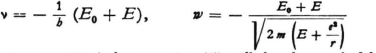
1 Schrödinger’s early works on wave mechanics are collected in his book Collected Papers on Wave Mechanics (Glasgow: Blackie and Son, 1928). The first group appeared in the Annalen der Physik (4) 79 (1926) pp. 361, 489, 734; 80 (1926) p. 437; 81 (1926) p. 109.
2 Schrödinger had inadvertently spelled the name of the mathematician Jacobi with a k.
3 This probably refers to Planck’s lecture, Physikalische Gesetzlichkeit im Lichte neuer Forschung, delivered on 14 February 1926 in Dusseldorf. Naturwissenschaften 14 (1926), p. 249. Reprinted in Max Planck, Physikalische Abhandlungen und Vorträge (Braunschweig: Friedr. Vieweg und Sohn, 1958) Vol. III, p. 159.
4 Grüneisen was at that time President of the Berlin branch of the German Physical Society.
5 See letter No. 19. It was actually 11 pages; see letter No. 20.
6 Schrödinger’s lecture, entitled “Foundations of an atomism based on the theory of waves”, was given on 16 July 1926 in Berlin with W. Nernst in the chair. A similar lecture was delivered on 23 July 1926 to the Bavarian branch of the Society with R. Emden in the chair.
7 M. Planck, Verhandlungen der Deutschen Physikalischen Gesell-schaft 13 (1911), p. 136. It is assumed here, by way of trial, that only emission from the atom takes place in quanta, but that absorption occurs continuously, an idea that later had to be given up. See especially Planck’s Scientific Autobiography (New York: Philosophical Library, 1949) pp. 43-46.
8 This sentence is written in the side margin of the letter, and was obviously added after its completion.
9 An exchange of letters on statistical mechanics had preceded these letters.
[The papers referred to are E. Schrödinger, Physikalische Zeitschrift 27 (1926), p. 95 and A. Einstein, Berliner Berichte (1924) p. 261. (1925) p. 3.]
10 A. Einstein, Berliner Berichte (1925) p. 3.
11 A. Einstein, Naturwissenschaften 14 (1926), p. 300.
12 See A. Einstein, Naturwissenschaften 14 (1926), p. 222. [English translation in A. Einstein, The World As I See It (New York: Philosophical Library, 1935), p. 204.
13 This concerns the easily established fact that the tea leaves scattered at the bottom of a cup collect in the middle when the tea is stirred.
14 In a letter to N. Bohr dated 13 May 1928 Schrödinger thanks him for an offprint of Bohr’s paper, The Quantum Postulate and the Recent Development of Atomic Physics (Naturwissenschaften, 16, [1928], p. 245). He remarks on the fact that the Heisenberg uncertainty relation prevents one from being able to distinguish between neighboring quantum states under certain circumstances, and mentions as examples the conjugate action and angle variables as well as the motion of a molecule in an ideal gas. He sees this as a limitation on the applicability of the old experimental concepts which will have to be replaced by a new system of ideas, which will, of course, be very difficult.
Bohr replies to this in a letter dated 25 May 1928, saying that he sees no basis for giving up the old concepts, and that all difficulties can be removed by means of the principle of complementarity. He does not consider the remark on angle variables to be sound because, in interpreting experiments with the help of the concept of stationary states, one always has to deal with those properties of an atomic system that depends upon the phase relations over a large number of consecutive periods. He does not quite understand the application of the uncertainty relation to a gas molecule because here the momentum quantity conjugate to the coordinate has no unique value.
15 See footnote 14 in the previous letter.
16 See Bohr’s account of his discussions with Einstein in Bohr’s book Atomic Physics and Human Knowledge (New York: John Wiley and Sons, Inc., 1958), p. 32.
17 Point Peconic, Long Island
18 Schrödinger’s witty conceptual experiment with a “smeared-out cat” is described in his article on the state of the quantum theory at that time in Naturwissenschaften 23 (1935), on page 812.
19` For Max Born’s position on quantum mechanics, see his article, The Interpretation of Quantum Mechanics in his book Physics in My Generation (London and New York: Pergamon Press, 1956) p. 140.
* It is very beautiful that you were able to carry out this calculation and that in doing so you arrived at the values required by the Balmer formula.
* If E0 + E is negative one can set
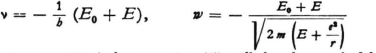
(both positive quantities), but equation (5) will then be satisfied by
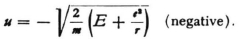
The wave velocity ω and the group velocity u would have opposite directions in this case.
* If one puts E = mc2 and 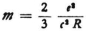 according to the usual formula (R is the radius of the electron), and if one also understands E to be energy in a circular Bohr orbit of radius r, so that
according to the usual formula (R is the radius of the electron), and if one also understands E to be energy in a circular Bohr orbit of radius r, so that 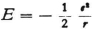 then
then 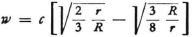
Since r > > R, we will have ω > > c. There is, naturally, nothing against that since we are dealing here with something quite different from the usual propagation of electromagnetic waves.
20 Victor August Julius, born 1851, Professor of Theoretical Mechanics and Mathematical Physics at the University of Utrecht from 1896 on, died 1902.
21 The time dependence is that of a sinusoidal vibration; since, however, it could not be established in what form this factor was written down in the original the gap was left open. Compare Letter 6.
22 This is clearly a slip. Schrödinger evidently means (1) and the unnumbered equation 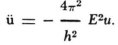
23 W. Heisenberg, Mathematische Annalen 95 (1926), p. 683.
24 P.A.M. Dirac, Proc. Royal Soc. A 109 (1925) p. 649; A 110 (1926) p. 561
25 G. Wentzel, Zeitschrift fur Physik 37 (1926), p. 80
* Each of the quantum integrals still contains an additive constant which remains undetermined. The only thing that can be concluded is that they are progressively larger by integral multiples of h. That is a serious deficiency and not an unimportant one like the additive energy constants.
* * i.e. would not represent the correct assertion of the theory.
26 The “festival day” referred to is the golden anniversary of Lorentz’s doctorate, celebrated in Leyden in December 1925.
27 Schrödinger participated in the 4th Solvay Conference in Brussels in April 1924; Lorentz presided over these Conferences.
* In order to picture the process to some extent, I imagined that there is an oscillator of frequency ν21 which absorbs the energy E2—E1 and then quietly radiates it; or also that when the atom has energy E2, it transforms itself into an oscillator ν21 for a time, and the latter becomes a Bohr atom again when its energy has decreased from E2 by radiating.
28 “Electron” probably appears here erroneously instead of “atom”
29 E. Schrödinger, Naturwissenschaften 14 (1926) p. 664
* It could probably also be derived from the equation of motion now under consideration (analogue of Huygens’ principle).
* I refer to them as lines and do not speak of “light rays” because there is no longer any question of the physical significance of the latter (limitation of a wide bundle of rays).
30 Lorentz uses the symbol H for h/2π, now usually denoted by 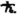 .
.
* * We can talk about this even without thinking of a propagation along the lines.
* Hard to say why. One could think here of de Broglie’s interpretation: internal vibrations of the electron and agreement in phase between this and the accompanying wave.