19. Lorentz to Schrödinger
Haarlem
27 May 1926
Dear Colleague,
I am finally getting around to answering your letter and to thanking you very much for kindly sending me the proof sheets of your three articles, all of which I have in fact received. Reading these has been a real pleasure to me. Of course the time for a final judgment has not come yet, and there are still many difficulties, it seems to me, about which I shall get to speak immediately. But even if it should turn out that a satisfactory solution cannot be reached in this way, one would still admire the sagacity that shows forth from your considerations, and one would still venture to hope that your efforts will contribute in a fundamental way to penetrating these mysterious matters.
I was particularly pleased with the way in which you really construct the appropriate matrices and show that these satisfy the equations of motion. This dispels a misgiving that the works of Heisenberg, Born, and Jordan, as well as Pauli’s, had inspired in me: namely, that I could not see clearly that in the case of the H-atom, for example, a solution of the equations of motion can really be specified. With your clever observation that the operators q and 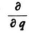 commute or do not commute with each other in a similar way to the q and p in the matrix calculation, I began to see the point. In spite of everything it remains a marvel that equations in which the q’s and p’s originally signified coordinates and momenta, can be satisfied when one interprets these symbols as things that have quite another meaning, and only remotely recall those coordinates and momenta. If I had to choose now between your wave mechanics and the matrix mechanics, I would give the preference to the former, because of its greater intuitive clarity, so long as one only has to deal with the three coordinates x,y,z. If, however, there are more degrees of freedom, then I cannot interpret the waves and vibrations physically, and I must therefore decide in favor of matrix mechanics. But your way of thinking has the advantage for this case too that it brings us closer to the real solution of the equations; the eigenvalue problem is the same in principle for a higher dimensional q-space as it is for a three dimensional space.
commute or do not commute with each other in a similar way to the q and p in the matrix calculation, I began to see the point. In spite of everything it remains a marvel that equations in which the q’s and p’s originally signified coordinates and momenta, can be satisfied when one interprets these symbols as things that have quite another meaning, and only remotely recall those coordinates and momenta. If I had to choose now between your wave mechanics and the matrix mechanics, I would give the preference to the former, because of its greater intuitive clarity, so long as one only has to deal with the three coordinates x,y,z. If, however, there are more degrees of freedom, then I cannot interpret the waves and vibrations physically, and I must therefore decide in favor of matrix mechanics. But your way of thinking has the advantage for this case too that it brings us closer to the real solution of the equations; the eigenvalue problem is the same in principle for a higher dimensional q-space as it is for a three dimensional space.
There is another point in addition where your methods seem to me to be superior. Experiment acquaints us with situations in which an atom persists in one of its stationary states for a certain time, and we often have to deal with quite definite transitions from one such state to another. Therefore we need to be able to represent these stationary states, every individual one of them, and to investigate them theoretically. Now a matrix is the summary of all possible transitions and it cannot at all be analyzed into pieces. In your theory, on the other hand, each of the states corresponding to the various eigenvalues E plays its own role.
Now permit me to make several comments in which, however, you probably will not find much new.
1. In your wave equation (I limit myself to the H-atom)
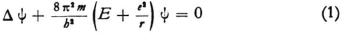
E is a constant independent of the coordinates; there are as many wave problems as there are energy values E, and of course the eigenvalues E are to be particularly considered here since only for these can the boundary conditions be satisfied. Your calculation of the eigenvalues* shows that one must understand E to be the energy of the electron, in the sense that the energy is set equal to zero when the electron is at rest at an infinite distance from the nucleus. Putting it another way, 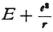 at any point x,y,z is the kinetic energy that the electron would have at that point for the prescribed value of E. This kinetic energy corresponds to the velocity
at any point x,y,z is the kinetic energy that the electron would have at that point for the prescribed value of E. This kinetic energy corresponds to the velocity

2. Since Equation (1) contains no time derivative one can only derive from it the wave length at a definite point; one has, namely,

varying from point to point.
The velocity of propagation, ω, of the waves, and the frequency, ν , related to it by the equation

cannot be derived from (1). A certain amount of arbitrariness remains here.
Now it is one of the basic ideas of your theory (and a very beautiful one) that the velocity, u, of the electron should be equal to the “group velocity”. This requires the relationship

and if one takes this into consideration one can also determine ν and ω.
Concerning equation (5) it is to be observed first, that we want to consider ν, ω, and u as all positive, and second, that at a definite point λ, u (and ω) can vary with ν, as follows from (2) and (3), because these quantities are somehow related to E. In carrying out the differentiation with respect to ν that appears in (5) one must, however, abandon the eigenvalues E. There does not seem to be anything against this; one can very readily imagine states (travelling waves) which do indeed satisfy the wave equation, but do not satisfy all boundary conditions.
From (4) and (5) it follows that

and therefore
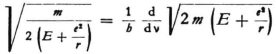
and
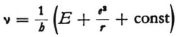
Since “const.” means independent of E, we can set the constant equal to 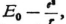 where E0 is not only independent of E but also of x,y,z . Thus,
where E0 is not only independent of E but also of x,y,z . Thus,

By this means the condition that the frequency be equal at all points of the field is satisfied. Further, from (3) and (4),*
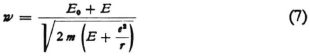
3. Your conjecture that the transformation which our dynamics will have to undergo will be similar to the transition from ray optics to wave optics sounds very tempting, but I have some doubts about it.
If I have understood you correctly, then a “particle”, an electron for example, would be comparable to a wave packet which moves with the group velocity.
But a wave packet can never stay together and remain confined to a small volume in the long run. The slightest dispersion in the medium will pull it apart in the direction of propagation, and even without that dispersion it will always spread more and more in the transverse direction. Because of this unavoidable blurring a wave packet does not seem to me to be very suitable for representing things to which we want to ascribe a rather permanent individual existence.
As you yourself remark, the blurring in question is far advanced in the field of the H-atom. A wave packet can hold together for some time only if its dimensions are large compared to the wave length. Since, however, the wave length determined by (3) is of the order of magnitude of the Bohr elliptic orbit, there can be no question of having a wave packet that is small compared to the dimensions of such an ellipse and which is moving along this line.
Naturally, if you assign a large positive value to the constant E in (6) and (2), (one can think of E = mc2), you can reach an arbitrarily high frequency ν with correspondingly large propagation velocity ω, but you cannot change the wave length given by (3) at all.* 4. If we decide to dissolve the electron completely, so to speak, and to replace it by a system of waves this has both an advantage and a disadvantage.
The disadvantage, and it is indeed a serious one, is this: whatever we assume about the electron in the hydrogen atom we must also assume for all electrons in all atoms; we must replace them all by systems of waves. But then how am I to understand the phenomena of photoelectricity and the emission of electrons from heated metals? The particles appear here quite clearly and without alteration; once dissolved, how could they condense again?
I do not mean to say by this that there cannot be many metamorphoses in the interior of atoms. If one wants to imagine that electrons are not always little planets that circle about the nucleus, and if one can accomplish something by such an idea, then I have nothing against it. But if we take a wave packet as model of the electron, then by doing so we block the way to restoring matters. Because it is indeed asking a lot to require that a wave packet should condense itself again once it has lost its shape.
The advantage that I spoke of consists of the following: if the electron continues to persist in a circular or elliptic orbit, one would then expect that in the wave equation, (1), (I am considering a point at which the electron is not located), there will appear not only the term e2/r that depends on the field of the nucleus, but also a similar term that refers to the electric field of the electron. One field is as good as the other and they are of the same order of magnitude. But if equation (1) is changed this way the calculation of the eigenvalues of E would break down and would give rise to unspeakable complications. If the electron as such is no longer there then one can more readily be satisfied that only the term depending on the nuclear charge appears in the equation.
5. We will now replace Bohr’s stationary states with energies E1, E2, etc. by “stationary wave systems” with frequencies
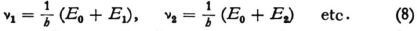
By giving the term E0 a large positive value you can make these fundamental frequencies so high that they cannot be observed at all. (You can also assume that they are incapable of radiating, i.e. that there is no connection at all between the field which consists of the corresponding system of waves and the ordinary electromagnetic field, even though they both fill the same volume.) The observed radiations have the frequencies
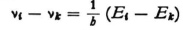
and the question arises as to how to account for this. Two ways suggest themselves to us—beats and combination tones.
There is not much to be said about the first. Let us suppose that we knew the fundamental equations from which the wave equation (1) results; I mean the true “equations of motion” which do not contain E at all, but contain time derivatives instead. If these fundamental equations are also linear then the superposition of two solutions, ψ1 = a1 cos (2 πν1t + b1) and ψ2 = a2 cos (2 πν2t + b2) will lead to beats; no instrument (resonator, grating) whose operation is completely determined by linear equations would respond to these beats as it would to vibrations of frequency ν1–ν2 · One can always imagine that somehow or other, although the process remains obscure for the present, a vibration takes place with the emission of radiation whose period corresponds to the frequency of the intensity maxima.
We can examine the origin of combination tones in somewhat more detail. To begin with, it is necessary that the fundamental equation be non-linear, but that is also sufficient. If, for example, a fundamental equation contains a term involving ψ2, and if the vibrations that denoted by ψ1 and ψ2 are present at the same time, then as a consequence a term of the form
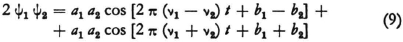
will appear, where the first quantity just represents the difference tone. In order to understand quite clearly how this leads to radiation, however, one would have to take account of the connection between the vibrating system and the electromagnetic field. As far as the term denoting a sum in (9) is concerned one can assume that it cannot be made observable because of its high frequency ν1 + ν2.
In addition one can also understand absorption pretty well if one uses combination tones, which would be difficult to manage if one wanted to reduce optical phenomena to beats.
Let us suppose that the first vibrational state, ψ1 = a1 cos (2 πν1t " b1) is already present in the atom and that now a force with frequency ν2-ν1 acts on it (incident light). This can excite vibrations like
ψ′ = á cos [2π (ν2–ν1)t +b′]
(provided that the resonance is not strong). As a result the quantity
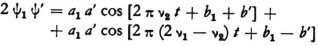
will appear in the term containing ψ2 in the fundamental equation, and one can consider both of its parts as expressions for certain forces that excite vibrations of frequencies ν2 and 2 ν1–ν2 · The first of these, because its frequency coincides with the second characteristic vibration, can set the system into sympathetic oscillation (in this proper mode), and a part of the energy of the incident light is finally used for this. The force whose frequency is 2 ν1–ν2 can remain ineffective because it corresponds to none of the characteristic vibrations of the system.
Naturally one could possibly try to pursue this kind of approach further.
What I do not like very much about this interpretation of radiation as produced by sum and difference oscillations is that the radiation is considered to be something of secondary importance, as something that depends on terms in the fundamental equations that one even neglects in first approximations (in deriving the wave equation (1).) Is it not really much simpler to hold onto Bohr’s stationary states and then perhaps to assume that a Planck oscillator of frequency (ν2–ν1) is present, (the atom could turn into one), and finally that this absorbs the energy h(ν2–ν1) in a quantum jump 2 → 1 and then it calmly radiates?
6. Perhaps I may add that many years ago, when the laws of spectra were not yet known, my compatriot V. A. Julius20 observed that in spectra containing many lines there are many pairs of lines for which Δν is almost the same. A probabilistic calculation, (similar to the one which served to prove that double stars are not accidental apparent approaches), then showed him that the number of differences Δν that differed from each other by less than a definite quantity 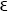 , is much larger than one should expect according to the laws of chance. After he had shown the reality of the equations Δν = Δ′ν=Δ″ ν=… this way he arrived at the idea that many spectral lines might originate in sum and difference oscillations.
, is much larger than one should expect according to the laws of chance. After he had shown the reality of the equations Δν = Δ′ν=Δ″ ν=… this way he arrived at the idea that many spectral lines might originate in sum and difference oscillations.
Rayleigh later made the observation that perhaps one could consider the simple appearance of the first power of the frequency in the spectral formulas (while dynamical laws lead rather to ν2) as an indication of kinematical relations.
After all these efforts I felt it to be a real simplification when Bohr showed that every frequency that is radiated corresponds to a definite energy difference, whereby the general structure of the spectral formulas immediately becomes clear. So I have lost my taste for explanations by means of sum and difference oscillations to some extent, but I can certainly reacquire it if your theory succeeds in other respects.
7. I find a real difficulty, as far as the combination oscillations are concerned, in the energy relationships. As far as the energy of the stationary wave system is concerned one can make any arbitrary assumption to begin with, since one can dispose of the amplitudes freely, even if one has already accepted Eq. (6) for the frequency. However, it seems obvious that if one replaces Bohr’s stationary states by stationary wave systems, one should assume certain definite energy differences between these. The fact that definite amounts of energy are necessary (in electron bombardment) to call forth definite radiation phenomena shows that the “energy levels” really exist, and if we no longer have the revolving electrons we have to look for the definite energy values in the individual stationary wave systems. The simplest thing would be to ascribe to these the energy values
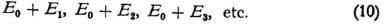
Here E1, E2, E3, … are the Bohr energy values (or also the eigenvalues of the wave equation), while E0 is identified with the E0 in (6), or if one prefers, it can be considered as different from the latter. In any case one probably has grounds for adding to the Bohr energy values a contribution, a positive one, E0, which is the same for all wave systems. The values E1, E2, etc. are certainly negative and it is natural to represent the energy of a system of waves as a positive quantity.
If it is now assumed that the wave systems can exist with only the energy values (10) (so that they can have only certain prescribed amplitudes), a difficulty arises.
Let us suppose that state 1 is the “natural” one, the state of the atom when it is left to itself and the one that corresponds to the lowest energy, and furthermore let us ask that radiation of frequency  be produced. According to Bohr we must first bring the atom into energy level 3, and we must therefore provide it with energy E3–E1 (by electronic collision, say). The measurements are in accord with this. According to the new theory, however, we have to realize both states 2 and 3, since the required radiation presupposes the simultaneous existence of both. The energy must then become E0 + E2 + E0 + E3 while it was originally E0 + E1. If we assume that the first state of oscillation 1 disappears in the electronic collision, we find that the energy that has to be provided is E0 + E2 + E3 – E1 which is hardly to be reconciled with the observations.
be produced. According to Bohr we must first bring the atom into energy level 3, and we must therefore provide it with energy E3–E1 (by electronic collision, say). The measurements are in accord with this. According to the new theory, however, we have to realize both states 2 and 3, since the required radiation presupposes the simultaneous existence of both. The energy must then become E0 + E2 + E0 + E3 while it was originally E0 + E1. If we assume that the first state of oscillation 1 disappears in the electronic collision, we find that the energy that has to be provided is E0 + E2 + E3 – E1 which is hardly to be reconciled with the observations.
One would naturally be able to escape this difficulty by assuming that the individual vibration states need not have quite the energies given by (10), but then what becomes of the energy levels?
Furthermore, according to Bohr just the energy E3–E2 is radiated in the transition 3 → 2; state 3 disappears and is replaced by state 2. Can one imagine that just the energy E3–E2 will be emitted in the radiation brought about by the difference oscillation, and then what becomes of the energy of both wave systems? Similar questions, which I need not go into, arise if the inverse process, absorption, is considered.
In conclusion I might say that one can feel that it is unsatisfactory in Bohr’s theory that the frequencies emitted are completely distinct from the frequencies of the periodic motions that really take place. It is fine that in your theory both kinds of frequencies are brought into a much simpler connection with each other, (namely ν emitted= ν2–ν1 where ν1and ν2 are “internal” frequencies); nevertheless it is not easy to understand this connection.
I should be very glad if you would write me sometime what you think about what is said above. Meanwhile please excuse me if perhaps I have not always correctly understood your meaning.
With kind regards
Yours faithfully,
H. A. Lorentz
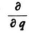 commute or do not commute with each other in a similar way to the q and p in the matrix calculation, I began to see the point. In spite of everything it remains a marvel that equations in which the q’s and p’s originally signified coordinates and momenta, can be satisfied when one interprets these symbols as things that have quite another meaning, and only remotely recall those coordinates and momenta. If I had to choose now between your wave mechanics and the matrix mechanics, I would give the preference to the former, because of its greater intuitive clarity, so long as one only has to deal with the three coordinates x,y,z. If, however, there are more degrees of freedom, then I cannot interpret the waves and vibrations physically, and I must therefore decide in favor of matrix mechanics. But your way of thinking has the advantage for this case too that it brings us closer to the real solution of the equations; the eigenvalue problem is the same in principle for a higher dimensional q-space as it is for a three dimensional space.
commute or do not commute with each other in a similar way to the q and p in the matrix calculation, I began to see the point. In spite of everything it remains a marvel that equations in which the q’s and p’s originally signified coordinates and momenta, can be satisfied when one interprets these symbols as things that have quite another meaning, and only remotely recall those coordinates and momenta. If I had to choose now between your wave mechanics and the matrix mechanics, I would give the preference to the former, because of its greater intuitive clarity, so long as one only has to deal with the three coordinates x,y,z. If, however, there are more degrees of freedom, then I cannot interpret the waves and vibrations physically, and I must therefore decide in favor of matrix mechanics. But your way of thinking has the advantage for this case too that it brings us closer to the real solution of the equations; the eigenvalue problem is the same in principle for a higher dimensional q-space as it is for a three dimensional space.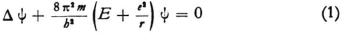
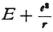 at any point x,y,z is the kinetic energy that the electron would have at that point for the prescribed value of E. This kinetic energy corresponds to the velocity
at any point x,y,z is the kinetic energy that the electron would have at that point for the prescribed value of E. This kinetic energy corresponds to the velocity




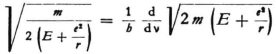
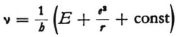
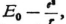 where E0 is not only independent of E but also of x,y,z . Thus,
where E0 is not only independent of E but also of x,y,z . Thus,
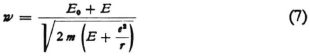
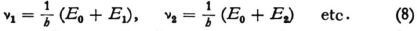
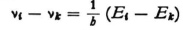
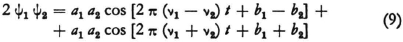
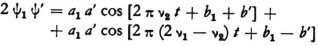
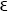 , is much larger than one should expect according to the laws of chance. After he had shown the reality of the equations Δν = Δ′ν=Δ″ ν=… this way he arrived at the idea that many spectral lines might originate in sum and difference oscillations.
, is much larger than one should expect according to the laws of chance. After he had shown the reality of the equations Δν = Δ′ν=Δ″ ν=… this way he arrived at the idea that many spectral lines might originate in sum and difference oscillations.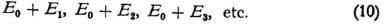
 be produced. According to Bohr we must first bring the atom into energy level 3, and we must therefore provide it with energy E3–E1 (by electronic collision, say). The measurements are in accord with this. According to the new theory, however, we have to realize both states 2 and 3, since the required radiation presupposes the simultaneous existence of both. The energy must then become E0 + E2 + E0 + E3 while it was originally E0 + E1. If we assume that the first state of oscillation 1 disappears in the electronic collision, we find that the energy that has to be provided is E0 + E2 + E3 – E1 which is hardly to be reconciled with the observations.
be produced. According to Bohr we must first bring the atom into energy level 3, and we must therefore provide it with energy E3–E1 (by electronic collision, say). The measurements are in accord with this. According to the new theory, however, we have to realize both states 2 and 3, since the required radiation presupposes the simultaneous existence of both. The energy must then become E0 + E2 + E0 + E3 while it was originally E0 + E1. If we assume that the first state of oscillation 1 disappears in the electronic collision, we find that the energy that has to be provided is E0 + E2 + E3 – E1 which is hardly to be reconciled with the observations.