axial load applied along humerus. If does not produce apprehension, maintain axial load and bring out of adduction and internal rotation  relocation of subluxed humeral head may occur.
relocation of subluxed humeral head may occur.
Further examination of the cervical spine is essential to exclude referred pain. Testing for generalized joint laxity using a Beighton's score is useful in cases of instability.
Exposure to underwear, offer a blanket to keep warm or to preserve dignity if examination of the whole spine not required. Usual format of inspection for scars (surgery/trauma), pigmentation (neurofibromatosis), tufts of hair (spina bifida).
Front, back (coronal plane), and side (sagittal plane) and look for scoliosis (lateral curvature of the spine). ‘Adam’s Forward bend test’—patient bends forward and ‘rib hump’ may be seen.
Thoracic kyphosis (slight forward curve), lumbar lordosis (short backward curve). Abnormalities can occur as:
• cervicothoracic (ankylosing spondylitis)
• thoracic (Scheuermann’s disease, osteoporotic wedge fractures)
• loss of lumbar lordosis: protective paravertebral muscle spasm
• hyperlordosis: spondylolisthesis, fixed flexion deformity of hips
• assess shoulder asymmetry and pelvic tilt. If one knee flexed could be nerve root tension (flexing knee relaxes sciatic nerve).
• Feel; spinous process and interspinous ligament for steps.
• Tenderness; correlating your anatomical landmarks with areas of tenderness gives you an idea of what the underlying structure/problem is. 1—bony structures, 2—intervertebral tissues, 3—paravertebral muscles and ligaments. The vertebral level should be noted.
All movements are performed actively by demonstrating/instructing. Passive movements can be performed at the end range of movement.
• Cervical: flexion, extension, rotation, lateral flexion.
• Spondylosis (reduced flexion and extension).
• Cervical disc prolapse (hold head to affected side, passive lateral flexion to contralateral side produces radicular pain).
• Thoracic spine: rotation (facet orientation and ribs restrict flexion, extension, and lateral rotation).
• Lumbar spine: flexion/extension (facet orientation restricts rotation).
• Flexion measured on forward bend as ‘fingers to knee, mid-shin, toes, etc. Is it smooth or hesitant?
• Lateral flexion measured in similar manner.
• Extension (support from behind, pain—facet arthrosis).
• Rotation by fixing the pelvis.
Antalgic, neurological (broad based in cervical myelopathy), short legged (compensatory scoliosis as a result of leg length discrepancy). A quick screening of lower lumbar nerve roots before walking may be useful:
• Squat and get up (L3—quadriceps).
• Stand on heels (L4—ankle dorsiflexion).
• Stand on one leg—Trendelenburg (L5—hip abduction).
• Tip toe (S1—plantar flexion of ankle).
• Spurling’s manoeuvre: narrows the involved neuroforamen (cervical radiculopathy). Axial pressure, hyperextend, lateral flexion, and rotated to affected side. Abduction of shoulder relieves pain.
• Adson’s manoeuvre: head extended and rotated to affected side, abduction of arm >15°—radial pulse obliterated.
• Roo’s manoeuvre: shoulders abducted, elbows flexed to 90°—fingers actively and repeatedly flexed and extended—recreates tingling.
• Lhermitte sign: flexion of the neck precipitates ‘lightning’ or paraesthesia of lower limbs.
• Hoffman’s sign: flick DIPJ of index/middle finger. If thumb flexes—+ve test.
• Inverted radial reflex: finger flexion seen with brachioradialis tendon reflex.
• Romberg’s: close eyes with feet together  unstable stance.
unstable stance.
• Sciatic stretch test: with patient supine passively, raise the leg straight to 30–50°—ask about pain in back or down the leg. Lower the leg gently and passive dorsiflexion reproduces symptoms (Lasegue’s sign). If patient sits up with knee straight (i.e. hip flexed at 90° then no nerve root tension). Can be done with patient sitting with knee flexed over the edge of the couch and actively extending each knee in turn (‘flip test’). ‘Crossover sign’ is when the patient’s symptoms are reproduced by a straight leg raise of the contralateral leg.
• Bowstring test: with patient supine, flex the hip and knee to 45°, then press on the popliteal nerve behind the knee, which recreates pain.
• Rib cage excursion: chest circumference in full inspiration and expiration ~difference of 7 cm. Reduced in ankylosing spondylitis.
• Schobers test: quantitative evaluation of lumbar spine flexion. Mark horizontal line at posterior superior iliac spines (PSIS) and second 10 cm above this. On forward flexion this distance should increase by at least 5 cm. Limitation by pain protection in ankylosing spondylitis.
Patient undresses to below waist and keeps underwear on to maintain dignity; offer a blanket for the supine part of the examination. Observe from front, back, and side. Compare both sides. Look for crutches or braces around the patient. Usual format of inspection for scars (surgery/trauma), sinuses.
• Front: muscle wasting. Can stand with feet flat on ground. Use blocks at this stage to correct leg length discrepancy. Palpate iliac crest to assess pelvic obliquity.
• Trendelenburg's test: originally described with examination from behind, it is best performed from the front. Place thumbs on each of the patient’s anterior superior iliac spines (ASISs). Ask them to put their hands onto your forearms for balance. Ask to stand on one leg and bend other knee to 90° without flexing hip. This is repeated on the contralateral side. The sign is negative if the pelvis remains level; if the pelvis drops (due to abductor muscle weakness, painful hip, dislocation/subluxation of the hip or shortening of the femoral neck), this is a positive sign. As the pelvis is acting as a fulcrum, the opposite side drops if there is weakness. This can be remembered as ‘the sound side sags’.
• Trendelenburg lurch: patient leans over the affected hip to shift body’s centre of gravity in that direction.
• Side: surgical scars, posture, and presence of skin contractures.
• Behind: gluteal muscle bulk and presence or absence of scoliosis.
Ask the patient to walk—abnormal patterns of gait may be due to muscle weakness, compensatory adaptation to pain, or loss of motion.
Joint warmth: less specific as deeper joint.
Bony points: ASISs, ischial tuberosity, and greater trochanter.
Perform active ROM then check additional passive movement.
• Flexion: patient supine and with knee flexed (prevents hamstring tightness), limited by soft tissues of abdomen and thigh. ROM 0–135°
• Thomas’s test: a patient with a fixed flexion deformity at the hip compensates when lying on their back by arching the spine and pelvis into an exaggerated lordosis. The lordosis can be eliminated by flexing the contralateral hip. Ask the patient to flex both knees and hold them with their hands in a supine position. Then extend knee fully with a hand under their back on the contralateral side to eliminate the lumbar lordosis. The angle between the couch and the thigh demonstrates the fixed flexion.
• Extension: patient prone—not usually assessed. ROM 0–30°.
• Abduction: patient supine and pelvis stabilized with examiner’s hand on opposite ASIS. ROM 0–45°.
• Adduction: patient supine and pelvis stabilized with examiner’s hand on opposite ASIS. Abduct contralateral leg. ROM 0–30°.
• Rotation in extension: either supine with knee straight and patella used as measurement gauge or patient supine with knee flexed to 90°.
• Internal rotation: ROM 0–40°. External rotation: ROM 0–60°.
• Rotation in flexion: earlier signs of hip pathology can be picked up in flexion. Supine with knee flexed to 90° (use tibia as protractor).
• Internal rotation: ROM 0–40°. External rotation: ROM 0–50°.
• If internal rotation full in extension but reduced in flexion—anterosuperior femoral head pathology, common after avascular necrosis (sectoral sign).
Best done after movement because if a fixed deformity is present, the contralateral limb must be placed in a similar position for a comparison to be made.
• Apparent: from midline point in the body (umbilicus, xiphisternum) to medial malleolus.
• True: ideally centre of femoral head, i.e. axis of movement, however, no palpable landmark. Hence from ASIS to medial malleolus.
• Galleazzi's test: is shortening above or below the knee? Flex both knees to 90° and keep heels together. Look from side (femur) and front (tibia) to see where the discrepancy lies. If above the knee, a few lines can be drawn to determine if the discrepancy originates from above or below the trochanter.
• Bryant’s triangle: point A—ASIS, point B – greater trochanter, point C is the intersection of a horizontal line from the ASIS and a vertical line from the greater trochanter. Compare both sides. Line from B to C shows supra-trochanteric shortening. Line from A to B shows amount of central dislocation compared to the other side.
• Nelaton’s line: ASIS to ischial tuberosity. Greater trochanter normally lies below or on line. If above, then femur displaced proximally/supra-trochanteric shortening.
• Schoemaker’s line: greater trochanter to ASIS continued onto the abdomen. If unilateral problem, then line intersects on the contralateral side; if bilateral, intersection is below the umbilicus.
• Anterior impingement test: patient supine, hip flexed to 90°, adducted and internally rotated. Positive if groin pain (labral tears).
• Posterior impingement test: patient supine at edge of couch, contralateral hip held flexed. Examiner fully extends, abducts, and externally rotates the examined hip. Test femoral neck against posterior acetabular wall.
• FABER (Flexion Abduction and External Rotation) test: ipsilateral foot placed on contralateral knee, ‘figure of four’ position. Push down on knee and contralateral ASIS. Pain from sacroiliac joint and posterior hip localize to different sites.
• Ischiofemoral impingement test/HEADER (Hip Extension ADduction, External Rotation) test: abnormal contact between ischium and lesser trochanter. Patient supine on edge of couch. Knee flexed to 90°, hip extended, adducted and externally rotated. Pain deep within groin/buttock.
• Ely’s test (rectus femoris): patient prone, knee passively flexed, if tight then hip rises away from couch.
• Ober’s test (fascia lata/iliotibial band [ITB]): patient lies on unaffected side, maximally flex both hips to flatten lumbar spine. Passively abduct the affected leg to 45° then into extension. If it does not fall into adduction and remains in abduction then ITB contracture.
Further examination of the lumbar spine and knee is essential to exclude referred pain. A complete neurovascular examination should be done to ascertain whether there is a true neurological deficit or if neurological symptoms are mimicking MSk symptoms.
Patient undresses to below waist and keeps underwear on to maintain dignity; offer a blanket for the supine part of the examination. Observe from front, back, and side. Compare both sides. Look for crutches or braces around the patient. Usual format of inspection for scars (surgery/trauma), sinuses.
• Front: look for alignment (genu valgum/genu varum) and shortening.
• Side: flexion/hyperextension deformities, recurvatum/procurvatum of tibia/femur.
• Behind: scars, bruising, swelling, Baker’s cyst.
Ask the patient to walk—look for varus thrust (medial OA, lateral ligament laxity), valgus thrust (lateral OA, medial ligament laxity). Look at foot progression angle (angle of foot with an imaginary straight line (~10–15° external) and patella progression angle (0°) to assess rotational deformity.
• Sat down: assess patella height with legs hanging on edge of bed. Ask to extend knees to assess tracking (J-sign with patella moving centrally then subluxes laterally in full extension).
• Supine: scars especially arthroscopic and swelling better seen here. Quadriceps and calf wasting can be circumferentially measured from a fixed bony point (tibial tuberosity).
• Joint warmth: using palms of hands compare bilaterally.
• Ballottement test: (massive effusion)—pressure on one side of knee is transmitted to hand on other side of knee.
• Patellar tap test: (moderate effusion)—hand over suprapatellar pouch to occlude press patella which bounces against trochlear.
• Wipe/bulge test: (small effusion)—suprapatellar pouch occluded. Stroke lateral gutter and watch fluid into medial gutter.
• Palpation of joint: with knee at 90° palpate along patella tendon, medial and lateral joint lines (?meniscal tear), femoral/tibial condyles, medial collateral ligament (MCL), and lateral collateral ligament (LCL), posterior aspect of knee (baker’s cyst).
Perform active movements then check additional passive movement.
• Passive hyperextension: by lifting both legs up by the ankles.
• Extensor mechanism: assessed by asking patient to ‘straight leg raise’. Any deficit of full active extension which is passively correctable is known as an ‘extensor lag’.
• If it appears as though there is a flexion deformity of the knee, place hand under knee and ask patient to push down. Passively try to straighten. If no movement—‘fixed flexion deformity’.
• Anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL): view both knees flexed to 90° from the side, look for posterior sag (PCL) injury by comparing the level of tibial tubercles.
• Quadriceps active test: if posterior sag noticed, fix the foot and ask patient to extend knee. If there is a posterior sag, the quadriceps pulls the tibial forward via the patella tendon.
• Anterior and posterior draw tests: knees flexed to 90°, check patient does not have painful feet and ask permission to lightly sit on feet to fix them. Then grasp tibia with both thumbs on tuberosity to pull tibia forward and backwards. Excessive movement backwards, positive posterior draw (PCL injury). Excessive movement forwards, positive anterior draw (ACL injury plus secondary restraints).
• Lachman test: most reliable for ACL. Grasp distal femur in one hand and with knee flexed at 20°, grasp the proximal tibia and try to displace anteriorly. If small hands, fix the patient’s femur on the examiner’s flexed knee. Note degree of displacement and feeling of ‘end-point’.
• Pivot shift test: measures rotational component of ACL and recreates forces resulting in giving way when landing on a planted foot. With patient supine, grasp the tibia in both hands and keep ankle in examiner’s armpit. With knee in full extension, apply internal rotation and valgus force with lateral hand (tibia subluxed anterolaterally) then flex to 20°—tibia reduces. Either obvious clunk (shift) or subtle (glide).
• MCL: hold leg above and below with knee in full extension, valgus force applied by pushing lower leg against lateral hand. If the knee opens up it implies a significant disruption to deep MCL and secondary stabilizers (cruciates, posteromedial capsule). Repeat in 20–30° flexion, which relaxes secondary restraints and allows assessment of superficial fibres of MCL.
• LCL: same as for MCL but apply varus stress. Less common and associated with a posterolateral instability.
• Dial test—postero-lateral corner (PLC) and PCL: patient prone, knees and ankles together, maximum passive external rotation at 30° and 90°. PLC injury asymmetry at 30°. PLC and PCL injury asymmetry at 30° and 90°.
• Apley's grind test (meniscus): patient prone, knee flexed to 90°, and axial rotatory force applied—not performed.
• McMurray’s test (meniscus): patient supine, knee flexed, one hand on top of knee, and one under the ankle. Varying degrees of compression from the top hand and rotation and flexion from the bottom hand to stress each compartment—not performed.
• Patella glide (patella instability): knee slightly flexed, patella maximally moved side to side. If patella spilt into quadrants, should move one medially and two laterally; if does not, then lateral and medial retinaculum is tight respectively.
• Clarke’s test (patellofemoral joint pain): pressure over superior pole of patella and ask patient to contract quadriceps—recreates pain—not performed.
Patient undresses to below waist and keeps underwear on to maintain dignity; offer a blanket for the supine part of the examination. Observe from front, back, and side. Compare both sides. Look for crutches, braces, orthoses, and footwear around the patient. Usual format of inspection for scars (surgery/trauma), sinuses.
• Behind: position of heel relative to the floor. The axis of the tibia compared to os calcis (tibiocalcaneal angle ~5° valgus). Achilles tendon swelling? Calf muscle bulk? Forefoot visibility (normally one and a half toes) ‘too many toes’ sign implies posterior tibialis tendon dysfunction. Ask patient to tip-toe (double heel raise). Assess gastro-soleus complex. If both heels in varus, posterior tibialis functioning well. If pes cavus consider Coleman block test.
• Front: ankle swelling—effusion/synovitis/osteophytes. Medial longitudinal arch—pes planus (flat)/pes cavus (  arch). Midfoot deformity. Forefoot deformity—hallux valgus, hammer toe (single interphalangeal fixed flexion), claw toe (fixed flexion of both IPJs)
arch). Midfoot deformity. Forefoot deformity—hallux valgus, hammer toe (single interphalangeal fixed flexion), claw toe (fixed flexion of both IPJs)
• Skin and nail changes (ulceration, pitting, etc.)
• Plantar aspect of foot and between toes: plantar keratoses and ulcers. Diabetic ulcers—under first, second, third metatarsal head and pulp of hallux.
Ask the patient to walk—disability, pain, and stiffness. Arthritic ankle—difficulty through rocker phase. First metatarsophalangeal joint (MTPJ) OA—difficulty with toe off.
Systematic and relate to underlying anatomy. Start at ankle and move distally.
• Medially: swelling/tenderness—synovitis, posterior tibialis irritation, posterior tibial nerve compression, flexor hallucis longus snapping.
• Anterior: synovitis/effusion in notch of Harty (space medial to tibialis anterior).
• Laterally: tibialis anterior, long extensors, peroneus tertius.
• Anterolateral aspect of ankle joint—thumb pressure with plantar flexed foot—pushes hypertrophic synovium into joint. Then dorsiflex foot if pain worsens (positive anterolateral impingement test).
• Move to inferior tibiofibular syndesmosis—tender? Injury.
• Squeeze test: compression mid-calf level (compress tibia and fibula in coronal plane). External rotation of the foot with tibia stabilized anteriorly is an alternative.
• Distal to joint line: anterior talofibular ligament (70° to axis of fibula to talus). Anterior sinus tarsi—irritation of subtalar joint.
• Posterior: posterior to fibula—peroneal tendons, peroneal retinaculum, calcaneofibular ligament (deep). Achilles tendon (tenderness, thickening), bony swelling (Haglund’s deformity). Passively plantar- and dorsiflex ankle while palpating Achilles tendon—if thickening moves, then from tendon not sheath.
• Midfoot: palpate from calcaneum (anteromedial tuberosity) to distal part of plantar fascia (plantar fasciitis/plantar fibromatoses)—worsened maximally dorsiflexing the great toe.
• Chopart’s joints (talonavicular and calcaneocuboid)—tenderness crepitus. Between the two is the sinus tarsi (subtalar joint irritation). Assess other mid tarsal joints.
• First MTPJ: hallux rigidus. Metatarsal shafts—stress fracture.
• Second MTPJ: Freiberg’s disease, synovitis.
• Tenderness between metatarsal heads, third webspace burning/paraesthesia radiating to toes—Morton’s neuroma. Mulder’s sign: reproduction of pain when squeezing the toes in a mediolateral direction and the production of a click by applying a dorsally directed pressure beneath affected webspace.
Perform active ROM then check additional passive movement.
• Ankle movement: patient seated, test with knee flexed and extended.
• ROM 20° dorsiflexion, 40° plantarflexion.
• Passive movement assessed in forefoot in supination to exclude dorsiflexion at Chopart’s and midtarsal joints. Inversion of heel to lock the subtalar joint will further isolate ankle movement. Cup the heel and rest sole of foot on forearm then assess movements.
• Subtalar movement: hold calcaneus with one hand and fix the talar neck with the thumb and index finger on the other hand. Apply varus and valgus stress to calcaneus. ROM 5° valgus to 5° varus with ankle in plantigrade position (foot 90° to tibia).
• Inversion and eversion: movement at subtalar, Chopart’s and mid tarsal joints. Inversion 20° and eversion 10°.
• Midfoot: contribution to inversion and eversion assessed by fixing. hindfoot. Also abduction and adduction 5° can be assessed this way.
• First tarsometatarsal joint: passive plantar and dorsiflexion—may contribute to hallux valgus.
• Hallux: dorsiflexion 80°, plantar flexion 40°. Grind test—axial load and torsion reproduces pain—hallux rigidus.
• Tibialis posterior: foot plantar flexed. Resisted inversion while palpating tendon behind medial malleolus.
• Tibialis anterior: maximum dorsiflexion and some inversion. Resisted dorsiflexion in this position while palpating tendon in front of medial malleolus.
• Peroneus longus: put foot in maximum plantar flexion and eversion. Ask patient to maintain position while examiner pushes foot inwards. Palpate tendons behind lateral malleolus.
• Peroneus brevis: foot placed in neutral position and resisted eversion performed.
• Coleman block test: used in cavovarus foot. Plantar flexion of first ray is a result of sparing of peroneus longus. To move the lateral four metatarsals into plantigrade, the heel goes into varus. Coleman block test determines if hind foot varus is fixed or mobile. Block placed beneath foot with first ray not supported. If heel mobile, it goes into valgus. If fixed deformity, it remains in varus.
• Thompson’s test: test for rupture of Achilles tendon, patient prone with feet dangling off edge of couch. Normally, the resting position with an intact Achilles is plantar flexion; if ruptured, the foot position is more neutral. Squeeze calf—if tendon intact, passive plantar flexion at ankle; if complete rupture, no response.
• Anterior draw test: used to test anterior talofibular ligament (ATFL) of lateral ligament complex. Grasp heel with one hand and distal tibia with the other hand. Attempt to translate heel anterior relative to tibia. Compare both sides—  translation = ATFL laxity.
translation = ATFL laxity.
Paediatrics has been dubbed ‘veterinary medicine’ and for a good reason. How do you carry out a comprehensive examination in a population group that tend to start screaming when they see someone with a stethoscope (and not helped by some parents who insist on telling their children that they will get an injection if they do not behave)? One-third to one-half of children presenting to EDs are <5 years old, who can be especially challenging when they are fretful, tired and febrile.1
But the art—and the fun—of paediatrics is reinventing the examination at each encounter to get the clinical information you need. Watch paediatricians who have thought of whiz ways to get children to cooperate in the clinical exam (e.g. asking a child to ‘flap your arms like a chicken!’ to test shoulder abduction/adduction and nerves C5, C6, C7, and C8).
The key is to be opportunistic. If the baby who has presented was crying, but is now fast asleep, examine with the stealth of a panther, gently brushing the fontanelle to check if it is bulging or sunken, listening to the heart sounds and chest, and gently palpating the abdomen. If the heart rate, respiratory rate (always measure your own vital signs), and examination are normal (see Table 50.11 for normal values), you can be more reassured when the child starts crying again when you undress them to complete your examination (e.g. looking for rashes and looking in the ears and throat).
Also, see it from the child’s point of view—you are a scary, large stranger. So get down to their level—paediatricians spend a lot of time crouching. Most examination of young children with stranger fear (6 months to 2 years) is best done on the parent’s lap, where they feel more secure. Examining the child’s teddy bear first might sound clichéd but it works. Distract them by asking them about who their favourite character from a Disney movie is or which football team they support. Remember that when children are ill, they often regress developmentally, so do not always expect age-appropriate behaviour.
Table 50.11 Paediatric normal values
| Age (years) | Respiratory rate at rest (breaths/min) | HR at rest (bpm) | Systolic BP (mmHg) |
| <1 | 30–40 | 110–160 | 80–90 |
| 1–2 | 25–35 | 100–150 | 85–95 |
| 2–5 | 25–30 | 95–140 | 85–100 |
| 5–12 | 20–25 | 80–120 | 90–110 |
| >12 | 15–20 | 60–100 | 100–120 |
Alert (GCS has been adapted for children)? Capillary refill time (normal <2 sec)? Assess the child’s nutritional status. Do they look like they are thriving, underweight, obese? Plot height, weight, and head circumference on a WHO growth chart. At the same time, you will be making a rapid assessment of development, with questions to parents/carers of the child’s function, covering, if required, the domains of gross motor, fine motor/vision, speech/language and social skills.
Ensure the parents remove the child’s shirt as observation is key to observe the work of breathing. Does the child look blue (cyanosed), or more likely a pale grey? Remember, cyanosis is difficult to spot until oxygen saturations are <85%. Do they have an oxygen mask or nasal cannula in place? IV line (infection)?
Count the respiratory rate: be systematic and work from the nose to the diaphragm—nasal flaring, tracheal tug (use of accessory muscles), intercostal recession (rarer in babies and toddlers who have a more elastic ribcage), sternal recession, subcostal recession. Listen (from the end of the bed) for stridor (inspiratory upper airway problem) and if stridulous, approach with extreme caution. Also look for any scars, central lines, or ports (e.g. in CF) and at the hands for clubbing/anaemia.
Percussing the chest is a useful sign in older children to spot consolidation or effusion but often difficult in toddlers and babies so is attempted infrequently in this age group. Percussion is essential if concerned about pneumothorax (hyper-resonance).
This should cover the same area as in adults, comparing each side. Listen for wheeze (expiratory), especially if prolonged, and crackles, and learn to distinguish these from transmitted upper airway sounds (very common in viral infections in children). Absent breath sounds (life-threatening asthma, pneumothorax).
Often counted as part of the paediatric respiratory exam. Leave this until last and ask someone to show you how to hold an otoscope and examine ears and throats—best done in older children at first while you gain confidence in what you are looking at.
Cyanosis, clubbing, central lines, scars as for respiratory examination. JVP difficult to assess in younger children because of anatomy (chubby necks). Growth and nutritional status important as the child pouring all her calories into coping with a large VSD will have little left over for normal growth.
Assess peripheral pulses. The femoral and brachial pulses are easier to find in babies and toddlers—palpating the femorals is essential to rule out coarctation of the aorta. Palpate for the apex beat—fourth intercostal space, slightly lateral to the midclavicular line from 0–7 years, then fifth intercostal space, midclavicular line. Palpate for a heave or thrill, as for adults.
Auscultate over the upper left sternal edge (pulmonary valve), lower left sternal edge (tricuspid valve), upper right sternal edge (aortic valve), and over the apex (mitral valve). Also, auscultate at the back between the scapulae in infants to pick up a murmur transmitted by a patent ductus arteriosus. Comment on any additional heart sounds (e.g. gallop rhythm, quality of the murmur (blowing, harsh)), systolic/diastolic, and grade any murmur detected. Examine elsewhere for signs of heart failure: sweaty, breathless, crackles at lung bases (pulmonary oedema), oedema (legs, sacrum), liver edge. There will likely be a history of poor feeding/growth.
Observation as for respiratory examination, especially nutritional status. You can check the tongue, dentition, and look for ulcers at the end with the ENT exam, if performed. Inspect for scars, herniae, gastrostomy, colostomy/ileostomy, and peritoneal dialysis port. Ask the child to lie flat while you kneel down by the bedside. Explain what you are going to do—‘If it hurts, just tell me to stop’. Auscultate for bowel sounds first, then look at the child’s face as you palpate, as you would for adults. Palpate and assess for hepatosplenomegaly. Essential to start at RIF for these—large spleens can be missed in infants and toddlers because the mass is mistaken for the child tensing their abdominal muscles. Percuss size if necessary. Palpate for flank pain and renal masses. Do not perform a rectal examination without a senior present—this is rarely done in paediatrics and left to the senior/surgeons. If examining for fissures, ensure chaperone is present. Any boy presenting with abdominal pain should be assessed for testicular torsion but ensure a chaperone is present and this is best done by a doctor.
Examining for trauma, abnormalities (e.g. hypospadias in boys, fused labia in girls), ambiguous genitalia, or for growth/endocrine problems. The Tanner staging system will be used to assess puberty in clinic (males: growth of penis, testes, and pubic hair; females: breast development, pubic hair). Examined when a senior is present and always ensure a chaperone is present.
Use the pGALS (paediatric gait, arms, legs, and spine) exam to quickly but comprehensively screen for MSk disorders in children. Box 50.1 is a summary but see Arthritis Research UK’s excellent teaching videos at  www.arthritisresearchuk.org/health-professionals-and-students/video-resources/pgals.aspx.
www.arthritisresearchuk.org/health-professionals-and-students/video-resources/pgals.aspx.
Box 50.1 pGALS examination
• Do you have any pain or stiffness in your joints/muscles/back?
• Do you have difficulty getting dressed without any help?
• Do you have any difficulty going up and down stairs?
• Observe walking. ‘Walk on your tip-toes/walk on your heels.’
• ‘Put your hands out in front of you.’
• ‘Turn your hands over and make a fist.’
• ‘Pinch your index finger and thumb together.’
• ‘Touch the tips of your fingers with your thumb.’
• Squeeze the metacarpophalangeal joints.
• ‘Put your hands together/put your hands back to back.’
• ‘Reach up and touch the sky.’
• ‘Put your hands behind your neck.’
• Feel for effusion at the knee.
• ‘Bend and then straighten your knee.’ (Active movement of knees and examiner feels for crepitus.)
• Passive flexion (90°) with internal rotation of hip.
• ‘Open your mouth and put three of your (child’s own) fingers in your mouth.’
• Lateral flexion of cervical spine—‘Try and touch your shoulder with your ear’.
• Observe the spine from behind.
• ‘Can you bend and touch your toes?’ Observe curve from side and behind.
Reproduced with permission from Foster HE, et al., Musculoskeletal screening examination (pGALS) for school-age children based on the adult GALS screen. Arthritis Care Research 2006; 55(5); 709–716.
This can appear complicated for the simple reason that young children, or children with developmental delay, cannot follow commands. But you can still pick up a huge amount of information from simple observation. Paediatric neurology is a textbook in itself which you are advised to look up. Most healthy children with normal development over the age of 5 years will be able to understand and perform the normal adult neurological examination of upper/lower limbs and cranial nerves—but make it fun or they will get bored! And many children will tolerate fundoscopy, so have a go. You will have to adapt for younger children or those with developmental delay of any type, and rely more heavily on observing what a child can do. It is important to ask the parents about function and a knowledge of developmental milestones is essential (e.g. it is normal for a 6-month-old baby to be unable to sit unsupported but worrying at 12 months—see Box 50.2).
In all children, look for dysmorphic features, inspect the skin (for neurocutaneous disorders such as NF1), and inspect the back (for any spinal disorder such as spina bifida, kyphoscoliosis). If there is a wheelchair, what extra supports have been added (hypotonia)? Look for orthoses. Always observe gait in children able to walk and assess cerebellar function by asking them to walk heel-to-toe. In the younger child, observe movement, fixing and following (vision), and any response to sounds. You can find out loads by watching them play—Lego, a puzzle, leafing through a book. To assess upper and lower limbs, check tone (stiff,  ; floppy,
; floppy,  ). Check for head lag and
). Check for head lag and  tone when pulling an infant gently to sit or holding them carefully on their chest and tummies (ventral suspension—ask to see these being done before trying them yourselves). Test reflexes, placing your finger or thumb between tendons and tendon hammer.
tone when pulling an infant gently to sit or holding them carefully on their chest and tummies (ventral suspension—ask to see these being done before trying them yourselves). Test reflexes, placing your finger or thumb between tendons and tendon hammer.
In babies, assess primitive reflexes: Moro (sudden—but gentle!—head extension causes all four limbs to extend; goes by 4 months of age); grasp (both fingers and toes curl to grasp object touching palm or sole; goes by 5–6 months); rooting (head turns towards touch near mouth—to breastfeed; goes by 4–6 months); asymmetric neck reflex (or ‘fencing’—the baby puts out the arm like a musketeer to whichever side head is turned; goes by 4 months); stepping (baby held upright will appear to step when feet touch surface; goes by 6 weeks of age).
Cranial nerves: use an object to see if child will fix and follow (II) and check pupillary reaction to light (III); assess eye movements with object or observe child looking around—symmetrical (III, IV, and VI)? Symmetrical smile/grimace (VII)? Startle/react to noise (VIII)?
Box 50.2 Red flags for child development
• Loss of developmental skills at any age.
• Concerns about vision (e.g. fixing or following an object).
• Persistently low muscle tone or floppiness.
• Asymmetry of movements or other features suggestive of cerebral palsy (e.g. hypertonia).
• Head circumference above the 99.6th centile or below 0.4th centile. Also, if circumference has crossed two centiles (up or down) on the appropriate chart or is disproportionate to parental head circumference.
(What the child cannot do.)
• Sit unsupported by 12 months.
• Walk by 18 months (boys) or 2 years (girls).
• Hold object placed in hand by 5 months (corrected for gestation).
• Reach for objects by 6 months (corrected for gestation).
Reproduced from Bellman Martin, Byrne Orlaith, Sege Robert. Developmental assessment of children. BMJ 2013; 346:e8687.
Plot height and weight and head circumference on growth charts.
(See Table 50.12.)
Table 50.12 Developmental milestones
| Age | Gross motor | Fine motor/vision | Speech/language | Social | |||
| Draw | Brick | Cut | Beads | ||||
| 6 weeks | Good head control—raises head to 45 when on tummy. Stabilizes head when raised to sitting position | Tracks object/face | Stills, startles at loud noise | Social smile (visual problem if not) | |||
| 6 months | Sits without support, rounded back Rolls tummy (prone) to back (supine). Vice versa slightly later | Palmar grasp (5 months) Transfer hand to hand | Turns head to loud sounds Understands ‘bye bye’/’no’ (7 months) Babbles (monosyllabic) | Puts objects to mouth (stops at 1 year) Shakes rattle Reaches for bottle/breast | |||
| 9 months | Stands holding on Straight back sitting (7½ months) | Inferior pincer grip Object permanence | Responds to own name Imitates adult sounds | Stranger fear (6–9 months–2 years) Holds and bites food | |||
| 12 months |
Walks
alone (9–18 months)  18 months is threshold for worry—i.e. Duchenne’s MD, hip problems, cerebral palsy, etc. 18 months is threshold for worry—i.e. Duchenne’s MD, hip problems, cerebral palsy, etc. |
2 | Shows understanding of nouns (‘Where’s mummy?’) 3 words (50% at 13 months) Points to own body parts (15 months), doll (18 months) | Waves ‘bye bye’ Hand clapping Plays alone if familiar person nearby Drinks from beaker with lid | |||
| Neat pincer grip (10 months) Casting bricks (should disappear by 18 months—persistence beyond this = abnormal) | |||||||
| 18 months | Runs (16 months) Jumps (18 months) | To and fro (15 months) | 4 | Shows understanding of nouns (‘Show me the xxxx’) 1 to 6 different words | Imitates every day activities | ||
| 2 years | Runs tiptoe Walks upstairs, both feet/each step Throws ball at shoulder level | Vertical line | 8 | Shows understanding of verbs (‘What do you draw with, what do you eat with?’) 2 words joined together (50+ words) | Eats skilfully with spoon (2½ years) | ||
| Puzzles—shape matching is >2 years skill. Random effort <2 years. Turns several pages of book at a time | |||||||
| 2½ years | Kicks ball | Horizontal line | Shows understanding of prepositions in/on (‘Put the cat on the bowl’) 3–4 words joined together | ||||
| 3 years | Hops on one foot for 3 steps (each foot) Walks upstairs, one foot per step; downstairs two feet per step | Circle |
Bridge
|
Single cuts | Griffiths beads | Understands negatives (‘Which of these is NOT an animal?’) Understands adjectives (‘Which one is red?’) | Begins to share toys with friends Plays alone without parents Eats with fork and spoon Bowel control |
| Turns one page of book at a time | |||||||
| 3½ years | Cuts pieces | Understands comparatives (‘Which boy is bigger than this one?’ while pointing to middle-sized boy! Or draw circles to illustrate point) | |||||
| 4 years | Walks upstairs/downstairs in adult manner | Cross Square (4.5 years) Triangle/person (5 years) |
12 blocks
Steps
|
Cuts paper in half | Small beads | Understands complicated instructions (‘Before you put x in y, give z to mummy’) Uses complex narrative/sequences to describe events. | Concern/sympathy for others if hurt Has best friend Engages in imaginative play, observing rules (4½ to 5 years old) Eats skilfully with little help Handles knife (at 5 years) Dressing and undressing |
Source: http://mrcpch.paediatrics.co.uk/development/ by Christopher Kelly.
Everything is delayed. Usually apparent by 2 years of age. Associated with cognitive difficulties. 25% have no known cause.
• Genetic: Down’s, cerebral dysgenesis, e.g. hydrocephalus.
• Metabolic: hypothyroidism, phenylketonuria .
• Congenital infection: rubella, CMV, toxoplasmosis.
• Neurocutaneous syndromes: tuberous sclerosis, neurofibromatosis.
• Extreme prematurity: intraventricular haemorrhage/periventricular leukomalacia.
• Birth asphyxia: hypoxic ischaemic encephalopathy.
• Metabolic: symptomatic hypoglycaemia, hyperbilirubinaemia.
• Infection: meningitis, encephalitis.
• Anoxia: suffocation, near drowning, seizures.
• Metabolic: hypoglycaemia, inborn errors of metabolism.
• Causes: cerebral palsy, congenital myopathy, spinal cord lesions, e.g. spina bifida, global developmental delay, idiopathic.
• Hand dominance: appears at 1–2 years/later therefore dominance before this time = abnormal (e.g. hemiplegia).
• Late walking: >18 months. NB: locomotor variants, e.g. bottom shuffling, commando crawling, may 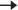 acceptable delay.
acceptable delay.
• Causes: hearing loss, global delay, anatomical deficit, e.g. cleft palate/cerebral palsy 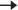 lack of palate coordination, environmental deprivation, normal variant/familial pattern.
lack of palate coordination, environmental deprivation, normal variant/familial pattern.
• Assessment of speech/language (history from mother).
• Interpersonal communication—pointing? Takes mother to what he wants? Eye-to-eye contact? Prefers to play on his own?
• Ritualistic/obsessive behaviour (spinning objects, rigidly ritualistic, dislikes changes in routine)?
• Other traits: dislikes crowded spaces/loud noise/having haircut.
• If neurological problems, then ask about obstetric history.
Reference
1. Downing A, Rugge G (2006). A study of childhood attendance at emergency departments in the West Midlands region. Emerg Med J 23(5):391–3.