Chapter 6
The Lungs and the Common Good
Eduardo Rosas Cruz was arrested late in the evening near Bakersfield, California, on July 28, 2014. He didn’t assault anybody, didn’t steal anything, and wasn’t driving under the influence. He wasn’t trespassing or jaywalking. In fact, he got arrested for something even the best-intentioned and hardest-working of us often forget to do: he didn’t take his medications.89
This arrest would appear to violate the medicolegal principle that a person of sound mind has the right to refuse any treatment or medication. But the case of Rosas Cruz was not typical. His lungs were infected with tuberculosis, and with the communal nature of our atmosphere, the state of California was within its legal rights to imprison him. The prosecutor, Stephen Taylor of San Joaquin county, put it succinctly: “Criminal prosecutions (can be) an extension of the practice of medicine.”90
The arrest of Rosas Cruz represents a rare intersection of crime and medicine. It is an extreme example, but it serves to demonstrate how the lungs exist at the juncture between our communal air, deadly infections, and our rights as citizens. Today, the intersection of these three issues is becoming more fraught. With growing urbanization, extreme mobility, and a plethora of new bacteria and viruses emerging, lung health serves as a bellwether for what is happening in society worldwide. The 2019-2020 emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, commonly known as COVID-19)—with the extraordinary subsequent shutdown of daily life, closed borders, and overwhelmed medical systems—is the perfect example of how a lung infection can take over society in a very short period of time.
The connectivity of our air can be hard to conceptualize. We can easily imagine the oceans as being connected: when a shipping container dumped thousands of Legos off the coast of Cornwall, England, in 1997, we were not surprised that some pieces were later recovered in Ireland, Galveston, Texas, and Melbourne, Australia. We can see the ocean currents. However, the air of our atmosphere, while invisible and intangible, is no less connected than our oceans. This is true at the local level, where infections can spread easily from lung to lung, but also at much greater distances. A recent study has shown the trees in Yosemite National Park are reliant on nutrients from the dust in China’s Gobi Desert that have caught a ride on the east-west jet stream, traveling some six thousand miles.91 Similarly, the Amazon relies on the dust of the Sahara Desert in Africa, which travels in the opposite direction but just as far.92
For humanity, the world that is our atmosphere has gotten smaller, more communal. The unseen air that is becoming increasingly more collective is something that needs to be respected, and the threats to it must be addressed at local, national, and international levels. No other organ teaches us this lesson better than the lungs, and no other diseases better than tuberculosis and COVID-19.
In November 2005, during my first year of pulmonary fellowship, I fielded a call about a sick patient who was on the general medical floor. He was twenty-two years old, a college kid who had come in with a fever and what looked like pneumonia, but he wasn’t responding to typical antibiotics. At night his fever spiked upward of 103 degrees Fahrenheit, and sweat poured out of him, saturating his bed linens. He had lost a great deal of weight, and his chest X-rays showed more inflammation every day.
We had no idea about a diagnosis, so we took him to the surgical suite, sedated him, and inserted a camera into his lungs, taking some small biopsies, tiny pieces of lung tissue, in the hope of finding a diagnosis. In retrospect, it is clear that the answer was right in his history, as almost all diagnoses in medicine are.
We learned that our patient had done something over the summer that thousands of young adults throughout the country had done—he had gone to an outdoor rock concert, this one with almost one hundred thousand people, all sharing the same cloud of air. Music festivals are among the most likely venues for infectious diseases to do their work: a lot of people from all over the world coming together into a tight space with variable sanitation facilities. Some cities, especially a hundred years or more ago, could also be described in this manner.
We did our bronchoscopy, got our tissue, and soon thereafter had our diagnosis: the young man had tuberculosis, and a lot of it. After his diagnosis, the patient was kept isolated in his room so he couldn’t inadvertently spread his bacteria, and per protocol and law we reported the disease to the Philadelphia public health department. Everything seemed to go smoothly; our young patient started his medications, his fever went away, and his cough got better. He was sent home, and every week he picked up his pills at a city-run clinic. He seemed reliable, and stated to the outpatient clinic that he was taking all his medications without an issue.
At home, however, the patient’s fever came back, as did his cough and shortness of breath. He came to the emergency room, where his breathing was found to be so bad, he ended up with a tube down his throat and on a ventilator, and was admitted to the ICU. Once we stabilized him, everybody involved tried to figure out what had happened to someone who had taken his medications and seemed on the road to recovery.
We got a CT scan (a more detailed X-ray), which showed that the inflammation in his chest had increased—after a month of treatment. Even worse, when we did another bronchoscopy, we saw that he still had TB organisms actively growing in his lungs.
We followed up with the health department about his bacterium to make sure it was susceptible to the antibiotics we were giving him. The health department in every state not only records every case of TB, but also reviews drug sensitivities (how well a strain of bacteria responds to medications). We were assured his strain of tuberculosis was sensitive, and that our patient had been taking his medications. We then checked him for basic immune deficiencies, and again nothing turned up.
With the obvious accounted for, we turned to the obscure, the area of medicine where judgment and experience come into play. Fortunately, we received sound advice and guidance from the infectious disease physicians. Stick to the basics, they stressed, only do them better. We continued our patient on the first-line anti-TB drugs he had been on, this time at a slightly higher dose, and added one dose intravenously since he had inflammation in his abdomen and likely wasn’t absorbing a lot of the medicine he had been taking. We supported his immune system with appropriate calories through a feeding tube and kept the pressure in his lungs low on the ventilator. In this case, there could have been an urge to change the plan radically, to alter the anti-TB drugs or give steroids or other immune modulators. The art of medicine is knowing when to give up and start anew versus when to stay with the basic plan, executed better. In this case, we stuck with the basic plan.
Gradually, the inflammation in the chest and abdomen calmed down. We were able to give the patient physical therapy, and he got off the ventilator and went to the general medical floor. We stuck to the basics of antibiotics and nutrition, and he did well. When in doubt in medicine, stick to the basics are indeed words to live by.
Although we didn’t recognize this until quite recently, infectious organisms have been spreading through the air and gaining entry into our lungs for centuries. Lung diseases such as influenza, anthrax, measles, and tuberculosis have all had a vast and wide-ranging effect on human life and culture. Newer illnesses, such as the severe acute respiratory syndrome (SARS) virus in Asia, and the Middle East respiratory syndrome (MERS) virus in Saudi Arabia, have also been shown to spread through the air, as has the COVID-19 virus from Wuhan, China, that first emerged in December 2019. The atmosphere is a communal space, and the lungs are an extension of it. Bacteria and other organisms have exploited this shared space for centuries to jump between people and multiply unseen.
This is not news to us, and most of us recognize that a potentially lethal disease could emerge from another person’s lungs and make its way into ours. If somebody coughs on the subway, that hacking will send everybody to the other side of the car. We are told to cover our coughs, to sneeze into our elbow, and then to wash our hands afterward. The lungs, mostly unrecognized for the work they do, are best known in modern culture as the petri dish and transmission vehicle for deadly diseases.
The science behind what happens in the air when we cough, sneeze, or talk has recently been examined, but there is still much we don’t know about how our secretions travel or how bacteria and viruses take advantage of different environmental conditions to move from host to host. Much of the original research into this subject was done in the late nineteenth and early twentieth century, when air was recognized as the vehicle for contagions; without any effective medicines or vaccines, this was one area that public health experts could focus on to control disease. With the COVID-19 crisis, much of this research is being revisited, and interest in the mechanics of contagion spread has increased.
One of the first people to demonstrate the transmission of organisms in the droplets of our secretions was German infectious disease doctor Carl Flügge. In 1897, Flügge placed samples of the harmless bacterium Bacillus prodigiosus into the mouths of volunteers and then documented the presence of the bacterium in droplets around those subjects after they talked or coughed.93 Today, droplets are defined as molecules that are greater than ten micrometers in size and contain the intact fluid from our secretions as well as potentially infectious organisms. Due to their size and weight, they generally travel no more than six feet after being expelled from our bodies and then settle on nearby surfaces. Droplets can remain infectious for hours to days, but they spread primarily by somebody touching the expelled secretion and then touching their own mouth or nose; the risk of directly inhaling droplets is usually limited to a six-foot zone. Given their relatively large size, the droplets themselves are filtered out prior to reaching the lungs, but the organisms contained in a droplet can spread to the lungs after replicating in the nose or throat.
In the 1930s, Dr. William Wells of Harvard University took the research of contagion spread a step further. At his laboratory, he built a chamber in which he atomized different liquids, and by projecting a strong beam of light he demonstrated their quick dispersion. He then added bacteria to the droplets, and while some, such as Staphylococcus aureus, disappeared quickly from the air, others, such as Bacillus subtilis, persisted after three days, which should not have been possible according to the current droplet science. Dr. Wells also conducted unique experiments at the Harvard School of Public Health, introducing sneezing powder into a lecture hall and then collecting the normal bacteria of his graduate students that was dispersed throughout the room. He also inoculated the air conditioner in the basement with Balantidium coli, and later recovered the organism from every corridor of the three-story building.94 With his findings, he promoted the term droplet nuclei, smaller molecules, less than ten micrometers in size (modern definitions may use less than five micrometers), in which the fluid has largely evaporated from the droplet, leaving an infectious particle that does not fall to the ground. This particle could clearly stay suspended in air for long periods of time, often traveling far afield. Given their smaller size and their potential to stay suspended in air for hours, droplet nuclei can easily be inhaled and make their way into our lungs, avoiding the filtering system of our nose and throat, and begin replication, initiating pneumonia directly.
The distance traveled by droplets and droplet nuclei depends on several factors: the location of the person emitting them (indoors or outdoors); the environmental conditions of temperature, humidity, and ventilation; as well as how the secretions are initially expelled from the person. Sneezing is the most powerful form of exhalation, and a single sneeze can produce as many as forty thousand droplets traveling at speeds of one hundred meters per second. A cough may expel three thousand droplets, while simple talking for a minute produces about six hundred droplets.95 Coughing and sneezing may also produce gas clouds, and a study in 2014 from MIT showed these clouds of gas can travel much farther afield than was initially suspected, easily making it into the ventilation units of a room.96 Different procedures in the hospital create their own unique infectious risks, such as when cardiopulmonary resuscitation is undertaken and somebody is pressing vigorously on the chest, or when a tube is inserted into the lungs to place a patient on a ventilator.
Respiratory infections today are generally broken up into those that spread through droplet transmission via inhalation at close proximity or contact with droplets on surfaces, and those that are able to survive in droplet nuclei and spread through strictly airborne means. Influenza and COVID-19 are believed to use droplets to spread, while organisms such as tuberculosis and the measles virus spread more through droplet nuclei.97 This influences the type of protective barrier that is necessary, with a six-foot separation and a mask that can stop relatively large particles recommended for COVID-19, while those with tuberculosis need to be kept in a negative pressure room to continuously suck out the air, and those taking care of them need a tight-fitting mask that has the ability to trap small particles.
However, given the many factors influencing the spread of a virus, such as particle composition, mode of being expelled from the body, and specific environmental conditions, the act of staying six feet away from a person infected with influenza or COVID-19 may not always be protective. A sneeze can travel up to twenty-seven feet, and a gas cloud from a cough can extend the life of a droplet from a fraction of a second to minutes. A 2020 report from China bears out this concern. According to this report, particles of COVID-19 were found in the ventilation systems of the hospital rooms of those infected.98 Based on the principle that droplets fall to the ground within six feet, this should not have been possible. We also have no clear understanding of how long protective equipment such as masks can last and under what circumstances, and if cleaning them affects their ability to protect. The eyes are another potential route of transmission. How much spread occurs through secretions coming in contact here is unknown, but accounts of an initial eye infection as the source of illness have been reported in those with COVID-19.99
The movement of the air in our atmosphere can be very beneficial, by transporting plant seeds and nutrients, and by diluting out toxins and smoke. Not surprisingly, bacteria and viruses have learned to exploit this system, specifically to travel from one host to another. The air is indeed collective, with us often unaware of unseen and unseeable threats.
In the first half of the twentieth century, there weren’t many specialists in medicine; but starting around 1950, with the development of new technologies, kidney doctors, heart doctors, and brain doctors all started carving out niches for themselves. For lung doctors, while little new technology existed, many cases of tuberculosis did. In fact, at the beginning of pulmonary specialization all lung doctors were TB doctors.
TB has been with humanity for so long, and its history with us is so extensive and varied, an argument could be made that no other infection or disease has affected humankind to a greater extent. It has touched many aspects of our culture, from our novels to our paintings to our people. If the history of civilization is surveyed in its entirety, no other infectious disease has killed more people than TB, over one billion in the last two hundred years alone.100 And it continues to kill more than one million people worldwide each year.101
Much as civilization itself, tuberculosis first appeared in East Africa some twenty thousand years ago.102 It has stuck with us ever since, and today it exists in the latent stage in almost two billion people, a quarter of the world’s population. Being in the latent stage means TB infected these people at some point, was controlled but not completely eradicated by the inflammatory system of their lungs, and has the potential to reactivate if their immune system weakens.
Looking back, we have a detailed historical record of TB’s existence. In analysis of ancient Egyptian mummies, paleopathology (medical analysis of ancient dead bodies) shows that some of the priests and priestesses were afflicted with TB. In 1891, in the ancient city of Thebes, forty-four well-preserved mummies were discovered dating back to about 1000 BCE. One, named Nesperehan, was an adult male with partial destruction of the lower thoracic and upper lumbar vertebrae, which created an acute angular deformity. Paleopathologists pin this type and location of spinal destruction clearly on tuberculosis.103
Modern techniques of DNA analysis have confirmed the existence of TB in precolonial South America as well. Surprisingly, the DNA imprint of the TB found in South America is not close to the typical European or African TB, but rather resembles the TB found in seals.104 The researchers’ conclusion was that seals brought TB to the Americas, picking it up from Africa and then swimming across the Atlantic and spreading it to those who hunted them along the coastline of South America.
Over the centuries, tuberculosis continued its march through civilizations, with names like phthisis in ancient Greece, tabes in ancient Rome, and schachepheth in ancient Israel. If the prevalence of tuberculosis ebbed slightly during the Middle Ages, that decrease was followed by a huge increase in Europe and North America during the eighteenth and nineteenth centuries. With more of the population concentrated in urban environments, and no detailed understanding of TB’s cause or mode of transmission, up to 90 percent of residents in certain cities got infected. For a time in nineteenth-century Europe and America, TB accounted for one out of every four deaths.
During this two-hundred-year period, TB became known as the “white plague,” due to its slow-acting way of sucking the life, weight, and health out of people, turning a patient from a person into a ghost. Painters and other artists began romanticizing the disease. The novelist Amantine-Lucile-Aurore Dupin (better known as George Sand) referred to her lover, the composer Frédéric Chopin, as “a poor melancholy angel,” and described how he “coughed with infinite grace.” The British poet Lord Byron, in 1828, wrote, “I would like to die from consumption. The ladies would all say, ‘Look at that poor Byron, how interesting he looks in dying!’”105
From Mimi, the heroine in Puccini’s La Bohème, to characters in Eugene O’Neill’s plays and Fyodor Dostoyevsky’s novels, tuberculosis played a big role in opera, literature, and other arts. One of the most fascinating case studies of TB in art, and its effect on the artist and his career, involved Edvard Munch’s painting The Sick Child, depicting his sister Johanne Sophie, who died at age fifteen from TB. Best known for his later Expressionist classic The Scream, Munch had an artistic breakthrough while working on The Sick Child: “I started as an Impressionist, but . . . Impressionism gave me insufficient Expression—I had to find an expression for what stirred my mind . . . The first break with Impressionism was The Sick Child—I was looking for expression.”106
The painting has two people in it, but only one face, that of Munch’s sister, a young girl with red hair and a caved-in chest, lying in bed and looking with yearning toward an older woman sitting in a chair next to her. The older woman is trying to comfort her, and their hands are symbolically intertwined, without much definition, symbolizing the meshing of their souls.
The older woman represents Munch’s aunt, Karen Bjolstad, but her face is down, completely unseen. The tragedy of the moment appears to weigh too heavily on her heart, the knowledge of what the child is going through and what she is facing with an incurable disease that is too much for her to handle. Perhaps this woman is also partly Munch himself, faced with the guilt of surviving tuberculosis as a child while his sister did not. It’s also obvious, to those who know Munch’s story, why his aunt and not his mother is holding his sister’s hand: his mother had died when Munch was six years old, also of tuberculosis.
The Sick Child is one of the most famous of Munch’s paintings, the canvas thick with broad paint strokes and emotion—greens and blues, representing illness and sadness, but also spots of red representing the deadliness of the disease, as well as its propensity to cause bloody sputum. It’s a painting that Munch not only worked on for a year, but one that he came back to many times to repaint and reimagine over the course of forty years. When he moved to Paris in 1896, he repainted The Sick Child several times in different colors, and later painted four more versions of it, two in 1907, one in 1925, and the last one in 1927, when Munch was sixty-two. The scars of the illness on his family, and on him, would not heal during his lifetime, the canvas becoming his medium for therapy.
Scientists have debated the cause of tuberculosis for millennia. Hippocrates believed the disease to be hereditary, because so many within a family seemed to contract it. Galen, a few hundred years later, believed it to be contagious, as well as incurable. Later, in 1546, the Italian Renaissance physician Girolamo Fracastoro wrote insightfully that TB is caused by “seminaria contagiosum,” or infectious seeds, and that the bedsheets and clothing of TB patients could be highly infectious. Galen’s miasma theory of disease, in which diseases were thought to originate in the air from normal rot of organic matter, also continued to be a popular explanation during this time.107
The case for TB as an infectious agent did not gain firm scientific support until the mid-nineteenth century. The first breakthrough study was done by French military surgeon Jean-Antoine Villemin, who noticed that recruits kept in the barracks were much more likely to contract the disease than those in the field. To test his hypothesis, he cut out an inflammatory lesion filled with pus from one of his patients who had died from TB and showed successfully that a rabbit exposed to this substance would develop the disease. He published his findings in 1865, in a paper entitled “Cause et nature de la tuberculose: son inoculation de l’homme au lapin,” or “Cause and nature of tuberculosis: its inoculation from a human to a rabbit.”108
Villemin was mostly disregarded, partly because he had only described the lung pathology of the disease and shown that it could be spread from person to person. He suspected the cause of TB was a bacterium, but he did not isolate the organism. What the world needed was to see the bacteria, evidence of which Robert Koch finally produced seventeen years later. With his findings and methods, Koch also laid the groundwork for the modern study of bacteria and for the widespread adoption of the germ theory of disease. His discovery received so much press, at a time when TB had such a strong hold on society’s consciousness, that it had wide-reaching implications for how we view disease, and how we function as a society.
Born in Hanover, Germany, in 1843, Dr. Koch excelled at his studies, graduating in 1866 from the Göttingen medical school with the highest distinction. He married, had a daughter, and then worked as an army physician during the Franco-Prussian War in 1872. After the war, he settled in Wollstein, a part of modern Poland, and opened a medical practice. As a gift for his thirtieth birthday, his wife gave him a microscope, which he immediately used to study the bacterium anthrax. Despite the heavy demands of a practice, he built a laboratory in his house and set to work proving that anthrax was the cause of the disease afflicting local farm animals. He subjected mice to the infected blood of sheep, then documented their subsequent illness and death. Afterward, at autopsy with the use of his microscope, he recorded evidence of the small, rod-like bacteria in their blood, spleen, and lymph nodes. Although in retrospect his experiments appear exceedingly simple, this was in fact the first time an infectious organism had definitively been demonstrated to cause a disease. It was the knockout blow to Galen’s miasma theory of disease, and the debate about the cause of many diseases shifted to infections.
After finishing his work on anthrax, Dr. Koch received an appointment at the Imperial Health Bureau in Berlin, where for the first time he was afforded proper laboratory space and research assistants. During the years 1880 and 1881, he continued to lay the groundwork for the modern study of infectious diseases. He developed new ways of growing bacteria, experimenting with different culture media, such as potato and a condensed algae protein called agar. He used steam and chemicals to promote or inhibit growth of bacteria within his new system. He also built on the technology of the microscope, using oil immersion to improve magnification, and employing condensers and different lighting conditions to improve resolution. Dr. Koch was the first to take photographs of bacteria, showing the world the hidden ecosphere that operated beneath the surface of what was visible. Within his laboratory he ignited the golden age of bacteriology, and with his findings he helped advance the understanding of the infectious basis of many diseases.
In August 1881, Dr. Koch traveled to London to attend the International Medical Congress with many of the leading scientists of the day. There he demonstrated his recent advances in bacteriological techniques, earning praise even from his rival infectious-disease scientist Louis Pasteur, who proclaimed, “C’est un grand progress, Monsieur!”109 When not presenting his work, Dr. Koch listened to lectures on different diseases, among them TB—a popular subject given its ubiquitous nature. Dr. Koch left London determined to apply his knowledge to identifying its cause.
A mere eight months later, on March 24, 1882, in a lecture to the Berlin Physiological Society, Dr. Koch presented his work of the previous few months, changing the field of TB forever. Thirty-six scientists were present, many of them well established in their careers and highly respected. But the lecture concluded with stunned silence, not even a whisper of a question or a hint of applause. They had just witnessed medical history and had recognized it as such. One of those present was future Nobel Prize winner Paul Ehrlich, who later stated: “The evening stands in my memory as my greatest scientific experience.”110
The lecture given by Dr. Koch is held in such high historical esteem not just because of the revelation that he had discovered the cause of the most lethal infectious disease in the history of mankind, but also because of his method of delivering that revelation. Starting off slowly, Dr. Koch meticulously explained the staining techniques he had used to finally culture this elusive bacterium, sharing his observation that older dyes, from the previous decade, worked better than newer dyes because the older ones contained ammonia, which TB cells liked to use as a building block to make their cell wall.
But what gave Dr. Koch’s lecture legendary status was what he brought with him. He transported his entire laboratory to the lecture room, including microscopes, test tubes, and slides with bacteria. He showed the audience his dissections from guinea pigs, which he had variably infected with TB from apes, humans, and cattle. The pathology of the different guinea pig lungs was the same, as were the cultures. He announced that everybody present was free to analyze his work with their own eyes, and to repeat his experiments in their own laboratories. Astonished, the scientists slowly came to the front to look in his microscopes and inspect his dissected guinea pig tissue. This was true open-access research, and it left an impression on those present. For the first time, people had proof that TB was caused by a bacterium, and it was right there for them to look at under a microscope—a rod shaped bacterium two to four micrometers in length.
Word of Dr. Koch’s discovery spread quickly through Europe and North America. On April 10, 1882, his lecture was published in the Berlin Medical Weekly, and from there news spread through the mainstream press, first published on April 23 in the London Times, then on April 24 in the Philadelphia Public Ledger, and featured on May 7 in the Sunday edition of the New York Times. The ghost murderer that had haunted humanity for thousands of years had finally been identified.
With its dense population, and as the destination of millions of immigrants in the past and present, New York City has historically been on the front line of the TB epidemic in the United States. This was never more true than in the late nineteenth century, when TB was the leading killer in the city, claiming ten thousand lives every year, or some twenty-seven patients on average every day.
At the end of the century, Dr. Herman Biggs changed the course of the infection and the fortunes of a city like few in history. Biggs was on the staff at Bellevue Hospital and also worked at the city’s Department of Health. Here he introduced new measures to control the rampant TB epidemic, many of which are still in place. However, he was confronted by a medical establishment that didn’t like being told what to do and how to practice medicine, challenges that persist today.
Dr. Biggs firmly believed in Dr. Koch’s germ theory, and that belief guided his first recommendations, which were also some of his most controversial proposals. In an effort to track the disease and ensure that patients received the most current care and resources, Biggs wanted all cases of TB to be reported to the city’s public health department. This divulging of patient information to the government was a radical idea and threw the medical establishment into a state of apoplexy. And as if this weren’t enough to provoke outrage, Biggs also wanted the public health authorities to track down all of the patients’ contacts, further infuriating the medical establishment.
Physicians at the New York Academy of Medicine swiftly organized against Dr. Biggs, calling his recommendations “mistaken, untimely, irrational and unwise,” and further labeling his measures “offensively dictatorial.” Citing the importance of patient-doctor confidentiality, they united against the “aggressive tyrannies of the Health Board.” The case went all the way to the New York state senate, with Dr. Biggs eventually convincing lawmakers that such measures were necessary for any attempt at controlling TB to be successful. In subsequent years, only about half of New York City’s doctors followed the recommendations, and Dr. Biggs didn’t push it further, but through compromise he established an important precedent of infection control.111
Other parts of Dr. Biggs’s program were less controversial. He set up a system in which all patients, in public and private hospitals, could obtain, free of charge, sputum analysis from the laboratory of the Department of Health to determine whether TB was present. He advocated for nutrition and rest, educated patients and their families to cover their mouths when they coughed, and instructed them to dispose of sputum in a sterile way. He also helped establish tuberculosis wards in hospitals throughout the city, where infected patients could be isolated from the general public.
Apart from medical initiatives, Dr. Biggs introduced other pioneering public health measures. The health department issued circulars educating the public about TB, which were translated into German, Hebrew, and Italian to accommodate the burgeoning immigrant population. In 1902, the Committee on the Prevention of Tuberculosis was founded, and the group expounded upon the importance of hygiene through public exhibits and parades. Together, these efforts represented the first mass education campaign against a single disease, like the campaigns we are familiar with against diseases such as HIV and Ebola, and later COVID-19.
The efforts of Hermann Biggs and others at the health department went a long way toward improving the rates of infection and death from TB in New York City. In 1900, there were 280 deaths per 100,000 people per year. By 1920, deaths were down to 126 per 100,000, and by 1940 the number was 49, less than 25 percent of the 1900 level.112 This was all without the use of any antibiotics. If nothing else, Dr. Biggs had proved that knowledge and prevention could be even more powerful than pharmaceuticals in controlling the spread of disease, especially an infectious one.
Dr. Biggs was not just a physician and epidemiologist; he also knew how to work with the political forces in power, squeezing money out of state budgets even in lean times. He worked with corrupt Tammany Hall and reformist mayors alike. He convinced them all that the improvement of society would pay for itself, and that ultimately the public got the health it paid for: “Public health is purchasable. Within natural limitations, a community can determine its own death rate,” said Biggs.113
In time, though, the lessons from Hermann Biggs were forgotten, and the ancient nemesis again gained a foothold in America’s most densely populated metropolis.
TB grows slowly. Compared to a typical bacterium such as streptococcus, which divides every thirty minutes, TB’s doubling time is sixteen to twenty hours. Cultures for normal bacteria are usually held for a few days. For tuberculosis, because it grows so slowly, one has to wait eight weeks for a definitive answer on a culture.
If patience is required when growing the bacteria in the laboratory, then patience is also the rule for killing them in the body. Today, a typical course of treatment for a bacterial pneumonia from streptococcus is five to seven days, usually with a single drug. For tuberculosis, the typical course is six to nine months, with multiple drugs being the standard. Recent attempts at shortening this time to even four months have failed.114 That’s a lot of time for something to go wrong, like a patient stopping their drugs prematurely, or drug resistance developing.
Tuberculosis also has a unique life cycle within our lungs, and a unique relationship with our immune system. When it is inhaled, it normally settles in the bottom portion of the lung. The main inflammatory white blood cell that is initially responsible for containing tuberculosis is the macrophage, known in the immunology world as the “big eater,” a large glob-like cell that scavenges pathogens such as TB, as well as inorganic chemical debris and even cancer cells. Unfortunately, the macrophage is not the most effective killer, especially in those who have a weakened immune system, such as somebody with HIV infection. For a few, this initial stage of infection can become significantly worse, and spill out of control. Without proper antibiotics, respiratory failure and death ensue.
Fortunately, this kind of rapid lethal progression occurs only in a small minority of patients. But what is strange about TB is that it has the ability to cause a minor initial infection that can be contained, but not completely killed, by the macrophage and then go dormant for years, or even decades. Tuberculosis goes into hiding in some patients, probably deep in the lymph nodes of the chest. It is likely transported to the lymph nodes by the macrophage, which ingest TB but is then unable to completely kill it. When a person gets older, or their immune system weakens for some other reason, TB can spring to life, burst through the macrophage, and cause a new and virulent infection. This is why some patients are offered prophylaxis, preventive medicines to kill any bacteria remaining in the lung or the lymph nodes. Again, patience is required, with nine months being the traditional standard treatment length necessary to eradicate whatever quiescent bacteria remain.
Treatment regimens for TB generally start with four drugs. If the bacteria have no resistance, the number of drugs can be tailored down to two after a few months. This protocol was created in response to data showing that TB has the ability to escape the reach of a single drug by developing resistance. Resistance was noted with use of the first drug for TB, streptomycin, which became available in 1945. Physicians observed that after an initial improvement, some TB patients would then get much worse. Drug-resistant TB had emerged. Even more ominously, multi-drug-resistant TB would develop in the 1950s, followed in 2006 by extensively drug-resistant TB. More recently, totally drug-resistant TB has surfaced, meaning none of the six or seven or eight drugs tested are likely to kill it.
The way TB develops resistance is unique. Typical bacteria, such as streptococcus or staphylococcus, engage in complex warfare with antibiotics and our immune system. In reaction to an antibiotic, these bacteria may develop pumps to actively drive the drug out of themselves. Or they change the makeup of their cell wall so antibiotics can no longer attach themselves. Some bacteria even develop a trap to ensnare the antibiotic once it comes inside its cell. Amazingly, the bacteria even “talk” to one another, trading pieces of their DNA that encode the pumps and traps and new cell-wall ingredients.
None of this subterfuge goes on in TB, no active attempt to evade drugs or trade genetic material or build new cell-wall components. TB bacteria become resistant through spontaneous mutations in their DNA, something that happens in all DNA, but at a very low frequency.115 If one of these mutations in the TB DNA happens to help it fight off our antibiotics, then the organism with that mutation gets selected out and thrives. The end effect is that drug-resistant TB is the result of random changes in DNA. It has been estimated that if a susceptible TB specimen is initially treated with two drugs, and those drugs are taken appropriately, there is almost no chance the bacteria can emerge as resistant. They simply do not have the ability to mount a complex defense against antibiotics, or to rapidly make significant changes to their physical makeup. But they do have the ability to capitalize on sloppy use of antibiotics. If antibiotic use is erratic, or if the course of antibiotics is too short, spontaneously mutated bacteria have the opportunity to become dominant and multiply, creating resistance. The conclusion on TB resistance is obvious—it is something we have created with carelessness.
With the public health efforts of Dr. Biggs and others, and the introduction of effective antibiotics in the 1950s and 1960s, the rates of TB throughout the country steadily declined, and TB was declared to be largely defeated, no longer a threat. New York City would prove that declaration of victory was premature. In 1980, the rate of tuberculosis was about twenty-one cases per hundred thousand people. By 1990, the number of cases in New York City had more than doubled, to almost fifty per hundred thousand, with a steep upward curve.116 Even before the data came out, this was an epidemic doctors in the city had recognized for several years. People had been coming into the hospital and dying of tuberculosis at rates not seen in decades.
As the TB data made its way into the newspapers, it triggered hysteria and misinformation about causes of the upswing. The early and easy explanation for the uptick in tuberculosis cases was the HIV epidemic—HIV causes a weakening of the immune system, thus allowing TB to multiply unchecked. Two doctors, Karen Brudney and Jay Dobkin, rejected this knee-jerk assessment. As physicians at Columbia-Presbyterian Medical Center, they were located at ground zero for the tuberculosis crisis and were uniquely situated to draw their own conclusions.
They worked, much as the organism they were tracking, in a slow and deliberate way, first confirming that a problem existed. They started by reviewing data from the year 1969, then looked at rates of TB over the following twenty years. This period had seen a dramatic increase, with central Harlem at the epicenter, showing a rate twenty times the national average.
The two infectious-disease physicians then made some general observations about what was going on in Harlem at the time, beyond the obvious HIV epidemic, which had started in the early 1980s. They noticed that in the previous ten years, the scourge of homelessness had hit Harlem particularly hard, with many people shepherded into overcrowded shelters. In the hospital, these homeless patients told doctors that the shelters were a breeding ground for TB.
With hints that something in society, other than HIV, was happening to cause the uptick in TB cases, Dr. Brudney and Dr. Dobkin started their study. The methods they used were unpretentious. Between January 1 and September 30, 1988, they interviewed all patients with confirmed TB at Harlem Hospital, and found out what was going on in their lives. They asked them about their housing situation, their current address, who paid the rent, and if there was heat or hot water. They asked the patients if they worked, if they were alcoholics, if they were drug abusers, how many sexual partners they had, and if they had a history of blood transfusions or a prior history of tuberculosis. The two physicians got to know their patients, like super-sleuths.
All the TB patients were also tested for HIV, and then at discharge given an appointment at the Harlem Hospital chest clinic, which was modeled after the Bellevue chest clinic service Dr. Biggs had started almost a hundred years earlier. If the patients were HIV positive, they also got an appointment with an infectious-disease doctor. The study then tracked who showed up at their appointments, or if a hospital admission had gotten in the way. After nine months of studying their subjects, the doctors tallied their data.
In total, 224 cases of TB were diagnosed in that period at Harlem Hospital—an astounding number in an American hospital by today’s standards. (By comparison, at the hospital where I work in Philadelphia, with a similar population and hospital size, we average one or two cases per year.) In the end, what the team saw in this population was not surprising to them, and it drove home Biggs’s observation that a city gets the public health it pays for.
Of the 224 cases, almost 80 percent of the TB-sufferers were male, and about half were alcoholics. About 70 percent were either homeless or in unstable housing. A quarter had been diagnosed with TB previously, and almost all of these patients admitted to not finishing treatment. Of the initial 224, 178 were able to be discharged, with the rest dying in the hospital. Of those making it to discharge, 89 percent did not complete therapy, with most never making it to a single outpatient visit. Although a majority of patients did in fact test positive for HIV, this clearly wasn’t the immediate cause of their unsuccessful TB treatment. What had done the most harm was a breakdown in social infrastructure and a lack of appropriate follow-up for a treatable disease.
Dr. Brudney and Dr. Dobkin dug deeper into the causes of this breakdown in TB control. Just as TB doesn’t grow overnight, the policies and cutbacks that allowed it to flourish again also did not happen in a day. In 1968, a task force appointed by Mayor John Lindsay reported on the state of TB and future directions. At the time, the city spent $40 million per year on TB, including on clinics and on one thousand inpatient beds. The 1968 task force recommended closing some inpatient beds but continuing strong financial support for outpatient clinics and increased home visits by nurses and home health aides.
Instead, with New York City’s fiscal crisis of the 1970s, almost all of the one thousand TB inpatient beds were eliminated, and the budget shrank to less than $25 million in the span of ten years. Financial support at the federal level also shrank, from a high of $1.4 million per year in 1974 to $283,000 in 1980. The recommended increase in home visits never materialized, and opportunities to screen for TB at drug clinics were missed. By 1979, New York City was seeing an increase in its TB cases, even before HIV arose.
Dr. Brudney and Dr. Dobkin published their work in 1991 in the American Review of Respiratory Disease. Titled “Resurgent Tuberculosis in New York City: HIV, Homelessness, and the Decline of Tuberculosis Control Programs,” their paper succinctly described how a volatile mix of homelessness, drug use, and alcoholism, along with a loss of funding, had gotten in the way of patients being treated appropriately.117 Tuberculosis, ever the opportunist, took advantage. In a time when we had our most powerful antibiotics, New York was doing worse than Dr. Biggs had done ninety years before, with education and no antibiotics at all.
Their paper was harsh in many ways, but it was also filled with hope in the form of opportunities for change. The resurgence was clearly not just the product of HIV, or some other mysterious affliction like Galen’s miasma of bad humors from poor sanitation. People were just not taking their medications, for a variety of personal and societal reasons. They had priorities other than their health, like paying the rent, or finding shelter, or looking for food, or obtaining drugs. Toward the end of their paper, Brudney and Dobkin suggested a relatively straightforward, time-tested approach of getting the epidemic under control: longer inpatient hospital stays, residential TB treatment facilities, and increased and aggressive community-based surveillance of medication administration.
Looking back, we know this paper represented a turning point in the fight against this new onslaught of an old disease. People clearly listened to the advice of Drs. Brudney and Dobkin, and of others on the front line of the new epidemic. Funding increased, as did directly observed therapy (DOT) to ensure people were taking their medications. The stories of creativity and persistence on the part of caseworkers are inspiring in their display of dedication. Caseworkers did DOT at health clinics and in homes, and also at more unusual places, like McDonald’s, and even under bridges where homeless people gathered.118 With an influx of money and motivated people, the rate of TB slowly but surely started to decline. As of 2016, the rate of TB in New York City had reached an all-time low of 6.9 cases per 100,000, down from 52 in 1992. Pockets of increased rates still exist, but nothing like the rate or absolute numbers seen in the late 1980s.
New York City remains on guard, especially as a slight uptick was reported in 2017 to 7.5 cases per 100,000 people, or from 565 total cases in 2016 to 613. Fortunately, this number came back down in 2018 to 559 cases.119 Newer surveillance methods utilizing unique technologies are helping. Remote observed therapy is now available, with patients logging onto their computers daily and taking their medicines on camera. The genetics of each TB bacterium can be tracked. With the help of DNA analysis, a 2013 outbreak in Sunset Park was localized to Chinese immigrants, with the strain probably imported from China. A small Internet café and a karaoke bar were the likely sites of transmission, and this information was used to find people asymptomatically infected, with the goal being to treat them and thus stop the spread.120
The strength and power of tuberculosis in this country are not what they once were. Each year since the early 1990s has seen a decline in the number of cases, and as of 2017, the overall rate of the disease was 2.8 per 100,000 people. The total number of deaths from TB in America was 528 in 2016, way down from 1,705 in 1992. Importantly, there has been no drop-off in awareness as occurred in the 1970s, and completion of therapy remains very high.
But if TB’s imprint on us here in the United States is becoming a whisper of what it once was, the numbers abroad tell a different story. The disease is raging out of control in many countries. Absolute numbers of cases are staggering in countries like South Africa, where in 2019 the incidence was 520 per 100,000; in the Philippines it was 554.121 Resistance has also become more of an issue, with multi-drug resistance rampant in countries like Kazakhstan, Ukraine, and Russia. The first cases of extremely drug-resistant strains of TB were reported in places like Iran, India, and Italy, but cases have since been reported in the United States as well.122
Our air today, much like our economy, is becoming more communal and globalized. Drug-resistant infections are a societal problem. Of all the cases of TB in the United States in 2018, 70 percent occurred in non-US-born people, a statistic that is increasing every year. Simply closing our borders to all immigrants is not a realistic solution, but we do screen immigrants and refugees for tuberculosis, for their own health as well as for the health of the country. We also should assume a greater role in helping control the multitude of TB epidemics throughout the globe.
Given what we know from Drs. Brudney, Dobkin, and Biggs, TB is a disease that can be managed. But until we follow through on all the measures at our disposal, the old torment that joined us on the plains of East Africa as we emerged as a species is going to continue to haunt us. It’s not as smart as we are, but it has one thing that we often lack—patience.
Herman Biggs’s observations about the costs and benefits of public health never felt more relevant than in 2020, when the world literally shut down to try and stop the spread of the novel COVID-19 virus. It is clear from early analysis that many missteps were made, both in the United States and in countries throughout the world. We found ourselves in a situation in which a respiratory virus brought the world to its knees, with everybody scared about contracting a lung infection.
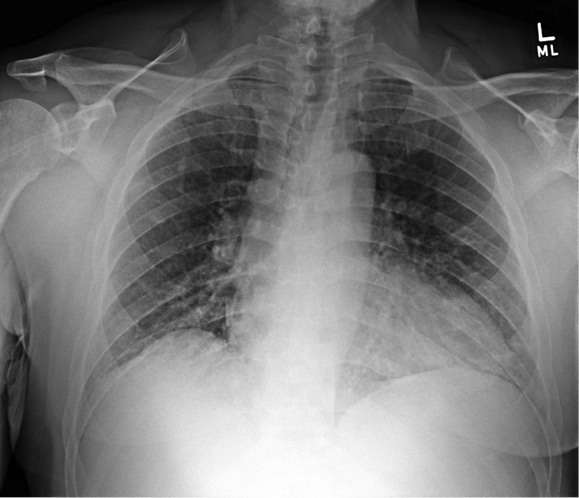

Figure 8: X-ray above with mild COVID-19 pneumonia at the bases, and X-ray below one day later with dramatic progression.
Coronaviruses do not historically cause significant disease in humans. A virus that typically infects birds, cats, pigs, and bats, it started causing trouble only when humans came into close contact with animals that harbored a rare, mutated version of the virus that could infect us. The first of these catastrophic events transpired in South China in 2002, when a coronavirus in civet cats jumped to humans.123 The close contact and slaughtering that takes place at exotic meat markets was the likely conduit of transmission. A home video of these markets shows meat from exotic animals stored in less than sanitary conditions, with no refrigeration or even ice.124 Despite the first SARS coronavirus sweeping through Guangdong province and then spreading globally in 2003, infecting eight thousand people and killing eight hundred, an ineffective effort was made on the part of the Chinese government to close unsanitary exotic meat markets.
Rumblings about a new coronavirus began to be heard in the United States in January 2020, and it soon became clear that a large outbreak in China had been building throughout December. The origin was again an exotic food market, this time in the city of Wuhan, in the Hubei province. The likely source was either a bat, a popular commodity at these markets, or possibly a pangolin, a small scaly anteater native to Asia and also available at open-air meat markets.125 Like the first SARS virus, COVID-19’s new preferred habitat was the human respiratory system, and it spread when somebody coughed and expectorated the virus into the air, or one of the droplets settled on a surface and was subsequently touched by another person.
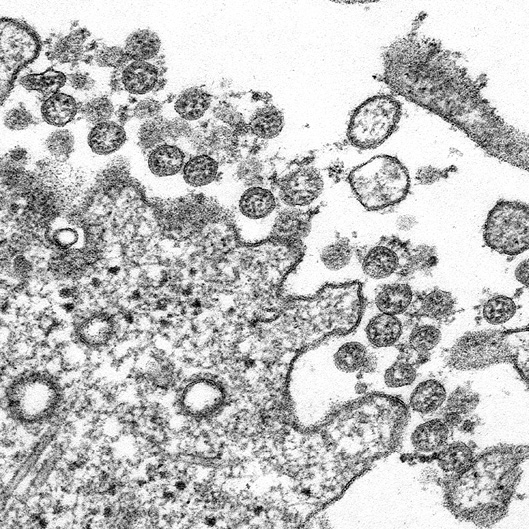
Figure 9: Coronavirus particles, as seen on an electron microscope, attempting to infect a cell.
In January and February 2020, leadership in the United States and at the CDC took opportunities to downplay the threat, then reinforced this careless response by neglecting to make preparations or take precautions, such as improving testing facilities or restricting travel. Dr. Helen Chu, at the University of Washington, was one of the people who, in January 2020, wanted to investigate the possibility of the novel coronavirus already circulating. She had been collecting nasal swabs locally in Seattle for an unrelated viral project, and she asked the state and federal governments for permission to test the swabs for coronavirus. Both said no. Growing nervous about an outbreak, Dr. Chu and her group went ahead and tested the samples anyway—and found a positive result in somebody with no known travel out of the country. The virus was circulating without anybody’s knowledge, or apparent concern.126
Although not all of the data is available, one key to a country controlling this disease appears to be fast and easily available testing. This would let people infected self-quarantine, while allowing for screening for those who had close contact with them. These people could then self-quarantine if their test was positive, slowing the spread of disease significantly. South Korea implemented this approach with apparent success, even using cell phone technology to track where infected people had been to appropriately sound the alarm. The South Korean government also quickly ramped up the ability to test ten thousand people a day, often at convenient drive-through testing stations that minimized potential exposures.127
In the United States, testing was available from state laboratories, but their technology was often woefully antiquated, and up until mid-March 2020, state lab results in Pennsylvania would take four or five days to come back. Even more concerning, up until the second week of March, the state of Pennsylvania could run only five or six tests a day because of limited equipment. Private labs such as LabCorp and Quest stepped in to help fill the void, but only after a month had been squandered. And even then, the private labs understandably did not want patients coming directly to them for specimen collection, leaving potentially infected people with no choice but to put on a mask and wait at the emergency room. Fortunately, rapid drive-through testing stations were eventually implemented.
The coronavirus story is one unfortunate illustration of how our air is communal, that the world is interconnected as never before, and warnings about potential global health threats need to be taken very seriously.
89. “California Tuberculosis Patient Found, Arrested,” San Francisco Examiner, July 29, 2014, https://www.sfexaminer.com/national-news/california-tuberculosis-patient-found-arrested/.
90. Associated Press, “Tuberculosis Patient Charged in Calif. for Not Taking Medication,” CBS News Online, May 16, 2012, https://www.cbsnews.com/news/tuberculosis-patient-charged-in-calif-for-not-taking-medication/.
91. S. M. Aciego, C. S. Riebe, and S. C. Hart, “Dust Outpaces Bedrock in Nutrient Supply to Montane Forest Ecosystems,” Nature Communications 8 (2017): 14800.
92. National Aeronautics and Space Administration, “NASA Satellite Reveals How Much Saharan Dust Feeds Amazon’s Plants,” NASA website, February 22, 2015, https://www.nasa.gov/content/goddard/nasa-satellite-reveals-how-much-saharan-dust-feeds-amazon-s-plants.
93. Nancy Tomes, The Gospel of Germs: Men, Women, and the Microbe in American Life (Cambridge, MA: Harvard University Press, 1998), 97.
94. William Firth Wells and Mildred Weeks Wells, “Air-Borne Infections,” JAMA 107 (1936): 1698–1703.
95. Lydia Bourouiba, “Turbulent Gas Clouds and Respiratory Pathogen Emissions,” JAMA, published online March 26, 2020, https://jamanetwork.com/journals/jama/fullarticle/2763852.
96. Peter Disikes, “In the Cloud: How Coughs and Sneezes Float Farther Than You Think.” MIT News Online, April 8, 2014, http://news.mit.edu/2014/coughs-and-sneezes-float-farther-you-think.
97. World Health Organization, “Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations,” WHO website, accessed May 9, 2020, https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
98. Sean Wei Xiang Ong, Yian Kim Tan, Po Ying Chia, et. al. “Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient,” JAMA, published online March 4, 2020, https://jamanetwork.com/journals/jama/fullarticle/2762692.
99. Alice Yan, “Chinese expert who came down with Wuhan coronavirus after saying it was controllable thinks he was infected through his eyes,” South China Morning Post, January 23, 2020, https://www.scmp.com/news/china/article/3047394/chinese-expert-who-came-down-wuhan-coronavirus-after-saying-it-was.
100. Tom Paulson, “Epidemiology A Mortal Foe,” Nature 502, no. 7470 (October 10, 2013): S2–S3.
101. World Health Organization, “Tuberculosis,” WHO website, accessed September 18, 2018, https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
102. I. Barberis, N. L. Bragazzi, L. Galluzzo, and M. Martini, “The History of Tuberculosis: From the First Historical Records to the Isolation of Koch’s Bacillus,” Journal of Preventive Medicine and Hygiene 58 (2017): E9–E12.
103. Anne C. Stone, Alicia K. Wilbur, Jane E. Buikstra, and Charlotte A. Roberts, “Tuberculosis and Leprosy in Perspective,” Yearbook of Physical Anthropology 52 (2009): 66–94.
104. Kirsten I. Bos, Kelly M. Harkins, Alexander Herbig, et al., “Pre-Columbian Mycobacterial Genomes Reveal Seals as a Source of New World Human Tuberculosis,” Nature 514 (2014): 494–497.
105. Clark Lawlor, Consumption and Literature: The Making of the Romantic Disease (Basingstoke, UK: Palgrave Macmillan, 2006), 111.
106. Arne Eggum, Edvard Munch: Paintings, Sketches, and Studies (New York: C. N. Potter, 1984), 46.
107. M. Monir Madkour, Kitab E. Al-Otaibi, and R. Al Swailem, “Historical Aspects of Tuberculosis” in Tuberculosis (Berlin Heidelberg: Springer-Verlag, 2004), 18.
108. Thomas M. Daniel, “Jean-Antoine Villemin and the Infectious Nature of Tuberculosis,” International Journal of Tuberculosis and Lung Disease 19 (2015): 267–268.
109. Edward S. Golub, The Limits of Medicine (Chicago: University of Chicago Press, 1997), 93.
110. Alex Sakula, “Robert Koch: Centenary of the Discovery of the Tubercle Bacillus, 1882,” Canadian Veterinary Journal 24, no. 4 (April 1983): 127–131.
111. Daniel M. Fox, “Social Policy and City Politics: Tuberculosis Reporting In New York, 1889–1900,” Bulletin of the History of Medicine 49, no. 2 (Summer 1975): 169–195.
112. Godias J. Drolet and Anthony M. Lowell, A Half Century’s Progress Against Tuberculosis in New York City (New York Tuberculosis and Health Association, 1952), https://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb1900.pdf.
113. H. Sheridan Baketel and Arthur C. Jacobson, “Public Health,” The Medical Times, 43 (June 1915): 200.
114. Corinne S. Merle, Katherine Fielding, Omou Bah Sow, et al., “A Four-Month Gatifloxacin-Containing Regimen for Treating Tuberculosis,” New England Journal of Medicine 371 (October 23, 2014): 1588–1598.
115. Tasha Smith, Kerstin A. Wolff, and Liem Nguyen, “Molecular Biology of Drug Resistance in Mycobacterium Tuberculosis,” Current Topics in Microbiology and Immunology 375 (2014): 53–80.
116. New York City Health Department, New York City Bureau of Tuberculosis Control Annual Summary, 2018, pdf file, https://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2018.pdf.
117. Karen Brudney and Jay Dobkin, “Resurgent Tuberculosis in New York City: Human Immunodeficiency Virus, Homelessness, and the Decline of Tuberculosis Control Programs,” The American Review of Respiratory Disease 144, no. 4 (October 1991): 745–749.
118. Natalie Shure, “How New York Beat Its TB Epidemic,” The Daily Beast, April 14, 2017, https://www.thedailybeast.com/how-new-york-beat-its-tb-epidemic.
119. New York City Health Department, New York City Bureau of Tuberculosis Control Annual Summary, 2018, pdf file, https://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2018.pdf.
120. Natalie Shure, “How New York Beat Its TB Epidemic,” The Daily Beast, April 14, 2017, https://www.thedailybeast.com/how-new-york-beat-its-tb-epidemic.
121. World Health Organization, “Tuberculosis Country Profiles,” WHO website, https://www.who.int/tb/country/data/profiles/en/.
122. World Health Organization, “Drug Resistant Tuberculosis,” WHO website, https://www.who.int/tb/areas-of-work/drug-resistant-tb/en/.
123. “China Scientists Say SARS-Civet Cat Link Proved,” Science News, January 20, 2007, https://www.reuters.com/article/us-china-sars/china-scientists-say-sars-civet-cat-link-proved-idUSPEK23793120061123.
124. “How Wildlife Trade is Linked to Coronavirus,” YouTube video, 8:48, Vox, March 6, 2020, https://www.youtube.com/watch?v=TPpoJGYlW54.
125. David Cyranoski, “Mystery Deepens over Animal Source of Coronavirus,” Nature Online, February 26, 2020, https://www.nature.com/articles/d41586-020-00548-w.
126. Sheri Fink and Mike Baker, “‘It’s Just Everywhere Already’: How Delays in Testing Set Back the U.S. Coronavirus Response,” New York Times, March 10, 2020, https://www.nytimes.com/2020/03/10/us/coronavirus-testing-delays.html.
127. Stephen Engelberg, Lisa Song, and Lydia DePillis, “How South Korea Scaled Coronavirus Testing While the U.S. Fell Dangerously Behind,” ProPublica, March 15, 2020, https://www.propublica.org/article/how-south-korea-scaled-coronavirus-testing-while-the-us-fell-dangerously-behind.