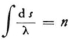21. Lorentz to Schrödinger
Haarlem
19 June 1926
Dear Colleague,
I read your last letter, for which many thanks, with lively interest, and it helped a great deal in making your interpretations clear to me. Now I see that the difficulties that I experienced turned partly on the fact that I had become very accustomed to the ideas of the current quantum theory, so that I could not immediately free myself from it sufficiently. That is why, for example, I objected that radiation appears as something “secondary” in your work.
You are quite right when you say that this is also the case in classical theory inasmuch as the term in the equation of motion of the electron that corresponds to the radiation resistance falls far short of the other terms, so that it can often be neglected in first approximation. But I was thinking of a quantum jump 2–1,in which (as I imagined along with Bohr) the definite finite quantity of energy E2–E1 is radiated with frequency 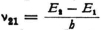 . Such transitions might occur rarely but the radiation is actually the main thing when each individual quantum jump does occur.* But if your interpretation (radiation as a difference tone) can be carried through successfully, and if we then no longer have to think of the radiation of just the quantity of energy E2–E1 I will also be satisfied.
. Such transitions might occur rarely but the radiation is actually the main thing when each individual quantum jump does occur.* But if your interpretation (radiation as a difference tone) can be carried through successfully, and if we then no longer have to think of the radiation of just the quantity of energy E2–E1 I will also be satisfied.
In this connection I was also very pleased with your remark about a moving electron’s “capacity for exciting radiation”. Here too I was thinking too much about the energy of the electron. If one can successfully explain the phenomena by connecting a definite frequency to the moving electron so that one is dealing with a resonance, it would be much more beautiful.
Meanwhile there are still many questions that arise here. Suppose we have a system with fundamental frequencies ν1; and ν2 , and that
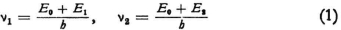
where E1 and E2 are the (negative) energies that we ascribe to the atom in two stationary states (according to Bohr), while E0 has a large positive value. One can now imagine that under the influence of an external irradiation whose frequency is ν2–ν1 the system will be caused to emit light of this same frequency (“resonance with the difference tone”). But how is resonance with an electron to occur? In de Broglie’s case (electron moving in a straight line) one must distinguish between the frequency in the interior of the electron and that of the waves that accompany the particle in its motion. I will keep to the first one here because I do not have a sufficiently clear idea of the waves in this case.
So far as the internal frequency is concerned, if this has the value ν0 for an electron at rest, then when the electron moves with a velocity ν its frequency will amount to
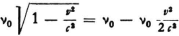
according to relativity theory. Probably one can hardly do anything else but take 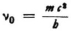 . The frequency then becomes
. The frequency then becomes
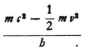
According to experiment the electron can only cause radiation of frequency ν2–ν1 if
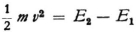
so that the last expression becomes

Now how can a system whose fundamental frequencies are given by (1) be caused to resonate so that it radiates 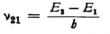 under the influence of a disturbance of frequency (2)? One does not understand it even if one puts Eo = mc2, which is the natural thing to do; and the matter is further complicated by the fact that the electron is flying through the electron28, so that it more quickly feels the oscillation field at different points with its rapid vibrations, so that probably something like a Doppler effect must still be taken into consideration.
under the influence of a disturbance of frequency (2)? One does not understand it even if one puts Eo = mc2, which is the natural thing to do; and the matter is further complicated by the fact that the electron is flying through the electron28, so that it more quickly feels the oscillation field at different points with its rapid vibrations, so that probably something like a Doppler effect must still be taken into consideration.
________________________
You gave me a great deal of pleasure by sending me your note, “The continuous transition from micro- to macro-mechanics”29 and as soon as I had read it my first thought was: one must be on the right track with a theory that can refute an objection in such a surprising and beautiful way. Unfortunately my joy immediately dimmed again; namely, I cannot comprehend how, e.g. in the case of the hydrogen atom, you can construct wave, packets that move like the electron; (I am now thinking of the very high Bohr orbits). The short waves required for doing this are not at your disposal. I already referred briefly to this point in my first letter and should now like to go somewhat further into it. Before that, however, permit me to communicate to you some calculations that were prompted by your note. Maybe the method that I used there can be applied in some case or other.
Since at present we hardly dare hope really to construct the wave packets in more complicated situations, I asked myself the question: if one assumes that there are wave groups that remain permanently confined to a small volume, can one then prove that they have to move in a field of force exactly as an electron would? This could naturally be proved immediately if the assertions of ordinary optics concerning propagation (light rays, group velocity) might be taken over to the present case. But one must be careful with this taking over; as you observe, optics talks about a continuous series of frequencies, but here we have only individual discrete frequencies. Your result already shows that in the case under consideration something else can be derived (and indeed more, namely a wave packet that really stays together permanently), than from the aforesaid optical theorems.
I tested the method first on the linear oscillator and then applied it to the H-atom.
___________________
In the original a calculation requiring 12 pages follows at this point whose result is that a wave packet does not remain intact on a high quantum number orbit in the hydrogen atom and hence cannot be used as a model of an electron.
___________________
This is the reason why it seems to me that in the present form of your theory you will be unable to construct wave packets that can represent electrons revolving in very high Bohr orbits. For we may surely take this much from classical optics*: a wave packet must include very many wave lengths. You had the advantage in your example of the linear oscillator of having arbitrarily short waves at your disposal.
___________________
In your letter you talk about having a certain quantity quadratic in ψ mean the electric charge density (and not perhaps an energy) where you imagine the electron to be “smeared out”. I should just like to ask whether it would not be nice (and desirable) if ∫ ρ d τ were to be a constant, if we are to identify a quantity appearing in the equations as the charge density? That would hardly be allowed to prove right if ρ= 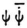 . Would it not be more natural to take ρ as having the value that I denoted by
. Would it not be more natural to take ρ as having the value that I denoted by 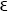 , and called the energy in the preceding calculation?
, and called the energy in the preceding calculation? 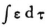 is indeed constant.
is indeed constant.
A second question: can you distinguish between positive and negative charge?
One difficulty, which I already alluded to, is that the V which appears in the formulas (with the term 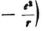 refers only to the field of the nucleus; can one confine himself to this potential if negative charge is also present, either continuously distributed in space or concentrated in an electron? If one alters the term
refers only to the field of the nucleus; can one confine himself to this potential if negative charge is also present, either continuously distributed in space or concentrated in an electron? If one alters the term 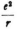 one runs the risk of losing the correct eigenvalues for E.
one runs the risk of losing the correct eigenvalues for E.
These are all obscure points. On the other hand it is once again gratifying that by making 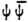 responsible for the emission of radiation, (you could obtain the same result with any quadratic quantity), you already allow the difference tones and the radiated frequencies to appear without the need for any further assumptions (non-linearity of the equations).
responsible for the emission of radiation, (you could obtain the same result with any quadratic quantity), you already allow the difference tones and the radiated frequencies to appear without the need for any further assumptions (non-linearity of the equations).
If you will permit me I should like to conclude with a brief summary of what, in my opinion, can be said about your theory now, so far as it is developed and so far as it can be maintained; I am thinking particularly of the H-atom in this connection. In doing so I put aside the energy packets and also do not talk about the blurring or dissolving of the electron.
1) In the field of the nucleus there can exist oscillating wave states which belong to a definite equation of motion. Rules are given for deriving these from the equations of motion of an electron.
The potential that appears in the equation of motion depends upon the nuclear charge. The charge of the electron has no influence on this potential.
2) The possible wave states have definite (very high) frequencies which are found by considering the boundary conditions (for r = 0 and r = ∞ ). At every point there are definite w and λ, depending on the point but independent of direction.
3) A quantity quadratic in ψ is made responsible for the emission of radiation. As soon as two of the states of motion already mentioned, with frequencies, ν1 and ν2, exist at the same time, this leads to the radiated frequency ν2–ν1 (and to a frequency ν2 + ν1, which is very high and which we are allowed to [or want to] disregard).
Thus far nothing is said about the electron. But it must somehow or other take part in the proceedings as already follows from the fact that the spectrum of an atom is fundamentally changed by the loss of an electron. For that reason I shall still add the following.
4) For any of the states of oscillation mentioned above there are certain specially distinguished lines* characterized by the condition,

for fixed end points, where ω is the velocity of propagation. The specially distinguished curves for the n-th state are precisely the n-quanta orbits of the electrons in the Bohr Theory.
Proof: one can replace (32) by

Now for the n-th state, which we want to consider, E is fixed at En, and in the wave equation
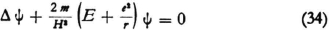
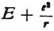 represents the kinetic energy
represents the kinetic energy 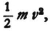 that an electron with the total energy En would have at the point in question.30 If one derives λ from (34) one obtains
that an electron with the total energy En would have at the point in question.30 If one derives λ from (34) one obtains 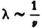 (with a constant coefficient); thus (33) is transformed into the condition that ∫ν d s = 0 , for prescribed En. But this is just the condition that determines the motion of the electron.
(with a constant coefficient); thus (33) is transformed into the condition that ∫ν d s = 0 , for prescribed En. But this is just the condition that determines the motion of the electron.
5) At the same time it can be seen that the specially distinguished curves are closed (ellipses or circles). Now they have the additional property that their circumference, expressed in wave lengths (I mean 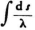 ) is an integer* *.
) is an integer* *.
Proof: From (34) we obtain for the wave length:
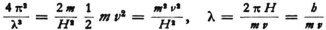
Hence
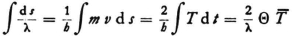
if Θ is the time it takes for the electron to go around once in the orbit under consideration and T is the time average of the kinetic energy. But for motion in a Kepler ellipse the theorem

is valid if E is the energy, (where the potential energy is zero at infinity.) We must therefore calculate
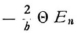
and can do this for a circular orbit, since the time of revolution Θ is the same for all n-quanta orbits, whether they are circles or ellipses. Now for a circular orbit of radius rn,
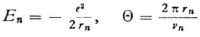
so that our expression becomes
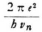
Now, since νn can be evaluated from the known formula 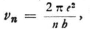 we obtain
we obtain
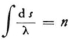
6) For some reason or other* the electron can move only along the specially distinguished curves. In connection with this we remain somewhat uncertain as to what the electron will do if two of the states of oscillation exist at the same time.
As you see, what has just been said comes close to de Broglie’s arguments. As compared to him you have made the advance of setting the wave states clearly before us, and that is an important step.
Nevertheless, if we have to give up wave packets and with them one of the basic ideas of your theory, the transformation of classical mechanics into a wave mechanics, something would be lost that would have been very beautiful. I should be very pleased if you could find a way out of this.
For the rest, I would be very satisfied if one could get as far with several other cases (relativistic correction, relative motion of the nucleus, Stark and Zeeman effects) as with the Balmer spectrum as summarized in 1 - 6 above.
With kind regards,
Yours faithfully,
H. A. Lorentz
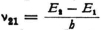 . Such transitions might occur rarely but the radiation is actually the main thing when each individual quantum jump does occur.* But if your interpretation (radiation as a difference tone) can be carried through successfully, and if we then no longer have to think of the radiation of just the quantity of energy E2–E1 I will also be satisfied.
. Such transitions might occur rarely but the radiation is actually the main thing when each individual quantum jump does occur.* But if your interpretation (radiation as a difference tone) can be carried through successfully, and if we then no longer have to think of the radiation of just the quantity of energy E2–E1 I will also be satisfied.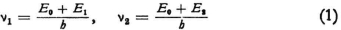
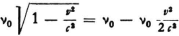
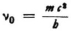 . The frequency then becomes
. The frequency then becomes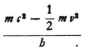
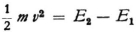

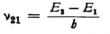 under the influence of a disturbance of frequency (2)? One does not understand it even if one puts Eo = mc2, which is the natural thing to do; and the matter is further complicated by the fact that the electron is flying through the electron
under the influence of a disturbance of frequency (2)? One does not understand it even if one puts Eo = mc2, which is the natural thing to do; and the matter is further complicated by the fact that the electron is flying through the electron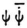 . Would it not be more natural to take ρ as having the value that I denoted by
. Would it not be more natural to take ρ as having the value that I denoted by 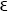 , and called the energy in the preceding calculation?
, and called the energy in the preceding calculation? 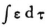 is indeed constant.
is indeed constant.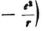 refers only to the field of the nucleus; can one confine himself to this potential if negative charge is also present, either continuously distributed in space or concentrated in an electron? If one alters the term
refers only to the field of the nucleus; can one confine himself to this potential if negative charge is also present, either continuously distributed in space or concentrated in an electron? If one alters the term 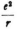 one runs the risk of losing the correct eigenvalues for E.
one runs the risk of losing the correct eigenvalues for E.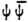 responsible for the emission of radiation, (you could obtain the same result with any quadratic quantity), you already allow the difference tones and the radiated frequencies to appear without the need for any further assumptions (non-linearity of the equations).
responsible for the emission of radiation, (you could obtain the same result with any quadratic quantity), you already allow the difference tones and the radiated frequencies to appear without the need for any further assumptions (non-linearity of the equations).

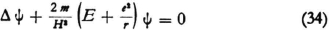
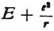 represents the kinetic energy
represents the kinetic energy 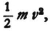 that an electron with the total energy En would have at the point in question.
that an electron with the total energy En would have at the point in question.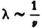 (with a constant coefficient); thus (33) is transformed into the condition that ∫ν d s = 0 , for prescribed En. But this is just the condition that determines the motion of the electron.
(with a constant coefficient); thus (33) is transformed into the condition that ∫ν d s = 0 , for prescribed En. But this is just the condition that determines the motion of the electron.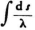 ) is an integer
) is an integer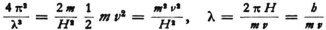
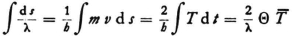

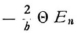
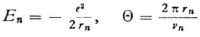
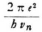
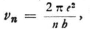 we obtain
we obtain