Tectonic Processes and Erosion
9.1 Introduction
It is now widely accepted that the compositions of the atmosphere and world ocean are dynamically controlled. The atmosphere and the ocean are nearly homogeneous with respect to most major chemical constituents. Each can be viewed as a reservoir for which processes add material, remove material, and alter the compositions of substances internally. The history of the relative rates of these processes determines the concentrations of substances within a reservoir and the rate at which concentrations change. Commonly, only a few processes predominate in determining the flux of a substance between reservoirs. In turn, particular features of a predominant process are often critical in controlling the flux of a phase through that process. These are rate-controlling steps.
Weathering and erosion of bedrock are fundamental to the geochemical cycles that control the composition of the atmosphere, the oceans, and sedimentary rock. Consequently, identification of rate-controlling aspects of the erosion process is crucial to the analysis of global biogeochemical cycles. This chapter sketches how rates of erosion are controlled at different spatial and temporal scales, starting with the surfaces of mineral grains and expanding to whole continents. Most of the discussion focuses on how tectonic processes, continental freeboard, climate and the susceptibility of various lithologies to erosion influence the rate of continental denudation. Furthermore, to understand the development of the Earth through time, atmospheric properties that might affect erosion rates, such as temperature, moisture availability, oxygen and carbon dioxide partial pressure, etc., are examined.
The world today is in a state of exceptionally rapid transition. Two phenomena are involved. One is the rapid oscillations between a glacial and an interglacial climate mode over roughly 100000-year intervals with transitions perhaps as short as decades. The other is the arrival of humans and the development of a technology fueled by geologic and biosphere energy sources. Both phenomena are responsible for tremendous shifts in geochemical cycles away from a dynamic equilibrium. In understanding rate-controlling steps we can better understand how rapid transitions affect the compositions of the ocean and atmospheric reservoirs.
Most of the examples in the following discussion are from the humid tropics. There are several fundamental reasons for focusing on the humid tropics as a study environment. Weathering and erosion are important to global geochemical cycles because of the chemical reactions that occur during weathering. Most of these reactions involve water, and although some are simple inorganic reactions, many are biologically mediated. The high temperatures, moist conditions, and luxuriant vegetation of this zone are ideal for rapid chemical weathering. Moreover, erosional processes in the humid tropics are particularly important on a global scale. According to Meybeck (1979), the humid tropics presently occupy about 25% of the Earth’s land surface and supply about 65% of the dissolved silica and 38% of the ionic load delivered by rivers to the ocean. Data compiled by Milliman and Meade (1983) and Milliman and Syvitski (1992) indicate that the same region contributes about 50% of the total river solid load to the ocean. These studies demonstrate that the bulk of this material is derived from active orogenic belts and island arcs. Finally, the equatorial region was least affected by the climatic fluctuations of the ice ages.
The study of chemical weathering in drier and cooler regions of the Earth is beset by numerous complications and ambiguities. In dry regions, chemical weathering is very slow, and commonly many of the characteristics of landforms and soils seem to have been inherited from earlier, moister times. Moreover, materials derived from chemical weathering and atmospheric deposition often precipitate out as mineral deposits in soils. Cooler climates have been strongly affected by the repeated glaciations of the Pleistocene. In glaciated areas, soils are exceedingly young, and soils of adjacent nonglaciated regions were affected by a variety of periglacial processes. Still wider regions near ice sheets were veneered with loess.
This chapter examines climatic and tectonic controls on erosion in the tropics and the implications of these observations regarding the composition of erosion products in general. The role of glaciations in continental denudation will then be examined and contrasted with tropical conditions. Finally, we will briefly examine human effects.
9.2 Erosion, a Capsule Summary
The energy that powers terrestrial processes is derived primarily from the sun and from the Earth’s internal heat production (mostly radioactive decay). Solar energy drives atmospheric motions, ocean circulation (tidal energy is minor), the hydrologic cycle, and photosynthesis. The Earth’s internal heat drives convection that is largely manifested at the Earth’s surface by the characteristic deformation and volcanism associated with plate tectonics, and by the hot-spot volcanism associated with rising plumes of especially hot mantle material.
Erosion is the process that tears down the subaerial landforms constructed by crustal deformation and volcanism. Matter derived from the earth’s crust generally moves from high elevation on land to low elevations in the ocean along pathways (rivers, glaciers, wind etc.) that are often long and complex. There are many pauses on the way (e.g., colluvial, alluvial, aeolian, lacustrine, and glacial deposits and lake and groundwaters), during which compositions can change. Erosion is typically most rapid in mountainous regions and coastal areas, and is slowest in flatlands. Matter is transported largely by rivers and to a lesser extent by winds and glaciers. During erosion, crustal material is initially mobilized by weathering.
9.2.1 Weathering and Crustal Breakdown
Weathering involves the chemical and physical breakdown of bedrock through its interaction with the hydrologic and atmospheric cycles. Chemical weathering is the breakdown of bedrock by chemical reactions. The products may include dissolved solids and new minerals (usually clays). Physical weathering is the physical breakdown of unweathered or partially chemically weathered bedrock; some old (primary) minerals remain intact. During erosion, these two types of weathering frequently operate in tandem. Chemical weathering weakens the rock; physical weathering finishes it off, making it available for transport processes. There are a few “rules of thumb”:
1. Chemical weathering is more important in warm moist regions, whereas physical weathering is more important in cold dry areas.
2. Contributions made by chemical weathering are greater in regions where there is much vegetation.
3. Contributions made by physical weathering are much greater in steep terrains (i.e., more primary minerals remain), and overall weathering rates are higher.
4. Equivalent igneous and metamorphic lithologies appear to weather about twice as rapidly in island-arc and younger volcanic terrains as compared to old cratonic settings (Stallard, 1995b).
The compositions of dissolved and solid erosion products are initially determined by the stability of the bedrock minerals at the site of weathering. The composition will change during transport to the ocean as the result of further weathering during storage in sedimentary deposits or during authigenic mineral formation in lakes, alluvial soils, and ground waters.
All minerals can be weathered chemically. The susceptibility of minerals varies considerably, however. This is illustrated nicely by the relative mineral stability for weathering under tropical conditions (Table 9-1). Note how stability appears to be closely related to composition. Similar mineral groups cluster together. For igneous and metamorphic minerals, stability is almost the reverse of the Bowen’s reaction series (Goldich, 1938); the first minerals to crystallize out of a magma are the most susceptible to weathering. Note also that the vulnerability to chemical weathering of ionically bonded chemical sediments is in reverse order to the typical marine evaporite sequence (see Holland, 1974).
Table 9-1
Mineral stability in tropical soilsa
| MOST STABLE | |
| Quartz > | Pure silica |
| K-Feldspar, micas > | Igneous and |
| Na-Feldspar > | metamorphic |
| Ca-Feldspar, amphiboles > | alumino- |
| Pyroxenes, chlorite > | silicates |
| Dolomite > | Carbonate |
| Calcite > | minerals |
Gypsum, anhydrite 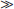 |
Evaporite |
| Halite | minerals |
| LEAST STABLE |
aaAdapted from Stallard (1985). Na-feldspar + Ca-feldspar = plagioclase.
Minerals can weather congruently to produce only dissolved weathering products or incongruently to produce both dissolved cations (Na+, K+, Mg2+, Ca2+), silica (Si(OH)4), and sometimes other constituents along with solid products that are cation-depleted and usually silica-depleted. Common minerals that always weather congruently are halite, anhydrite, gypsum, aragonite, calcite, dolomite, and quartz. Halite, anhydrite, and gypsum are so unstable that they almost never occur in the solid load of rivers. Calcite and dolomite sometimes occur in alkaline rivers that are supersaturated with respect to these minerals, for example, the Yellow River of China. Quartz is the most persistent of all common primary minerals and occurs in most river sediments. Minerals that contain iron and aluminum usually weather incongruently to produce clays and iron/aluminum sesquioxides (oxides and hydroxides of Fe3+ and Al3+). Magnesium, potassium, and to a lesser extent calcium and sodium are retained in cation-rich clays such as the smectites, vermiculites, illites, and chlorites.
The most stable minerals are often physically eroded before they have a chance to chemically decompose. Minerals that decompose contribute to the dissolved load in rivers, and their solid chemical-weathering products contribute to the secondary minerals in the solid load. The secondary minerals and the more stable primary minerals are the most important constituents of clastic sedimentary rocks. Consequently, the secondary minerals of one cycle of erosion are often the primary minerals of a subsequent cycle. When a weakly cemented sedimentary rock consisting of chemically stable minerals is exposed to weathering, it often breaks down physically, and although erosion might be very rapid, the solid products undergo little chemical alteration. If physical erosion processes are not very intense, most of the erosion products are dissolved, and stable primary minerals and secondary minerals will accumulate over the bedrock to form soil. Additional details on mineral transformations by weathering are provided in Chapter 7.
9.2.2 Weathering and the Atmospheric Gases
It is chemical weathering that makes continental denudation so important to geochemical cycles. Solution transport by rivers into the ocean is the largest single flux of many elements into the seawater reservoir. Carbon dioxide and molecular oxygen, compounds of fundamental biogeochemical importance, are consumed by weathering reactions involving primary minerals. The rate of consumption by weathering reactions, although much slower than the rate of the cycling through organisms, may be important in controlling the long-term concentrations of CO2 and O2 in the atmosphere and ocean (Garrels and Lerman, 1981; Berner et al., 1983).
The consumption of CO2 during weathering is indirect. Most members of two important classes of minerals, the silicates, and the carbonates, consume hydrogen ions and release alkali (Na+, K+) and alkaline-earth (Ca2+, Mg2+) cations during weathering. The primary proton donor is carbonic acid (H2CO3), formed by the hydrolysis of carbon dioxide. An accumulation of carbonate and bicarbonate ions in solution results. Organic acids, which are photosynthetic derivatives of CO2 are often important in shallow soil horizons, and mineral acids (sulfuric, nitric, hydrochloric) from atmospheric, biological, or rock-weathering sources also can be important. The net effect of the reactions is to consume protons and, when weak acids are the proton donors, to produce alkalinity. Carbon dioxide is returned to the atmosphere primarily by carbonate deposition, metamorphic reactions, volcanism, and mid-ocean ridge hydrothermal circulation.
Oxygen is consumed largely by the oxidation of reduced iron in silicates and sulfides, of sulfur in sulfides, and of organic carbon in sedimentary rocks. Oxygen is returned to the atmosphere as a result of biological fixation of reduced carbon, sulfur, and iron and the subsequent burial of these compounds. Not all chemical weathering reactions involve CO2 or O2. Certain minerals, such as quartz (SiO2) and the major evaporite minerals (NaCl, CaSO4, and CaSO4·XH2O) dissolve without reacting with anything except water.
Atmospheric CO2 and O2 are also coupled through the formation and burial of and exhumation and weathering of organic carbon. When carbon dioxide is converted by photosynthesis to organic matter oxygen is released. If the organic matter is buried, then O2 cannot recombine with the carbon to make CO2, and the process is a net source of O2 to the atmosphere. When buried carbon is reoxidized through weathering or burning, then O2 is consumed and CO2 is released. The burning of fossil fuels represents a tremendous artificial acceleration of this release.
9.3 Soils and the Local Weathering Environment
The initial partitioning of elements into dissolved and solid phases during continental denudation is controlled by the chemical and physical processes associated with soil genesis. The description of weathering processes as they occur in soil profiles is discussed in Chapter 7. A vast body of observational data, not to be reviewed here, has shown that soils are remarkably complex on the local scale. Soil systems are neither physically nor chemically homogeneous. Both solid and dissolved matter is washed down through soil profiles; capillary action operating in tandem with evaporation can lead to upward transport of dissolved material; frost action, root growth, tree falls, and burrowing can mix material within a profile; transport of fluids in soils on non-porous substrates is largely lateral; soil porosity is controlled by both chemical and physical processes; freezing can have profound effects on the structure of soil and the composition of residual fluids; biochemical processes generate many thermodynamically unstable reactants; the chemical activities of many chemical species (e.g., H2O, CO2, O2) change tremendously on time scales that are infinitesimally short geologically (minutes to days); and reactions frequently occur in thin films and on grain surfaces.
9.3.1 Weathering Reaction Kinetics
It was once said that one of the reasons why weathering reaction kinetics has not been studied in more detail is that the reactions are too slow to be studied by graduate students. In recent years, great advances have been made in modeling, experimental design, and in examining mineral surfaces (White and Brantley, 1995). Quantum tunneling microscopy has permitted the examination of the placement of atoms on reactive surfaces permitting the testing of molecular-scale models of reaction chemistry. The resolution of electron microscopy has also improved as have the type and quality of tandem chemical measurements. Physically and chemically based models can now represent aspects of hydrology and chemical weathering, but as yet, integrated models that capture weathering processes in a small watershed do not exist. One factor is the incredible complexity of soil, in part imposed upon it by biological processes and season changes in temperature and hydrology.
Several simple experimental systems that simulate some aspect of the groundwater environment have been used to study the breakdown of individual minerals. These kinetics studies have encompassed quartz (Brantley et al., 1986; Dove, 1995), feldspars (Blum and Stillings, 1995), pyroxenes and amphiboles (Brantley and Chen, 1995), carbonates (Berner, 1978), and glasses (White, 1983). Simultaneously, theoretical studies of weathering processes have advanced considerably (Casey and Ludwig, 1995; Lasaga, 1995; Nagy, 1995). Relative stability observed in laboratory weathering is consistent with field-based observations; however, experimental rates appear to be faster than those in natural systems. In situ studies (White, 1995) and complex models (Sverdrup and Warfvinge, 1995) have tried to bridge the differences between field and laboratory.
Studies of mineral grains from laboratory experiments and from natural soils, using electron microscopy and the recently developed atomic force microscopy, provide some interesting observations about reaction mechanisms and rate controls during weathering. A continuum of rate-limiting mechanisms can be defined between reactions that are transport (diffusion) controlled and those that are surface reaction controlled (Berner, 1978). In the former case, reactions are limited by diffusion of solution products away from the crystal surfaces. This situation occurs where reactions are sufficiently rapid that fluid concentrations near the primary mineral are close to equilibrium values for the driving reaction, which need not be the final equilibrium for the system. Under a microscope, grain surfaces appear smooth with rounded edges (Fig. 9-1). In the latter case, reaction rates are controlled by local surface energy on the crystals. Surface reaction control is important under circumstances where surrounding fluids are decidedly undersaturated relative to the equilibria that are driving the weathering reaction. Surfaces appear rough and pitted, commonly on zones of obvious crystallographic defects (Fig. 9-1, see also Gilkes et al., 1980; Brantley and Chen, 1995; Hochella and Banfield, 1995). Most studies of primary minerals in natural soils show extensive pitting which is suggestive of surface reaction control.
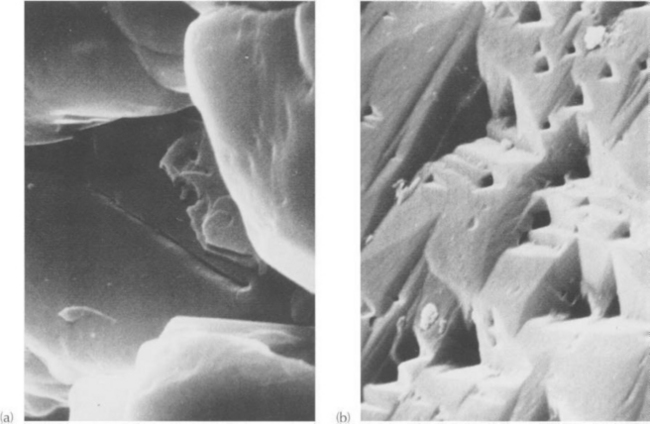
Fig. 9-1 Electron micrographs of quartz grains from a soil profile described in (a) Stallard (1985) and (b) Brantley et al. (1986). The quartz grains in (a) are from a sample close to fresh bedrock near the bottom of the profile. The sample in (b) is from near the top of the profile. Note that the surfaces of the grains in the left-hand photograph are smooth, whereas the right-hand grains are deeply etched. Presumably the etched grains are dissolving in soil waters that are strongly undersaturated with respect to quartz. Such etching indicates that reaction rates are affected by variations in surface energy. The smooth grains are probably dissolving at or close to equilibrium conditions.
There are several important caveats to the use of this observational model. There is, for example, a considerable range of undersaturation, down to 50% in the case of quartz, where surface defects are not especially reactive, and crystals appear smooth (Brantley et al., 1986). The formation of weathering rinds and the build-up of clays and other secondary weathering products in the immediate vicinity of the weathering grains can strongly affect the transport processes. Finally, in soils, fluid compositions change rapidly and a wide range of reactions may be important during weathering (Drever and Smith, 1978; Eberl et al., 1986; Herbillon and Nahon, 1988; Trolard and Tardy, 1989; van Breeman, 1988a,b).
9.3.2 Variable Chemistry of Soil Fluids, a Complicating Factor
Weathering rates are most sensitive to the throughput of water. In soils, this is a decidedly discontinuous process. Typically, water flows through soil following rainfall or snowmelt. Once saturated, the flux of water is largely dependent on the physical properties of the soil and not on the rate of supply. Water that cannot be accommodated by flow through the soil, because of soil saturation or high rate of supply, is rerouted into overland runoff and interacts minimally with primary minerals. Following wetting, typical soils drain rapidly. At most times, soils are drying. This scenario is evident in discharge curves for small river catchments, where precipitation and snowmelt events show up as spikes over a very long background discharge which reflects slow groundwater inputs.
Groundwater environments can be represented as a simple flow-through system. For the situation where chemical weathering of mineral grains is transport controlled, the weathering rate of a mineral should be directly dependent on the rate of throughput of water. For the situation where rates are controlled by surface reactions, weathering rate should be independent of the rate of throughput of water.
Soil water flow is decidedly episodic. During dry times the water solutions in the soil are probably fairly concentrated and not very reactive. Time-averaged reaction rates should be roughly proportional to the fraction of time reacting minerals are in contact with thermodynamically undersaturated (and reactive) water. In a study of the relationship between denudation rate and runoff for rivers draining igneous and metamorphic rock in Kenya, Dunne (1978) obtains the relationship of (denudation rate in tons/km2 per year) = 0.28 (runoff in mm/year)0.66.
The fact that soils dry between episodes of water flow complicates weathering reaction scenarios. During drying, solute concentrations in water films increase, and the areal extent of the films decrease. The chemical activity of water drops. As a result, chemical equilibria that might be important for controlling weathering reactions within wet soils are replaced by new equilibria reflecting the elevated concentration of solutes. A different suite of clays and sesquioxides might become stable and silica (opal), calcite, and various evaporite minerals can precipitate. During subsequent wetting, both primary minerals and secondary minerals formed under drying conditions may react. Features, such as etch pits may form during episodes of wetting and thermodynamic instability, even if they would not normally form under average or typical conditions. Moreover, secondary minerals that formed under dry conditions may persist through wetting cycles. The formation of calcium carbonate nodules in soils, caliche, is an example of this. The mineral constituents of such soils may not at equilibrium. The overall rate of chemical and physical weathering and the composition of weathering products changes with increasing runoff (see models in Stallard, 1995a,b). At low runoff, eroded solids are cation-rich and the ratio of silicon to cations in solutes is low. At high runoff, eroded solids are cation-depleted and silicon forms a large fraction of the solute load.
Freezing, which also produces residual fluids with elevated concentrations of dissolved solutes, presumably does not have as significant an effect as drying because lower temperatures are involved. Freeze–thaw cycles, however, can break apart rocks and expose fresh mineral surfaces.
9.3.3 Local Responses to Atmospheric Variables
The influence that variations of temperature and levels of atmospheric CO2 and O2 have on chemical weathering are more subtle. Temperature appears to have a direct effect on weathering rate (White and Blum, 1995). The silica concentration of rivers (Meybeck, 1979, 1987) and the alkalinity of ground waters in carbonate terrains (Harmon et al., 1975) are both positively correlated with temperature variations. It is not clear, however, whether temperature-related variations in weathering rates are largely due to variations in vegetational activity that parallel temperature variations.
Partial pressures of O2 and CO2 in soils are controlled largely by soil biology. Oxygen is consumed and CO2 is produced in soils by decay and root respiration. Plausible variations of the atmospheric concentration of these gases have little effect on their partial pressures in soils. The rate of the hydrologic cycle, however, is thought to respond directly to global mean temperature and is therefore indirectly sensitive to the partial pressure of atmospheric CO2, which as a “greenhouse” gas, can affect global mean temperatures (see Berner et al., 1983).
In summary, of all the local variables that can affect weathering rate, the supply of water is clearly the most important. Biology is very important as the supplier of proton donors and complexing agents for weathering reactions, as a mediator in the moisture budget for the soil, and as a controller of soil structure. Beyond suggesting that surface reactions are important in controlling the weathering rate for most minerals and confirming mineral stability sequences, laboratory models of weathering chemistry are just beginning to recognize the importance of soil structure, coatings, and biological processes in controlling weathering rates.
9.4 Slope Processes and the Susceptibility of Lithologies to Erosion
Weathering, atmospheric deposition, and the fixation of atmospheric gases are the ultimate sources of the material transported by rivers. These processes operate over the surface of the river catchment, and the resultant water and weathering products must be transported downslope before arriving in a channel. Examination of erosion processes on hillslopes provides insight as to how chemical weathering rates are controlled at an intermediate spatial scale and how chemical elements are partitioned between the dissolved and solid loads of rivers. Excellent models of watershed hydrology, such as TOPMODEL (Bevin and Kirkby, 1979; Bevin et al., 1995), have developed, and interfacing these with studies of physical, geochemical, and biological processes in soils and on hillslopes should be a major research direction in the future.
The erosion process on slopes can be envisioned as a continuum between the weathering-limited and transport-limited extremes (Carson and Kirkby, 1972; Stallard, 1985, 1995a). Erosion is classified as transport limited when the rate of supply of material by weathering exceeds the capacity of transport processes to remove the material. Erosion is weathering limited when the capacity of the transport process exceeds the rate at which material is generated by weathering. These two styles of erosion represent an interesting parallel to controls of weathering reaction rates on mineral surfaces, discussed earlier, wherein a similar continuum was defined between surface-reaction control and transport (diffusion) control (Stallard, 1988).
The weathering and transport processes associated with either end of the continuum are quite different. Where erosion is weathering limited, erosion rate is controlled by the rate at which chemical and physical weathering can supply dissolved or loose particulate material. In essence, erosion rates are controlled by susceptibility to weathering. Soils are thin, because loose material moves rapidly downslope. Much of this material is only partially weathered, because most rocks lose their structural integrity before they are completely chemically decomposed. Processes characteristic of weathering-limited regions include rockfalls, landslides, or anything that tends to maintain a fresh or slightly weathered rock surface (Table 9-2). These processes often operate at a threshold slope angle. In humid climates, weathering-limited conditions are associated with thin soils and steep straight slopes which often undergo parallel retreat at a threshold angle (Fig. 9-2).
Table 9-2
Erosion regimes, features, and processes associated with transport-limited and weathering-limited erosion
| Transport limited | Weathering limited |
| Thick soils | Thin or no soils |
| Slight slopes that are convexo-concave | Steep slopes that are straight and at a threshold angle |
| Erosion rates | Erosion rates |
| independent of lithology | depend on bedrock susceptability |
| Processes: | Processes: |
| soil creep | rock slides |
| removal of dissolved phases | strong sheet wash |
| soil avalanches | |
| removal of dissolved phases |
Weathering limited: potential transport processes greater than weathering supply. Transport limited: supply by weathering greater than the capacity of transport processes to remove material (until weathering is slowed by feedback).

Fig. 9-2 Photographs illustrating (a) long straight slopes in the Andes and (b) convexo-concave slopes transitional into very flat terrain. The view of the Andes is taken from Huayna Picchu, the small peak next to Machu Picchu. The view is up the valley of a small tributary to the Urubamba River. Note that the V-shaped valley formed by fluvial erosion becomes a U-shaped glacial valley at its highest end. The glacier was active during the last ice age. (b) The Guayana Shield in southern Venezuela, taken from an airplane. In the foreground is the Orinoco River downriver from the Casiquiare Canal, a natural channel that connects the Orinoco and the Amazon River systems. The hills in the foreground are granite and about 200 m high.
In contrast, under transport-limited conditions, weathering rates are ultimately limited by the formation of soils that are sufficiently thick or impermeable to restrict free access by water to unweathered material. Erosion rates are low, and soils and solid weathering products are cation deficient. In regions where transport-limited erosion predominates, soils are thick and slopes are slight and convexo-concave (Fig. 9-2). With time, these slopes tend towards increasing flatness. Soil creep is a process typical of transport-limited situations. Most soil mass movement and wash processes, however, are intermediate between weathering limited and transport limited in character.
Erosion associated with chemical weathering caused by circulating soil/ground water is intermediate between being transport limited and weathering limited in character. Chemical erosion would be transport limited whenever reactions at the mineral-grain level are also transport limited. As discussed earlier, this occurs when the flushing rate for water is sufficiently slow that equilibrium is reached with respect to some controlling reaction. Chemical weathering of carbonate rocks is probably transport limited as soil and ground waters are nearly saturated with respect to carbonate minerals (Holland et al., 1964; Langmuir, 1971). In the case of silicate weathering, transport-limited erosion would occur where the silicates are particularly unstable or where water movement is restricted by low porosities or lack of hydraulic head in soils or bedrock.
9.4.1 Soils, Slopes, Vegetation, and Weathering Rate
For a given set of conditions (lithology, climate, slope, etc.), there is presumably an optimum soil thickness that maximizes the rate of bedrock weathering (Fig. 9-3) (Carson and Kirkby, 1972; Stallard, 1985). For less than optimum soil thicknesses, there is insufficient pore volume in the soil to accept all the water supplied by precipitation and downhill flow. Excess water runs off and does not interact with the subsurface soil and bedrock. In contrast, water infiltrates and circulates slowly through thick soils (especially where forested). If profile thicknesses greatly exceed the optimum, long residence times for water at the base of the profile lead to a reduction of weathering rate as a result of equilibration with respect to secondary phases. With increasing thickness, soil profiles also become more structured with definite horizons. Some of these can be rather impermeable. Water is routed through weathered material, and weathering rates are thereby reduced.
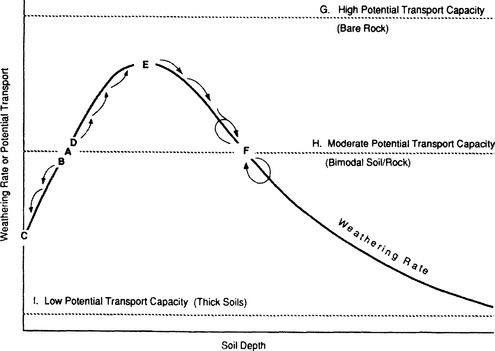
Fig. 9-3 Conceptual model to describe the interaction between chemical weathering of bedrock and downslope transport of solid erosion products. It is assumed that chemical weathering is required to generate loose solid erosion products of the bedrock. Solid curve portrays a hypothetical relationship between soil thickness and rate of chemical weathering of bedrock. Dotted lines correspond to different potential transport capacities. Low potential transport capacity is expected on a flat terrain, whereas high transport is expected on steep terrain. For moderate capacity, C and F are equilibrium points. (Modified with permission from R. F. Stallard, River chemistry, geology, geomorphology, and soils in the Amazon and Orinoco basins. In J. I. Drever, ed. (1985), “The Chemistry of Weathering,” D. Reidel Publishing Co., Dordrecht, The Netherlands.)
Figure 9-3 portrays a hypothetical model of how chemical weathering and transport processes interact to control soil thicknesses. The relationship between soil thickness and rate at which chemical weathering can generate loose solid material is indicated by the solid curve. The rate at which transport processes can potentially remove loose solid weathering products is indicated by horizontal dotted lines. The rate of generation by chemical weathering initially increases as more water has the opportunity to interact with bedrock in the soil. As soil thickens, the optimum thickness for maximum rates of chemical weathering is exceeded, and weathering rates decrease with increasing soil thickness (E-F). The decrease presumably occurs because the rate of chemical weathering is limited by diminished access of reactive waters to unweathered materials. Recent work by Heimsath et al. (1997) indicates that an exponential decrease in weathering rate with increasing soil thickness is reasonable. If the potential transport rate exceeds weathering rate, loose soil material should not remain on the slope. If weathering rate exceeds potential transport rate, soils develop. As soils develop, weathering rates vary, leading to an evolution of the soil profile.
For soil profiles that are less than the optimum thickness, there is a destabilizing feedback between soil thickness and weathering rate. Assume that a thin soil is in a dynamic equilibrium such that weathering inputs balance transport losses (A in Fig. 9-3). Weathering rate can be reduced relative to transport rate by either increasing the strength of transport processes or by thinning the soil (B). Either way, transport removal would exceed weathering supply, and the soil would continue to thin. Eventually only a hard cohesive saprolite or bedrock would remain (C). If the soil is thickened, or if the capacity of transport processes is reduced, the soil would tend to accumulate (D–E). Finally, a situation involving stabilizing feedback occurs (F), and a thick soil forms such that transport of loose material balances weathering inputs. If soil thickens beyond point F, weathering rates decrease, and transport processes restore the soil thickness back to the stable value. This model suggests that soil distributions should be distinctly bimodal: either thin, weathering limited (G), or thick, transport limited (I). For some intermediate values of potential transport rate (H), either hard rock or a moderately thick soil could exist.
When soil thickness is at the stable value (F), erosion is transport limited. Chemical weathering is also transport limited. This is, however, not because of reaction kinetics; instead this limitation is primarily controlled by physical factors, most probably, restricted access of water to the primary minerals.
The effects of vegetation are complex. Vegetation reduces short-term physical erosion by sheltering and anchoring soils. This effect is equivalent to reducing potential transport in Fig. 9-3. A cover of vegetation does not necessarily reduce denudation rates. Vegetation can maintain a layer of soil on steep slopes, particularly under wet conditions. As the soil thickens, it often becomes unstable, detaches, and slides down the slope (Garwood et al., 1979; Pain, 1972; Scott, 1975a; Scott and Street, 1976; Stallard, 1985; Wentworth, 1943; Larsen and Simon, 1993; Larsen and Torres Sánchez, 1998) (Fig. 9-4). Under such circumstances, weathering rates can be exceptionally high because of the extra moisture and bioacids; likewise, denudation rates are very high because of the continuous resupply of fresh rock. The effect of erosion following fires, tree falls, and land clearing on slopes is similar to that of slides (McNabb and Swanson, 1990; Meyer et al., 1995; Scott, 1975b; Stallard, 1985, 1995a, 1998). On slight slopes, over extended periods of time (up to millions of years), vegetation may reduce weathering rates by allowing very thick soils to accumulate. For a given soil thickness, however, weathering should be faster because of the supply of bioacids.

Fig. 9-4 Photograph of landslides (soil avalanches) that occurred following earthquakes in Panama on July 17, 1976, near Jaque. In the background is a bay of the Pacific Ocean. The effects of this earthquake are described by Garwood et al. (1979), who estimated that about 42 km2 (about 10%) of the region near the epicenter of the earthquake was devegetated. The bedrock is mostly island-arc basalts and andesites. (Photography by N. C. Garwood.)
9.4.2 Elemental Partitioning: The Role of Slope Processes
There are two principal ways to selectively partition different elements between the dissolved and solid loads in rivers: by selective chemical weathering of particular primary minerals, and by the formation of secondary phases that are enriched or depleted in certain elements, relative to bedrock (Stallard, 1985, 1995a).
Different styles of erosion are associated with different degrees of partitioning of elements between dissolved and solid load. As rocks weather chemically, they lose their structural integrity. When only kaolinite, gibbsite, or other cation-depleted phases form, as commonly happens with transport-limited erosion, cation ratios in solution should match those in bedrock. During weathering-limited erosion, unstable primary minerals are selectively removed, causing elemental partitioning. For example, in moist vegetated areas on crystalline rocks or indurated sediments, solifluction, soil avalanching, and sheet runoff remove weakly cohesive material (solum and soft saprolite), leaving a cohesive hard saprolite behind (Simon et al., 1990; Stallard, 1985; Stallard and Edmond, 1983). Where transport processes are sufficiently intense, more stable minerals such as zircon, quartz, potassium feldspars, and micas survive chemical weathering and are eroded. Some of these resistant minerals contain substantial K and Mg, but none contain much Na or Ca. Thus, K and Mg are enriched relative to Na and Ca in solid erosion products; Na and Ca are enriched in solution relative to K and Mg (Fig. 9-5). If Mg is incorporated into the lattice of many secondary clays, as often happens, this further accentuates its retention in bulk solids (Stallard et al., 1991).
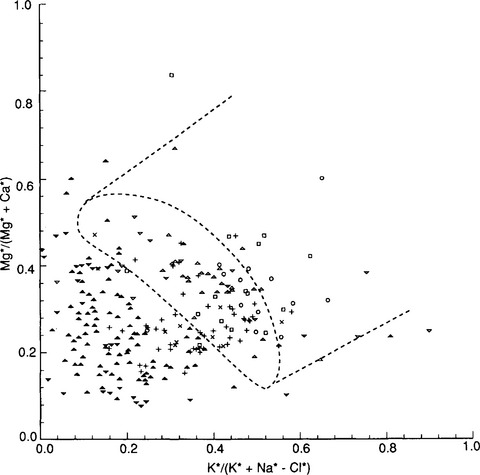
Fig. 9-5 Relation of Mg2+/(Mg2+ + Ca2+) to K+/(K+ + Na+ – Cl−) for dissolved material in surface waters of the Orinoco River and Amazon River basins, the Isthmus of Panama, and the Island of Taiwan and for rock types representative of bedrock in these basins. Symbols:  , samples from rivers that drain mostly mountain belts developed on felsic continental crust;
, samples from rivers that drain mostly mountain belts developed on felsic continental crust;  , samples from rivers that drain mostly island-arc mountain belts developed on mafic oceanic crust; +, samples from rivers that originate in continental mountain belts, but that drain large areas of craton; ×, rivers that drain alluvial and nonalluvial sedimentary rocks in foreland and intracratonic basins; ◻, rivers that drain only alluvial sediments in foreland and intracratonic basins;
, samples from rivers that drain mostly island-arc mountain belts developed on mafic oceanic crust; +, samples from rivers that originate in continental mountain belts, but that drain large areas of craton; ×, rivers that drain alluvial and nonalluvial sedimentary rocks in foreland and intracratonic basins; ◻, rivers that drain only alluvial sediments in foreland and intracratonic basins;  , rivers that drain hilly to mountainous shield on felsic crust;
, rivers that drain hilly to mountainous shield on felsic crust;  , rivers that drain hilly to mountainous shield on mafic crust or deeply eroded island arc;
, rivers that drain hilly to mountainous shield on mafic crust or deeply eroded island arc;  , rivers that drain peneplaned shield. The dashed oval represents the range of analyses for common igneous rocks; the dashed lines that extend away from the igneous-rock field represent the composition field for common sedimentary rocks. (Reproduced with permission from R. F. Stallard, Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck (1988). “Physical and Chemical Weathering in Geochemical Cycles”, pp. 225–246, Kluwer Academic Publishers, Dordrecht, The Netherlands.)
, rivers that drain peneplaned shield. The dashed oval represents the range of analyses for common igneous rocks; the dashed lines that extend away from the igneous-rock field represent the composition field for common sedimentary rocks. (Reproduced with permission from R. F. Stallard, Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck (1988). “Physical and Chemical Weathering in Geochemical Cycles”, pp. 225–246, Kluwer Academic Publishers, Dordrecht, The Netherlands.)
Dissolved phases are assumed to best reflect the weathering processes occurring at the erosion site, because water is not usually stored for long periods (many years) in soils or during fluvial transport. Sediment moves slowly through river systems; the larger the river system the longer sediment takes to move through (Church and Slaymaker, 1989; Meade et al., 1990). Weathering products, however, can weather chemically during transport downslope and through fluvial systems. Solids degrade when they accumulate at the base of slopes (colluvium) or during storage on floodplains (alluvium); this obviously affects dissolved components (Johnsson et al., 1991; Stallard et al., 1991). Soil solutions also evolve as they flow downslope. Contact with fresher materials is prolonged, and evapotranspiration can concentrate solutions (Carson and Kirkby, 1972; Stallard, 1988; Tardy et al., 1973). Both of these phenomena can cause the formation of new suites of clays and sesquioxides, and the precipitation of carbonates.
Aggradation or degradation of biomass or soil reservoirs may also produce effects that appear to be fractionation. This is because the elemental ratios in vegetation or soil reservoirs can be very different from those of bedrock. Sufficiently large and rapid changes in these reservoirs are sometimes evident in river chemistry. For example, the uptake and release of potassium in association with the seasonal growth and loss of leaves can affect the composition of streams that drain temperate deciduous forests (Likens et al., 1977; Vitousek, 1977).
9.5 Landforms, Tectonism, Sea Level, and Erosion
On a larger scale, landscape development reflects those mechanisms that expose bedrock, weather it, and transport the weathering products away. Present and past tectonism, geology, climate, soils, and vegetation are all important to landscape evolution. These factors often operate in tandem to produce characteristic landforms that presumably integrate the effects of both episodic and continuous processes over considerable periods of time.
Many important erosion-related phenomena are episodic and infrequent, such as flash floods, landslides, and glaciations, while others such as orogenesis and soil formation involve time scales that exceed those of major climate fluctuations. In either case, the time scale of human existence is too short to make adequate observations. Consequently, it is difficult to directly estimate the rates or characterize the effects of such phenomena on erosion products. The key to understanding weathering and erosion, on a continental scale, is to decipher the relationship between landforms, the processes that produce them, and the chemistry and discharge of riverborne materials.
To a first approximation, landscape formation processes can be viewed as being controlled by climatic and tectonic factors. Climate delimits where different types of weathering can occur, while tectonics controls how the effects of weathering are expressed. The role of tectonic setting is emphasized in this discussion because tectonic history is often critical to controlling denudation rates and the composition of erosion products. The object of the discussion is not to focus on the particulars of landscape development. Instead, landforms are primarily used to distinguish between different erosional regimes and to identify features that are useful for characterizing denudation and uplift rates, especially indicators of past sea level. The linkage between tectonic processes and landforms is embodied in the concept of a morphotectonic region.
9.5.1 The Effects of Erosional Regime
Many landforms have a convex upper slope, a straight main slope, and a concave lower slope. Carson and Kirkby (1972) argue that the main slope is dominated by weathering-limited processes; whereas, the upper and lower slopes are primarily areas of transport-limited erosion or even deposition in the case of the lower slope. If the overall slope is largely transport limited, it will undergo parallel retreat at the threshold angle. With parallel retreat, topographic form is maintained and characteristic landscapes are thereby generated.
The weathering regime exerts a major control on the production rate and the composition of erosion products from different lithologies (Stallard, 1985, 1988). For weathering-limited conditions, erosion rate is controlled by the susceptibility of the bedrock to weathering. The tectonic history, physical properties (porosity, shear strength, jointing, etc.), and chemical properties of the bedrock exert a major influence. For a given climate, within a specific catchment, each rock type should contribute an amount of material to river transport that is proportional to both its extent of exposure and its susceptibility to weathering. (Deep groundwater transport important in karst terrains would contribute additionally.) Solid erosion products should include abundant partially weathered (cation-rich) rock and mineral fragments. If the bedrock is especially physically unstable, it will contribute strongly to the solid load of rivers; likewise, chemically unstable bedrock will contribute strongly to the dissolved load.
In transport-limited conditions, however, susceptibility is not so important due to the isolating effect of thick soils. In the extreme situation of a flat landscape lowering at a uniform rate, the erosional contribution by a particular rock type should be related only to the area exposed. Solids would be cation-deficient.
9.5.2 The Effects of Deposition, Storage, and Burial
Sediment storage and remobilization involves huge mass fluxes and can respond rapidly to changes in climate and human activities. Accordingly, sedimentation on land may play a major role in the carbon cycle (Stallard, 1998). Sediment-laden rivers flowing over flat terrain commonly develop extensive floodplains. At times, floodplains coalesce into broad depositional alluvial plains such as the Llanos of South America. The sediments in those deposits weather chemically. Less stable minerals in the sediment are broken down and alluvial soils develop. Eventually only the most stable minerals, such as quartz, remain, and the clays are transformed into cation-deficient varieties. Sediment in such rivers, especially the sand, may go through many cycles of deposition, weathering, and erosion before it is transported out of the system. Compositionally, this sediment resembles that derived from transport-limited erosion. Elemental fractionation between the original bedrock and erosion products still occurs because of the permanent burial of some cation-rich material and the uninterrupted transport of much of the fine-grained suspended sediment out of the system (Johnsson et al., 1988, 1991; Stallard, 1985, 1988).
9.5.3 The Effects of Tectonic Processes
Tectonic processes provide the material of which landscapes are made. Tectonism produces uplift followed by erosion, or subsidence followed by deposition. Sediment storage makes the calculation of denudation rates rather time-scale dependent. Sediment deposited at one time can be re-eroded through increases in river discharge caused by wetter climates or lowering of base level, perhaps brought about by uplift. What might be considered eroding bedrock on one scale can be just stored sediment in transit to the ocean on another, longer, scale. In the following sections, tectonically active and tectonically quiet areas are discussed separately to highlight the differences in erosional style between these two types of settings. An important aspect of this difference is the degree to which erosion is influenced by eustatic sea level fluctuations.
To a first approximation, fast tectonic processes occur at plate boundaries (Fig. 9-6). The primary exceptions to this are incipient rifts and the so-called “hot spots” or “mantle plumes,” both of which are associated with uplift and volcanic activity in plate interiors. Collisional plate boundaries are of greatest interest, because these are the sites of the intense faulting, folding, and volcanism associated with the world’s great mountain belts. Most divergent plate boundaries are underwater mid-ocean ridges, and are of no direct concern. Incipient divergent boundaries on land are associated with rift zones and their characteristic volcanism and block-faulted mountains. Transcurrent shearing (neither convergent nor divergent) plate boundaries are characterized by intense seismic activity, but less impressive mountain building. Hot spots are evidenced by local, sometimes intense volcanism, apparently fixed with respect to the mantle and not with the plate upon which it is occurring.
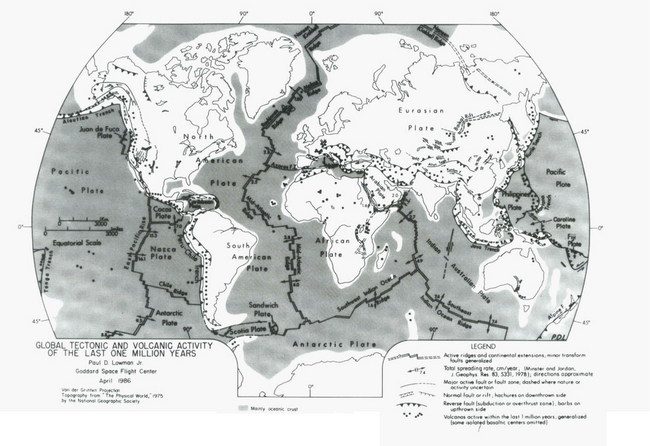
Fig. 9-6 Global tectonic map for the last one million years. (Reproduced with permission from P. D. Lowman, Jr. (1981). Bull. Internat. Assoc. Engineer. Geol. 23, 37–49.)
The term “craton” is generally used to refer to tectonically quiet or stable continental areas. Major subdivisions include “shields” where long-term erosion has exposed extensive areas of old crystalline basement, “platforms” where shields have a flat-lying sedimentary veneer, intracratonic basins where slow long-term subsidence has led to thicker sedimentary deposits on the craton, and “passive continental margins” where continental crust has rifted, separated, cooled, and subsided (Pitman, 1978; Sloss and Speed, 1974). Between cratons and mountain belts there is often a “foreland basin” where basement has slowly subsided as a result of sedimentary loading and tectonic downwarping. Intracratonic basins, passive margins, and foreland basins, represent loci of long-term sediment accumulation and storage (Ronov et al., 1969; Sloss, 1963, 1979; Soares et al., 1978; Vail et al., 1977; Vail and Herdenbol, 1979). Intracratonic basins subside through especially long stretches of geologic time. The Amazon Trough, for example, has been active for all of the Phanerozoic (Soares et al., 1978).
9.5.4 Effects of Sea-Level Change on Erosion
The ocean surface represents the master base level for continental erosion and sedimentation. Given a sufficient period of time, in the absence of tectonic processes, continents would presumably be eroded flat to about sea level. It is not surprising, therefore, that most tectonically quiet areas on continents tend to have low elevations and are often flat, whereas tectonically active areas, mostly mountain belts, have high elevations and steep slopes (Figs 9-2 and 9-4).
High and low stands of sea level are directly recorded as sedimentary coastal onlap sequences and as erosional terraces. These records are complicated in regions of crustal instability, and the rate and nature of crustal deformation determines whether evidence of short-term or long-term sea-level fluctuations are preserved and how easily this evidence is interpreted. Because continental basement warps and fractures through time, and because evidence of sea level is erased by erosion, the interpretation of this evidence to produce sea-level curves for the Phanerozoic has been a subject of considerable debate.
Eustatic sea-level fluctuations occur on a wide range of time scales (Vail et al., 1977; Vail and Herdenbol, 1979). The shortest duration changes appear to be associated with cyclic glacio-eustatic sea-level changes. Amplitudes are on the order of 100 m and appear to involve the superposition of several cycles ranging in period from 20 to 400 kyr. These cycles are related to changes of glacial ice and seawater volume apparently driven by climatic processes sensitive to Earth’s orbital and rotation motions. On a thousand-fold longer time scale are sea-level fluctuations associated with the development of coastal onlap sequences and depositional sequences in intracratonic basins of North America, the Russian Platform, and Brazil. These fluctuations have amplitudes of 100 to 200 m and periods ranging from 10 to 80 Myr. Within regions of sluggish tectonics, the effects of sea-level change on these time scales seem to be especially important in affecting both landscape morphology and the nature of sedimentary deposits. All of the above variations are superimposed on two very long-term sea-level fluctuations that occurred over the last 700 Myr (Fischer, 1983). These have amplitudes of 300–400 m with low stands in Eocambrian and Triassic-Jurassic times. Lows apparently coincide with major episodes of continental break up. Sea level again seems to be approaching such a minimum.
The clearest sedimentary records of sea level change occur where there is a good and steady supply of sediment and where the land is slowly and steadily sinking relative to mean sea level (Pitman, 1978). Areas that commonly satisfy this requirement are passive margins, intracratonic basins, and the foreland basins of major orogenic belts. When sea level rises, thicker sedimentary units are deposited more inland, generating a coastal onlap. With sufficiently high sea level, epeiric seas begin to flood the cratons, often filling foreland and intracratonic basins before spilling out over normally high ground; this is a major transgression. At low sea level, very little deposition occurs on the cratons. Instead, most of the previously deposited sediments are removed by erosion and redeposited along the continental margins. Passive margins seem to have the most easily interpreted subsidence history, but the longest records are found in intracratonic basins. In the tectonically active areas, deformation and subsidence are typically too rapid to preserve information about eustatic sea level.
Several investigators have argued that there is a major tectonic component to the subsidence histories of passive margins and intracratonic basins. Sloss and Speed (1974) and Sloss (1979) note that subsidence episodes appear to be globally synchronous and coincident with high sea levels. They argue that simple sedimentary loading caused by deposition at times of high sea levels is not an adequate explanation and suggests that some common deep driving mechanism controls both sea level and subsidence.
In regions where land is steadily rising relative to mean sea level, the effects of sea-level fluctuations are sometimes recorded as erosional features on land. Whenever the rate of sea-level rise matches the rate of uplift, there is an apparent sea level still stand. Both deposition and erosion are controlled by this almost fixed base level, and a terrace may form. If sea level falls and again rises, the terrace will have risen sufficiently so that it is preserved upslope. Episodic uplift can produce terraces, but these should not be synchronous with similar terraces developed in other regions, unless episodes of uplift were being controlled by some deep-seated process. The best-formed terraces from the late Pleistocene–Holocene sea-level fluctuations occur on coasts where uplift has been rapid (Fig. 9-7). These take the form of combined wave-cut benches, beach ridges, and carbonate banks. Deformation and erosion in these areas is often so rapid, however, that no evidence of longer-term (even early Pleistocene) base-level changes is preserved. The erosional effects of long-term sea-level fluctuations, however, are spectacularly recorded on some cratons.
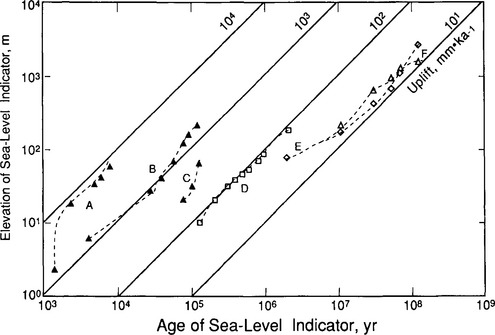
Fig. 9-7 The elevation of the terraces and other sea level indicators compared to their age for several locations. To derive actual uplift rates, one needs to know the age of the terrace and the eustatic sea level at that time. (Stallard, 1988). (A) Elevation data from the Hengchun Peninsula, Tainan, and Eastern Coast Range of Taiwan, corrected for glacial-eustatic effects and grouped by intervals of about 1500 yr. Uplift seems to have been discontinuous, but has averaged about 5000 m/Myr (Peng et al., 1977). (B) Calculated composite terrace elevations for the Huon Peninsula, New Guinea, using data from Bloom et al. (1974). Uplift has been continuous and has ranged from 940 to 2560 m/Myr; the calculation used 1620 m/Myr. (C) terrace elevations for the Island of Barbados (Clermont traverse) from Matthews (1973); uplift rate has been about 400 m/Myr. (D) Depositional terraces in the Amazon Trough from Klammer (1984). Ages are estimated from those of interglacials on the standard oxygen isotope curve. Uplift is calculated to have been about 80 m/Myr. Klammer argues that the apparent change in elevation has been caused by drop in sea level since the Pliocene rather than by uplift. (E, F) Erosion surface from the Guayana and Brazilian Shields, respectively (King, 1957; McConnel, 1968; Aleva, 1984). (Reproduced with permission from R. F. Stallard (1988). Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck, “Physical and Chemical Weathering in Geochemical Cycles,” pp. 225–246, Kluwer Academic Publishers, Dordrecht, The Netherlands.)
9.5.5 A General Concordance Between Erosion Rates and Uplift Rates
Figure 9-7 displays uplift curves, representing the elevation of various sea-level indicators versus time, for tectonically active (A, B, C) and quiet (D, E, F) regions. Note that uplift rates for Taiwan, a fold and thrust belt, exceed estimates for shield regions in South America by almost three orders of magnitude. If we assume that the hypsographic curves for any of these regions have remained fixed through time, which implies that the region has had a steady-state appearance, then uplift rates equate with denudation rates. However, because the climatic and tectonic processes that govern landform development do not operate constantly and continuously, current denudation rates may differ significantly from these values.
Dissolved solids concentrations (dissolved phases derived from the weathering of bedrock) can be used to estimate ranges of denudation rates for particular regions. Histograms of dissolved solids concentrations for different morphotectonic regions of the Amazon and Orinoco basins are presented in Fig. 9-8. Solution chemistry of rivers provides the best gage of current weathering for a particular terrain because sediment compositions are more difficult to interpret because of sediment storage as alluvium or colluvium. These denudation rates are in general agreement with more rigorous calculations (Edmond et al., 1995; Gibbs, 1967; Lewis et al., 1987; Paolini, 1986; Stallard, 1980). Comparison with Fig. 9-7 shows a reasonable match between denudation rates and uplift rates for a particular type of terrain. The most concentrated water samples and highest denudation rates are observed in river basins in tectonically active areas.
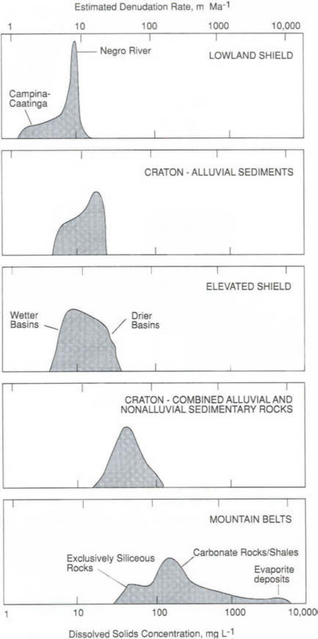
Fig. 9-8 Histogram of dissolved solids of samples from the Orinoco and Amazon River basins and corresponding denudation rates for morphotectonic regions in the humid tropics of South America (Stallard, 1985). The approximate denudation scale is calculated as the product of dissolved solids concentrations, mean annual runoff (1 m/yr), and a correction factor to account for large ratios of suspended load in rivers that drain mountain belts and for the greater than average annual precipitation in the lowlands close to the equator. The correction factor was treated as a linear function of dissolved solids and ranged from 2 for the most dilute rivers (dissolved solids less than 10 mg/L) to 4 for the most concentrated rivers (dissolved solids more than 1000 mg/L). Bedrock density is assumed to be 2.65 g/cm3. (Reproduced with permission from R. F. Stallard (1988). Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck, “Physical and Chemical Weathering in Geochemical Cycles,” pp. 225–246, Kluwer Academic Publishers, Dordrecht, The Netherlands.)
9.6 Erosion in Tectonically Active Areas
In many active mountain belts, both rapid long-term uplift and erosion are sustained by tectonic recycling of sediments. For example, the island of Taiwan (Fig. 9-7A), which is a fold and thrust belt, has been formed over the last several million years during an ongoing collision between the Luzon island arc and the Eurasian continental margin (Chi et al., 1981; Suppe, 1981). During this orogeny, metamorphosed and diagenetically altered sediments have been uplifted, eroded, and deposited, only to be reincorporated into the cycle once more (Manias et al., 1985). The central range of Taiwan has some of the highest denudation rates (13000 tonne/km2 per year solid + 650 tonne/km2 per year dissolved = 5150 mm/kyr) measured for river catchments anywhere on Earth (Li, 1976). The high denudation rate is a reflection of the poorly lithified, highly tectonized nature of the sedimentary rocks that compose the island. Sediment-yield data compiled by Milliman and Meade (1983) and Milliman and Syvitski (1992) indicate that island arcs and mountain belts in the tropical and subtropical west Pacific may contribute as more than 22% of all solid material discharged by rivers into the ocean. Furthermore, the tropical mountainous areas in southeast Asia and India may contribute another 33%.
9.6.1 Erosion and Orogeny
Mountain building usually involves compressional deformation of the crust. Studies of the physics of orogeny suggest that there is a feedback between the nature of the building process and denudation rates. Suppe (1981), Davis et al. (1983), and Dahlen et al. (1984) have modeled the effects of brittle deformation in accretionary fold-thrust mountain belts such as Taiwan and the Andes. The basis of their model is the hypothesis that rock deformation is governed by pressure-dependent, time-dependent brittle fracture or frictional sliding – “Coulomb behavior.” In such belts, sediments are scraped off subducting lithosphere, and a large wedge of deformed sediment, separated from under-riding crust by a basal decollement, is built. The process resembles the behavior of snow being pushed in front of a bulldozer blade. The mountain belt develops a regional profile that tapers towards the subducting plate. If the angle of the taper (0–6°) is less than a critical value, the wedge sticks to the basal decollement. Compression, due to the addition of material at the toe of the wedge, is taken up by the internal deformation of the wedge such that the wedge steepens. Once the angle of regional taper reaches the critical value, the entire wedge can slide over the basal decollement, deforming as material is added so as to maintain the critical taper. If too much material (∼15 km) piles up, the deepest material starts to deform, greatly reducing basal resistance to sliding. Flat high plateaus, such as the Andean Altiplano and the Tibetan Plateau, may develop over the areas that are deforming in this manner.
Models indicate that the overall topographic profile of such belts evolves into a stable form such that erosional outputs balance accretional inputs (Suppe, 1981; Davis et al., 1983). A mountain belt cannot reach unlimited height as more material is added. Two factors mitigate against this: (1) erosion and (2) changes in rock deformation from brittle to ductile with increasing depth. There should be a continuous supply of easily eroded material so long as accretion continues. Since erosion thins the wedge and reduces the taper, the intensity of deformation should increase with increasing erosion rates. It seems reasonable, therefore, that the lithological susceptibility to erosion and climate-related erosional intensity may in turn influence the form of the entire mountain range, not just the form of the slopes. Suppe (1981) argues that the width and regional slope of mountain belts is susceptible to the climate regime. For example, mountain belts might tend to be wider where erosion rates are reduced, such as in a region of dry climates. There must be some minimum regional denudation rate for a steady-state profile to form. Under the limiting case of no erosion, an accretionary mountain belt should continue to widen so long as accretion continues.
9.6.2 River Chemistry and Bedrock Susceptibility in Mountainous Regions
The composition of dissolved and solid material transported by rivers in mountain belts of the humid tropics is consistent with weathering-limited erosion. In the Andes, sediments constitute the principal basement lithology, and the river chemistry correlates with catchment geology (Stallard, 1980, 1985, 1988, 1995,a,b; Stallard and Edmond, 1983). For example, black shales have particularly high Mg:Ca ratios, and Bolivian rivers that drain black shales are exceptionally magnesium-rich. Rivers that drain evaporites have the highest total cation (TZ+) concentration, followed by those that drain carbonates, and finally by those that drain only siliceous rocks. This is illustrated by the trimodal nature of the histogram of dissolved solids from Andean rivers in Fig. 9-8 and by the ternary diagram in Fig. 9-9. Here, Si(OH)4, alkalinity, and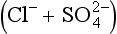 are used as input markers for the respective lithologies, bearing in mind that alkalinity is also produced by weathering of silicate minerals, and
are used as input markers for the respective lithologies, bearing in mind that alkalinity is also produced by weathering of silicate minerals, and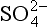 − by weathering of pyrite and other sulfides. Total cations increase systematically from silica to alkalinity to (chloride + sulfate). Many Andean rivers are slightly supersaturated with respect to calcite (Stallard, 1988, 1995b; Stallard and Edmond, 1987); this saturation ultimately limits the supply of alkalinity to the rivers. Similar limits do not apply to Na, K, Mg, and Ca because of additional inputs from silicates and evaporites. Erosional contributions by the weathering of carbonates and especially by evaporite minerals greatly exceed those expected if inputs are assumed to be proportional to the fraction of the catchment area in which these lithologies are exposed. Much of the evaporite input is sustained by actively extruding salt diapirs (Benavides, 1968; Stallard, 1980; Stallard and Edmond, 1983).
− by weathering of pyrite and other sulfides. Total cations increase systematically from silica to alkalinity to (chloride + sulfate). Many Andean rivers are slightly supersaturated with respect to calcite (Stallard, 1988, 1995b; Stallard and Edmond, 1987); this saturation ultimately limits the supply of alkalinity to the rivers. Similar limits do not apply to Na, K, Mg, and Ca because of additional inputs from silicates and evaporites. Erosional contributions by the weathering of carbonates and especially by evaporite minerals greatly exceed those expected if inputs are assumed to be proportional to the fraction of the catchment area in which these lithologies are exposed. Much of the evaporite input is sustained by actively extruding salt diapirs (Benavides, 1968; Stallard, 1980; Stallard and Edmond, 1983).
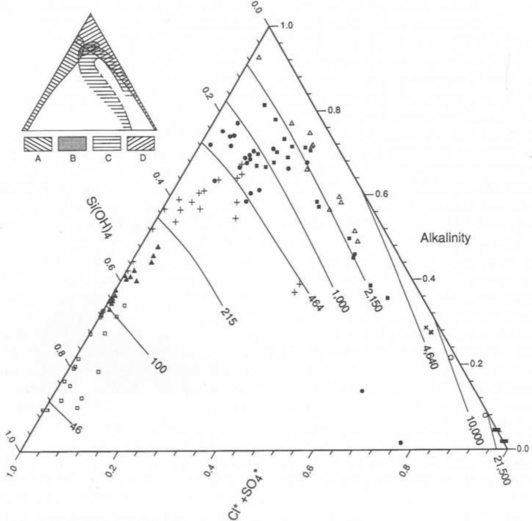
Fig. 9-9 Ternary diagram showing proportions of dissolved Si(OH)4, carbonate alkalinity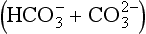 , and
, and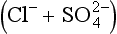 in the Orinoco River and Amazon River basins. Charged species are in equivalents; Si(OH)4 is in mole units. Curves, in the large figure, are numbered in total cation concentration (mequiv/L). Unlike previous figures, symbols represent the total cation concentration interval that includes the sample’s concentration. The predominant symbol within each interval corresponds to samples whose concentrations plot within that interval. In the small figure, the patterned areas correspond to the predominant source of samples whose concentrations plot within the areas: A, streams that drain cratonic areas; B, streams that originate in mountain belts, but that drain large areas of cratons; C, streams that drain mountain belts with extensive black shales; D, streams that drain mountain belts with extensive carbonate rocks and evaporite deposits. (Reproduced with permission from R. F. Stallard (1988). Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck, “Physical and Chemical Weathering in Geochemical Cycles,” pp. 225–246. Kluwer Academic Publishers, Dordrecht, The Netherlands.)
in the Orinoco River and Amazon River basins. Charged species are in equivalents; Si(OH)4 is in mole units. Curves, in the large figure, are numbered in total cation concentration (mequiv/L). Unlike previous figures, symbols represent the total cation concentration interval that includes the sample’s concentration. The predominant symbol within each interval corresponds to samples whose concentrations plot within that interval. In the small figure, the patterned areas correspond to the predominant source of samples whose concentrations plot within the areas: A, streams that drain cratonic areas; B, streams that originate in mountain belts, but that drain large areas of cratons; C, streams that drain mountain belts with extensive black shales; D, streams that drain mountain belts with extensive carbonate rocks and evaporite deposits. (Reproduced with permission from R. F. Stallard (1988). Weathering and erosion in the humid tropics. In A. Lerman and M. Meybeck, “Physical and Chemical Weathering in Geochemical Cycles,” pp. 225–246. Kluwer Academic Publishers, Dordrecht, The Netherlands.)
The presence of unstable and cation-rich minerals in the suspended load and bed material of rivers that drain the Andes indicates that erosion is extraordinarily rapid. In these rivers, the solution enrichment of Na+ relative to K+ and Ca2+ relative to Mg2+, when compared to bedrock ratios (Fig. 9-5) indicates that K- and Mg-rich solid phases are being eroded. Moreover, the chemical and mineralogical compositions of the sediment correlate with geology. The sands in tributaries that have their headwaters in the mountain belts are commonly litharenites (DeCelles and Hertel, 1989; Franzinelli and Potter, 1983; Johnsson et al., 1988, 1991; Potter, 1978, 1994; Stallard et al., 1991). Especially unstable minerals such as calcite, amphiboles, and pyroxenes are present in samples from some of these rivers. Micas and 2:1 clays, including illites, vermiculites, and smectites are abundant in the fine-grained fraction (Gibbs, 1967; Irion, 1976, 1984; Koehnken, 1990; Stallard et al., 1991). Chlorites (2:1:1 clays), quartz, feldspars, and amphiboles are also common in the silt-size fraction. Pyrophyllite occurs in some rivers. When compared to average igneous rocks, fine-grained sediments typically are enriched in Al relative to soluble cations, in Mg relative to Ca, and in K relative to Na (Stallard, 1985; Stallard et al., 1991). Andean rivers often acquire an intense red color only after crossing regions with red beds. Furthermore, the suspended load of rivers in the Peruvian Andes that have not yet crossed the red beds is enriched in vermiculite and mica, whereas in the lower courses of the same rivers, it is enriched in smectite and kaolinite. Finally, the marine shales, which are abundant in the Bolivian, Colombian, and Venezuelan Andes, are very micaceous, and illite is particularly abundant in rivers that drain these regions.
Weathering products in island-arc and young volcanic terrains present an interesting contrast to those from tectonically assembled, continental mountain belts. When solutes from rivers draining siliceous rocks are compared, concentrations in the island-arc and younger volcanic settings are about twice those in continental mountain belts (Stallard, 1995b). The rocks in island-arc and volcanic settings are much younger than most continental rocks. One possible cause of higher concentrations is that rocks in the former settings have more volatiles in the mineral lattices and in fluid inclusions. These volatiles, in turn, make the rocks more susceptible to chemical weathering. Many volcanic rocks are undersaturated; they do not contain quartz. When these rocks weather, coarse saprolite pellets form. These are transported like sand and gravel. Within the pellets are grains of unweathered minerals (Johnsson and Stallard, 1989). When buried and compressed these pellets should transform into a sedimentary rock consisting of sand-size mineral grains suspended in a fine matrix – a greywacke.
9.7 Erosion of the Cratons
Erosion on cratons has been difficult to describe. This is in part because denudation rates are very low and because sea-level fluctuation may be important to the erosion process. Cratons seem to undergo major episodes of erosion following drops of sea level. When the level drops to a stable stand of several million years, much of the landscape is eroded down to the new level and an erosion surface or a planation surface forms.
Two principal models have been put forward to describe the development of extremely eroded topography following a drop in base level in a region that had once been planed flat (James, 1959). One is the classic Davis cycle (Davis, 1932), the other is a model developed by King after Penck (King, 1953, 1967). These differ in their prediction of how the raised topography is dissected to form hills and valleys. According to Davis, rivers first cut steep valleys into the landscape, then their valleys broaden, and regional slopes are flattened until the last remnants of the original surface are worn away at interfluves. At this point the landscape is said to be mature. Slopes continue to flatten until formerly elevated interfluves become low swells. This is a peneplain. In King’s model, initial slopes are formed along the edges of the uplift and the sides of large penetrating river valleys. Slopes then evolve into steepened equilibrium forms that separate the old erosion surface from an incipient new surface. As time proceeds, these slopes undergo parallel retreat into the older surface that is consumed while the younger surface is extended. The end product is a pediplain. The steep slopes are the locus of most of the erosion and the remaining terrain is almost flat, being drained by rivers having little erosive capacity. In the Davis model, no remnants of previous surfaces can exist. In King’s model, however, successive erosional levels can remain stacked and can even actively expand through parallel retreat into the older surfaces long after the initiating change in base level has been superseded.
A search for stacked erosion surfaces became the centerpiece of work by King (1967), who compiled spectacular continental-scale examples from South America, Africa, India, and Australia. In a sense, these surfaces are rather like super-terraces, occurring within a narrow range of altitudes for a particular region. Surfaces nearest sea level are well defined and undissected, whereas the most elevated surfaces are usually remnants of small extent or are simply delineated by a large number of hills having peaks of similar height topped by deeply weathered soils (Fig. 9-10). In South America (Fig. 9-7E and F), Africa, and other tropical cratons at least five levels seem to be identifiable. King argued that to a first approximation the surfaces are globally synchronous (within about 10 Myr) and that the oldest ones are of great age, perhaps predating the rifting of the South Atlantic (late Jurassic to early Cretaceous).

Fig. 9-10 Radar image of erosion surface in Venezuela (Petroleos de Venezuela S. A., 1977). The region bounded by this photograph is approximately 50 km by 100 km. The Orinoco crosses the left side of the image. The confluence of the Meta is just off the lower left corner of the image. To the west of the Orinoco are the Llanos of the Andean Foreland Basin; to the east of the Orinoco is the Guayana Shield. The erosion surfaces appear as areas of slightly dissected, raised topography in the Guayana Shield. An irregular NNW–SSE trending escarpment that starts to the left of center and runs to the lower edge separates the two surfaces.
Subsequent geomorphic studies and work related to bauxite exploration have produced better dating and descriptions of relationships among erosion surfaces in South America (Aleva, 1979, 1984; Krook, 1979; McConnell, 1968; Menendez and Sarmentearo, 1984; Zonneveld, 1969). The most economic bauxite deposits occur on the Neogene (55 Myr) surfaces. Although bauxite from older surfaces is of high grade, they are of small extent and very dissected. The bauxite becomes progressively more ferruginous on younger surfaces. Where the substrate is quartzose, quartz persists through the entire weathering profile, which may be as much as 50 m thick on the 55 Myr surface (Menendez and Sarmentearo, 1984).
These surfaces are dated by classical stratigraphic techniques. Deposits of fluvial, lacustrine, or eolian sediments that contain dateable pollen or vertebrates are sometimes preserved on the surface. This is not common and dating usually is accomplished by tracing a surface into an area of subsidence such as a passive margin or an intracratonic basin. Occasionally, the surfaces have been sufficiently downwarped to be buried under marine sediments. More often, such as off the northeastern South American coast, erosion surfaces coincide with hiatuses in the sedimentary section; these are frequently overlain by deposits of quartz and bauxite gravels and sub-arkosic sands. This is thought to be indicative of the early dissection of thick and highly weathered soils following a drop in base level. Such hiatuses are not unlike those associated with sea level high stands seen in coastal onlap sequences. Accordingly, Aleva (1984) notes that the major surfaces appear to coincide with major high stands on the late Mesozoic–Cenozoic sea level curve. The most recent and youngest surfaces are ten or so glacio-eustatic terraces from the Amazon valley described by Klammer (1984) (Fig. 9-7D).
The Brazilian and Guayana shields are among the best exposures of elevated crystalline basement in the humid tropics. The topography can be spectacular with steep slopes, either of bare rock or thinly vegetated, often topped by high plateaus, some of which are quite expansive. The highest landforms are 2000–3000 m tablelike mountains called “Tepuis” (Fig. 9-11). These are topped by thick orthoquartzites. Inselbergs are locally common. Great talus piles do not accumulate below cliffs and at the base of slopes. Much of this material probably just dissolves. A karst-like topography developed on the orthoquartzite, gneisses, and granites is found on some of the higher and presumably most ancient areas (Blancaneaux and Pouyllau, 1977; Szczerban, 1976; Urbani, 1986). The karst on quartzite is established to such a degree that stalactites consisting of opal, cristobalite, tridymite, and quartz have formed locally (Chalcraft and Pye, 1984). Aerial and radar photographs show that all but the youngest surfaces are dissected to various degrees, and that this dissection is geologically controlled. Many rivers appear to follow fault systems and dike swarms (Fig. 9-10, Schubert et al., 1986; Stallard et al., 1991). This would indicate that the older surfaces are no longer expanding in the manner described by King, since the dissected surfaces can no longer serve as fixed base levels for overlying scarps. Especially resistant lithologies such as quartzites and some intrusives persist as high elevations. Thus, even though weathering rates are very slow (Fig. 9-8C), the observation that lithology plays an important role in landscape morphology indicates that weathering-limited erosion is occurring.

Fig. 9-11 Photograph of Ayun Tepui, taken from Canaima, Venezuela. The cliffs, which are about 2000 m high, consist of orthoquartzite. The irregular top is a karst topography formed by the slow dissolution of the quartz. It is estimated that the top of this tepui, and similar ones elsewhere on the Guayana Shield, have been exposed since the Jurassic.
9.7.1 Erosion and the Slow Uplift of the Shields
The development of topography consisting of stacked erosion surfaces on many shields indicates that vast areas of cratons are undergoing a slow but sustained rise relative to sea level. The remnants of erosion surfaces are evidence of the former thickness of the cratons. It is interesting to speculate that this tendency to rise has held true for many shield areas throughout much of geologic time, thereby explaining the high metamorphic grade of rocks exposed on the surface of most shield areas. In this sense, shields would be dynamical opposites to intracratonic basins. In this context, continental platforms are areas that neither rise nor sink dramatically. Tectonic modes change with time. For example, the thick quartzites that top many of the highest peaks of the Guayana shield are evidence that this area once behaved as either a platform or a basin.
Part of the rising and sinking of cratons may be due to isostatic adjustments in response to erosion and to sediment accumulation. Part, however, may be due to deep-seated processes. Burke and Wilson (1972), Crough (1979), and Morgan (1983) have argued that uplift and erosion of shields is caused by random repeated passages of continental crust over mantle plumes. As a segment of crust passes over a mantle plume, it is heated and becomes more buoyant. This event produces a fairly rapid uplift followed by a slow reduction of elevation as a result of combined erosion and cooling. If erosion is sufficiently intense, the formerly elevated segment of crust becomes a depositional basin once it has completely cooled. Morgan (1983) used this idea to examine the relationship between hot spots and regional geology in the continents bracketing the Atlantic. He hypothesized the recent passage of a hot plume under the Guayana shield to explain its presently elevated nature.
The globally synchronous development of erosion surfaces and slow uplift rates of shields indicates that models based on localized heating of the crust and mantle are not adequate to explain the uplift process. To produce near simultaneous surfaces on separate continents, King (1967) invoked global epeirogeny – uplift driven by some sort of deep-seated process. The change in base level does not have to act continuously once scarps are initiated. They may become self sustaining to some degree, for as they retreat, isostatic adjustment would raise the topography (King, 1956), thereby stabilizing the effects of the initial base level change. The isostatic uplift would be cumulative, thus explaining the stacked topography. Global sea-level drop seems to be a more reasonable initiating event, but it is still a matter of speculation as to whether the uplift is continuous or episodic and isostatically self sustaining or driven by deep-seated, global, tectonic processes. There is, however, an interesting parallel between King’s view of erosion surfaces and the idea of Sloss and Speed (1974) and Sloss (1979) that there is a tectonic component to the subsidence of sedimentary basins at times of high sea level.
9.7.2 Weathering-Limited Erosion on the Elevated Shield
Rivers from the shields transport very little dissolved or solid load, and the solids tend to be more resistant mineral phases (Franzinelli and Potter, 1983; Gibbs, 1967; Irion, 1976; Johnsson et al., 1988, 1991; Koehnken, 1990; Potter, 1978; Stallard, 1995a,b; Stallard et al., 1991). The major cations of the dissolved load are in bedrock proportions (Fig. 9-5), indicating that bedrock is strongly leached during weathering and most of the cation load is in solution. River sands include more potassic feldspars, as might be expected if weathering-limited erosion is occurring on the deep slopes. Erosion rates in these terrains are much lower than in active mountain belts like the Andes, even though elevations are as much as 3000 m. Both lithology and basement structure contribute to the low erosion rates in elevated crystalline-basement terrains (Stallard, 1988; Stallard et al., 1991). Rocks are frequently massive and composed of minerals like quartz, potassium and sodium feldspars, and micas, which are somewhat resistant to weathering. Uplift apparently involved ductile (plastic) deformation and was followed by a long history of weathering that may have removed all unstable lithologies. The basement of active orogenic belts is not so massive owing to faulting, brittle deformation, and volcanism. Furthermore, active uplift provides a continuous supply of fresh unstable lithologies.
9.7.3 Transport-Limited Erosion on the Lowlands
Vast tracts of the South American and west African lowlands have predominantly transport-limited denudation regimes (Stallard, 1988; Stallard et al., 1991). These regions represent the flattest and youngest erosional/depositional surfaces of the late Neogene. On the shield, the substrate is crystalline basement, while in the intracratonic basins, the Andean foreland basin, and the coastal plains, much of the sedimentary substrate consists of strongly “preweathered” fluvio-lacustrine sediment (Stallard, 1985, 1988; Stallard and Edmond, 1983; Stallard et al., 1991). Soils and solid loads of rivers are rich in quartz, kaolinite, and iron sesquioxides, all of which are cation-depleted phases. River sands are quartzose (Franzinelli and Potter, 1983; Johnsson et al., 1988, 1991) and the suspended load is largely kaolinite plus quartz. Aluminum sesquioxides (gibbsite) are much less common. In rivers with pH > 5 particles have iron sesquioxide coatings; at lower pH values coatings are absent (Stallard et al., 1991). Dissolved major cations are in bedrock proportions. Dissolved solid concentrations, and by inference denudation rates, are exceedingly low (Fig. 9-8). This agrees with the diminution of weathering rates in conjunction with the development of thick soils. Dissolved loads tend to be lower on the shields than on the sediments, even though rocks of the shield are composed of less stable minerals; however, the low permeabilities of the rocks and many of the soils of the shields compared to those of the sediments apparently counterbalances this.
Where easily weathered lithologies such as carbonates and evaporites are near the surface, such as in the lower Amazon valley, their contribution to the rivers appears minor, probably because thick residual soil covers have developed. This indicates that susceptibility to weathering is indeed less important in controlling lowland erosion rates. Carbonate platforms, such as southern Florida and the Yucatan in North America, are exceptions because of deep water circulation throughout the carbonate karst.
9.8 The Effects of Transients: Continental Ice Sheets and Human Technology
As seen in the previous discussion, susceptibility of bedrock to erosion is the primary factor controlling erosion rate in steep terrains, whereas, supply of reactants to reactive minerals limits erosion in flat terrains. Geochemists are frequently called upon to assess the impact of some human-induced environmental perturbation. Would pulverized granite make a good fertilizer in areas of thick, cation-depleted, tropical soils? What affect does permanent deforestation of a mountain slope have on erosion on a short time scale? On a time scale that is 10 times longer? What about acid rain? What about long-term climatic warming or increased atmospheric carbon dioxide? What happens when a formerly moist region dries out? Humans move as much solid matter about as do all other geomorphic processes (Hooke, 1994). Human modifications of soil genesis, erosion, plant productivity, and terrestrial sedimentation may be enough to account for the burial of a billion tonnes of carbon annually (Stallard, 1998). A billion tonnes of carbon is a significant fraction of the carbon released from the burning of fossil fuels. The Earth has recently undergone a remarkable perturbation, continental glaciation. Glaciations are an especially interesting context within which to evaluate the concepts of erosion regime.
9.8.1 Glacial Weathering and Erosion
During the past 2.4 million years the Earth has gone through several episodes of continental glaciation (see Hughes, 1985). Each of the last seven of these episodes (600 000 years) shows a characteristic pattern of waxing and waning (Broecker and Denton, 1989). Each episode started slowly. Extensive ice sheets gradually build up in Canada (the Laurentide ice sheet), northern Europe (the European or Fenno-Scandian ice sheet), and across northern Asia. Smaller ice sheets form in Iceland and southern South America. Temperatures cool globally, and the altitude of mountain glacial formation descends about 1000 m. The ice caps of Greenland and Antarctica became more active. The glacial buildup lasts about 100000 years and ends abruptly, over a few hundred years. Within the buildups and terminations there are short episodes of glacial advance and recession. The last ice age ended about 13 500 years ago.
Glaciers are powerful agents of physical erosion. In a detailed geomorphological study of the glaciated Canadian Shield, Sugden (1978) concluded that erosional style was related primarily to the basal thermal regime of the ice. From the center of divergence on an ice cap, Sugden identified five idealized zones. Erosion is minimal under the cold-based ice at the center of the cap – shear from ice flow is minimal and the ice is anchored to the bedrock. Surrounding this was a zone of basal melting then a zone of basal freezing, and finally a zone of melting. Various processes drive erosion in these zones (Fig. 9-12). Sugden concludes that the excavation of debris is an important factor influencing the amount of erosion accomplished by an ice sheet. The excavation process contrasts strongly with fluvial systems or alpine glaciers, for which downslope transport is important. Sugden argues that landforms on the Canadian Shield are equilibrium forms with respect to the thermal regime of the ice sheet at maximum glaciation.
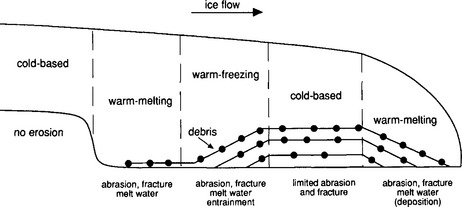
Fig. 9-12 Idealized model of the relationship between styles of glacial erosion and the basal thermal regime of ice, The most important are surface temperature, ice thickness (pressure melting), and the rate of ice deformation (frictional heating). Cold-based ice is generally protective and has little erosive power unless it contains debris inherited from an up-glacial basal freezing zone. In warm-melted zones, basal slip between ice and rock promotes fracture, plucking, and abrasion. If ice subsequently passes into a freezing zone, bedrock particles can be entrained during the freezing process. This further enhances excavation of bedrock. During a glaciation, patterns of erosion may shift as ice sheets develop and retreat, but peak erosion seems to occur near glacial maxima. The top surface of an ice sheet is relatively flat. Erosion is more pronounced at basement topographic lows (either valleys or lowlands), relative to adjacent basement highs, because the thicker ice over the lows promotes pressure melting, and because low areas tend to be zones of ice convergence. Less porous rocks, such as crystalline shield may be more susceptible to glacial erosion than porous rocks such as sediments, because diversion of melt water throught the substrate may promote binding of the ice to the bedrock. Such ice would act more like cold-based ice. adapted from Sugden (1978). Many factors affect the basal thermal regime. (Adapted with permission from D. E. Sugden (1978). Glacial erosion by the Laurentide ice sheet. J. Glaciol. 83, 367–391, International Glaciological Society.)
The estimation of depth of erosion by continental ice sheets has been a controversial endeavor. In mountainous regions such as the North American Rockies, alpine glaciers have scoured deep valleys into a topography that was, prior to glaciation, much smoother and more rolling. For continental ice sheets, it is much harder to find a convenient frame of reference, such as pre-existing topography, from which depth of erosion can be judged. White (1972) argued that the ice sheets had removed much of the sedimentary cover of the Canadian Shield and drew a cross-section indicating that there may have been as much as 1000 m of erosion. This conflicted with geologic, pedological, and geomorphological evidence that some areas of the Canadian Shield had been minimally eroded during the ice ages and that the oldest glacial deposits are rich in material from crystalline basement (Gravenor, 1975; Sugden, 1976). More recently, careful compilations of the quantities of glacial sediment deposited in the oceans around North America and in depositional basins on North America by Laine (1980) and Bell and Laine (1985) indicate that about 120 m or more of physical erosion were caused by continental ice sheets. If present river yields are used, about 20 m of chemical and 20 m of physical erosion would have occurred over the same time period (3 Myr assumed). Clearly, continental ice sheets are potent agents of erosion – on shields, ice evidently is more powerful than the processes associated with the humid tropical weathering.
The above-mentioned river yields are undoubtedly affected by the recent glacial scouring transient. Throughout the glaciated region there is still an abundance of loose glacial debris, much of which is fresh bedrock and mineral grains. This great abundance of fresh bedrock minerals at the ground surface contrasts markedly with the situation in flat regions undergoing transport-limited erosion where soil minerals are cation-deficient. Gravenor (1975) presents data that indicate that prior to glaciation the shield may once have had a soil mantle that even included bauxites. Presumably, both chemical and physical erosion must be proceeding much faster now than under steady-state conditions involving a thick soil. Thus, Bell and Laine (1985) used worst-case values – generally a good practice. What might be a more realistic estimate of weathering rates without glaciations?
9.8.2 Late-Glacial/Deglaciation Geochemical Anomalies
Ice ages end much more quickly than they develop, and the marine chemical record indicates that this was an unusual time. Late in the last glaciation and during glacial retreat, the oceans had 87Sr/86Sr ratios (Dia et al., 1992) and Ge/Si ratios greater than those of today (Froelich et al., 1992). The source of excess 87Sr is radioactive decay of 87Rb in potassium-bearing minerals; thus, an especially strong source of 87Sr is the weathering of potassium-bearing minerals in old continental crust such as the Canadian Shield. In today’s world, water that has high Ge/Si ratios are either draining terrains undergoing transport-limited erosion (Murnane and Stallard, 1990) or rivers contaminated by ash from coal burning, where the Ge is associated with organic matter and sulfides (Froelich et al., 1985). If we use today’s world as a reference frame, the late-glacial data indicate a strong contribution of 87Sr and Ge from a shield terrain undergoing intense tropical-style weathering (Froelich et al., 1992; Gibbs and Kump, 1994). This contradicts all that we know about global climate of the time (see Broecker and Denton, 1989).
Investigators studying warm-based mountain glaciers and glaciated areas have noted that even in granitic areas, waters are particularly high in Ca2+, K+, and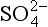 ions relative to other major soluble cations (Axtmann and Stallard, 1995; Drever and Hurcomb, 1986; Mast et al., 1990; Stallard, 1995b). One source of Ca2+ appears to be intergranular calcite, one of the last minerals to crystallize out of the cooling granite melt. Volumetrically, this calcite is a minor constituent. Calcite is also far more susceptible than other minerals in granite (Table 9-1). A primary reason for the Ca2+ abundance may be that the calcite is making a disproportionate contribution to the steam waters because of the recent exposure of fresh granite by glacial activity. Similarly, the excess
ions relative to other major soluble cations (Axtmann and Stallard, 1995; Drever and Hurcomb, 1986; Mast et al., 1990; Stallard, 1995b). One source of Ca2+ appears to be intergranular calcite, one of the last minerals to crystallize out of the cooling granite melt. Volumetrically, this calcite is a minor constituent. Calcite is also far more susceptible than other minerals in granite (Table 9-1). A primary reason for the Ca2+ abundance may be that the calcite is making a disproportionate contribution to the steam waters because of the recent exposure of fresh granite by glacial activity. Similarly, the excess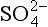 may be from the weathering of disseminated trace sulfides. The K+ appears to come from the physical breakdown of micas in general and the oxidative weathering of vermiculite. Calcium-bearing minerals release strontium with a low 87Sr/86Sr ratio, while the ratio derived from the micas is exceedingly high, especially in old rocks. The weathering sources of Ca2+ and K+ during soil development persist well after glaciers have departed (Blum et al., 1994; Mast et al., 1990; Stallard, 1995b), but are relatively weak compared to sub-glacial sources (Axtmann and Stallard, 1995). In the case of the Laurentide region, soil development took several thousand years and still continues (Harden et al., 1992). Micas and sulfides are potential sources of excess Ge/Si. Thus, the peculiarities of late Ice Age seawater may be cause by the different styles of chemical weathering associated with glaciers (Stallard, 1995b). This points to the need to examine biogeochemical processes in detail before drawing analogies between different processes in various terrestrial environments (erosion regime) and the data that need to be explained (marine chemistry).
may be from the weathering of disseminated trace sulfides. The K+ appears to come from the physical breakdown of micas in general and the oxidative weathering of vermiculite. Calcium-bearing minerals release strontium with a low 87Sr/86Sr ratio, while the ratio derived from the micas is exceedingly high, especially in old rocks. The weathering sources of Ca2+ and K+ during soil development persist well after glaciers have departed (Blum et al., 1994; Mast et al., 1990; Stallard, 1995b), but are relatively weak compared to sub-glacial sources (Axtmann and Stallard, 1995). In the case of the Laurentide region, soil development took several thousand years and still continues (Harden et al., 1992). Micas and sulfides are potential sources of excess Ge/Si. Thus, the peculiarities of late Ice Age seawater may be cause by the different styles of chemical weathering associated with glaciers (Stallard, 1995b). This points to the need to examine biogeochemical processes in detail before drawing analogies between different processes in various terrestrial environments (erosion regime) and the data that need to be explained (marine chemistry).
9.9 Conclusion
Weathering processes exert a major control on many aspects of the chemistry of the Earth’s surface environment. Considerations of temporal and spatial scale are very important in evaluating how the weathering processes affect the chemical response of the atmosphere and ocean reservoirs. Continental denudation, the transfer of material from the land surface to the ocean, is a particularly important aspect of this response. In small stream basins, solution chemistry is controlled by processes that act on a hillslope scale. There is commonly little storage of water in most large river systems; thus for large river systems, hillslope processes remain important. Storage on a time scale of months to millions of years must be considered for solid material. Long-term burial results in a net loss to the river, and chemical weathering during storage can be important for large river systems.
In regions where the erosion regime is weathering limited, susceptibility of the bedrock to chemical and physical weathering controls erosion rates. This susceptibility relates directly to the chemical and physical properties of the rock. Susceptibility also depends on local climate. Moreover, weathering rates are affected by the soils that form on the rock, and the nature of the vegetation that grows on the soil. Vegetation, by supplying bioacids that chemically degrade the rock, can increase the rate of erosion of rocks that are resistant to physical erosion.
Susceptibility of bedrock to erosion is not necessarily controlled by the reactivity of the mineral grains that make up the bedrock. In the case of limestones, the weathering reactions proceed until equilibrium is reached. The rate is controlled by the supply of reactants (protons). Rocks composed of silicate minerals are different. For example, the massive, poorly jointed granites of the Guayana Shield erode more slowly than the tectonized, highly cracked and sheared granites of the Andes. The difference is not caused by contrasting mineralogies, but by differences in the permeability of water and stability of steep slopes. Again, the supply of reactants is very important, but because the weathering does not proceed to equilibrium or to completion, the reactivity of individual mineral grains is also important. Thus, when compared to the bedrock proportions, K is enriched relative to Na and Mg relative to Ca in solid erosion products. Likewise, Na+ and Ca2+ are enriched in solution relative to K+ and Mg2 +. In flat terrains, these enrichments do not occur because, the mobilization of major soluble cations from bedrock is complete. The presences of volatiles and glasses may cause rocks from island arc terrains and younger volcanics to be more susceptible than equivalent older rocks from cratons.
In regions where erosion is transport limited, weathering rates are controlled by the supply of reactive fluids to unstable minerals. This is controlled by soil properties, regional base level, and ultimately, sea level.
The concept of weathering regime may be useful for interpreting the effects of important phenomena in Earth history. These include the evolution of life, changes in patterns of plate tectonic interactions, major meteorite impacts, glaciations, and so on. Continental chemical denudation depends on the proportions of the Earth’s surface that are eroding under the two types of regimes: weathering-limitation associated with steeper slopes; transport-limitation associated with flat areas. Susceptibility has to be evaluated for each climate regime. In the case of glacial and periglacial conditions, chemical weathering processes are sufficiently different that they may be misinterpreted if one uses models developed for warmer parts of the world.
On the time scale of the entire history of the Earth, denudation rate is controlled by the tectonic processes that supply fresh bedrock to the subaerial Earth-surface environment. There are two important aspects to this. One is the uplift of mountains and volcanism to produce steep terrains that undergo weathering-limited erosion. The other is the rise and fall of global sea level that affects erosion and sedimentation on the cratons. Low sea level equates with erosion of the cratons. High sea level equates with cessation of erosion and even sedimentation on the cratons. Glaciers are the most potent agents of physical erosion on cratons and perhaps in mountain terrains were the bedrock is resistant to chemical weathering. The role of continental ice sheets in excavating shield over the history of the Earth deserves further consideration.
Questions
1. Provide some simple reasons for the rules of thumb for physical and chemical weathering given in Section 9.2.1.
2. Describe how vegetation can increase and decrease the weathering rate.
3. What is the distinction between physical and chemical weathering?
4. Describe the factors that limit mountain height. How does this relate to the areas of the Earth that deliver the largest dissolved and suspended loads to the oceans?
5. How might chemical and physical weathering have differed from today’s world before the advent of rooted land plants? What about before any land plants, assuming that there were once green films?
6. Ice ages ended quickly. Processes that promote the increase of carbon dioxide in the atmosphere are positive feedbacks in ending an ice age. Is subglacial chemical weathering a positive or a negative feedback? What circumstances would allow one to give the opposite answer? (Hint: look at the weathering reactions in Appendices 1 and 2 in Drever and Hurcomb (1986).)
Aleva, G. J. J. Bauxite and other duricrusts in Surinam: A review. Geol. Mijnbouw. 1979; 58:321–336.
Aleva, G. J. J., Laterization, bauxitization and cyclic landscape development in the Guiana ShieldJacob L., Jr., eds. Bauxite: Proceedings of the 1984 Bauxite Symposium. Los Angeles, California, February 27, 1984–March 1, 1984. American Institute of Mining, Metallurgical and Petroleum Engineers, Dordrecht, Holland, 1984. 297–318.
Axtmann, E. V., Stallard, R. F., Chemical weathering in the South Cascade Glacier basin, comparison of subglacial and extra-glacial weatheringTonnessen K. A., Williams M. W., Tranter M., eds. Biogeochemistry of Seasonally Snow-Covered Catchments. Boulder, Colorado, USA, July 3, 1995–July 14, 1995. International Association of Hydrological Sciences Publication; 228. International Association of Hydrological Sciences, New York, 1995. 431–439.
Bell, M., Laine, E. P. Erosion of the Laurentide region of North America by glacial and glaciofluvial processes. Quatern. Res. 1985; 23:154–174.
Benavides, V. Saline deposits of South America. Geological Society of America Special Paper. 1968; 88:249–290.
Berner, R. A. Rate control of mineral dissolution under earth surface conditions. Am. J. Sci. 1978; 278:1235–1252.
Berner, R. A., Lasaga, A. C., Garrels, R. M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983; 283:641–683.
Bevin, K. J., Kirkby, M. J. A physically based variable contributing area model of basin hydrology. Hydrolog. Sci. Bull. 1979; 23:419–437.
Water Resource Publications. Bevin, K. J., Lamb, R., Quinn, P. F., Romanowicz, R., Freer, J., TopmodelSingh V. P., ed. Computer Models of Watershed Hydrology. Highlands Ranch, Colorado, 1994. 1995:627–668.
Blancaneaux, P., Pouyllau, M. Formes d’altération pseudokarstiques en relation avec la géomorphologie des granites précambriens du type Rapakivi dans le territoire Fédéral de l’Amazone, Vénézuéla. Cah. ORSTOM Sér. Pédol. 1977; 15:131–142.
Blum, A. E., Stillings, L. L., Feldspar dissolution kineticsWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Washington, DC, 1995. 291–351.
Blum, J. D., Erel, Y., Brown, K. 87Sr/86Sr ratios of Sierra Nevada stream waters: Implications for relative mineral weathering rates. Geochim. Cosmochim. Acta. 1994; 58:5019–5025.
Brantley, S. L., Chen, Y., Chemical weathering rates of pyroxenes and amphibolesWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Washington, DC, 1995. 119–172.
Brantley, S. L., Crane, S. R., Crerar, D. A., Hellmann, R., Stallard, R. F. Dissolution at dislocation etch pits in quartz. Geochim. Cosmochim. Acta. 1986; 50:2349–2361.
Broecker, W. S., Denton, G. H. The role of ocean-atmosphere reorganization in glacial cycles. Geochim. Cosmochim. Acta. 1989; 53:2465–2510.
Burke, K., Wilson, J. T. Is the African Plate stationary? Nature. 1972; 239:387–390.
Carson, M. A., Kirkby, M. J., Hillslope, Form and Process. Cambridge University Press, Washington, DC, 1972. 475.
Casey, W. H., Ludwig, C., Silicate mineral dissolution as a ligand-exchange reactionWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Cambridge, England, 1995. 87–117.
Chalcraft, D., Pye, K. Humid tropical weathering of quartzite in southeastern Venezuela. Z. Geomorphol., N. F. 1984; 28:321–332.
Chi, W. R., Namson, J., Suppe, J. Stratigraphic record of plate interactions in the coastal range of eastern Taiwan. Geol. Soc. China Mem. 1981; * 491–530.
Church, M., Slaymaker, O. Disequilibrium of Holocene sediment yield in glaciated British Columbia. Nature. 1989; 337:452–454.
Crough, S. T. Hotspot epeirogeny. Tectonophysics. 1979; 61:321–333.
Dahlen, F. A., Suppe, J., Davis, D. Mechanics of fold-and-thrust belts and accretionary wedges: Cohesive Coulomb theory. J. Geophys. Res. 1984; 89:10087–10101.
Davis, D., Suppe, J., Dahlen, F. A. Mechanics of fold-and-thrust belts and accretionary wedges. J. Geophys. Res. 1983; 88:1153–1172.
Davis, W. M. Piedmont Benchlands and Primärrümpfe. Geol. Soc. Am. Bull. 1932; 43:399–440.
DeCelles, P. G., Hertel, F. Petrology of fluvial sands from the Amazonian foreland basin, Peru and Bolivia. Geol. Soc. Am. Bull. 1989; 101:1552–1562.
Dia, A. N., Cohen, A. S., O’Nions, R. K., Shackleton, N. J. Seawater Sr-isotope variations over the past 300 ka and global climate cycles. Nature. 1992; 356:786–788.
Dove, P., Kinetic and thermodynamic controls on silica reactivity in weathering environmentsWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Washington, DC, 1995. 235–290.
Drever, J. I., Hurcomb, D. R. Neutralization of atmospheric acidity by chemical weathering in an alpine drainage basin in the North Cascade Mountains. Geology. 1986; 14:221–224.
Drever, J. I., Smith, C. L. Cyclic wetting and drying of the soil zone as an influence on the chemistry of ground water in arid terrains. Am. J. Sci. 1978; 278:1448–1454.
Dunne, T. Rates of chemical denudation of silicate rocks in tropical catchments. Nature. 1978; 274:244–246.
Eberl, D. D., Srodon, J., Northrop, H. R., Potassium fixation in smectite by wetting and drying. Geochemical Processes at Mineral Surfaces. ACS Symposium Series; 323. American Chemical Society, Washington, DC, 1986. 296–325.
Edmond, J. M., Palmer, M. R., Measures, C. I., Grant, B., Stallard, R. F. The fluvial geochemistry and denudation rate of the Guayana Shield in Venezuela, Colombia, and Brazil. Geochim. Cosmochim. Acta. 1995; 59:3301–3325.
Fischer, A. G. The two Phanerozoic supercycles. In: Berggren W., Van Couvering J., eds. Catastrophies in Earth History: The New Uniformitarianism. Washington, DC: Princeton University Press; 1983:129–150.
Franzinelli, E., Potter, P. E. Petrology, chemistry, and texture of modern river sands, Amazon River system. J. Geol. 1983; 91:23–39.
Froelich, P. N., Hambrick, G. A., Andreae, M. O., Mortlock, R. A., Edmond, J. M. The geochemistry of inorganic germanium in natural waters. J. Geophys. Res. 1985; 90:1133–1141.
Froelich, P. N., Blanc, V., Mortlock, R. A., Chillrud, S. N., Dunstan, W., Udomkit, A., Peng, T. H. River fluxes of dissolved silica to the ocean were higher during glacials: Ge/Si in diatoms, rivers, and oceans. Paleoceanography. 1992; 7:739–767.
Garrels, R. M., Lerman, A., Phanerozoic cycles of sedimentary carbon and sulfur. Proc. Nat. Acad. Sci. USA; 78. 1981:4652–4656.
Garwood, N. C., Janos, D. P., Brokaw, N. V. L. Earthquake-caused landslides: A major disturbance to tropical forests. Science. 1979; 205:997–999.
Gibbs, M. T., Kump, L. R. Global chemical erosion during the last glacial maximum and the present: Sensitivity to changes in lithology and hydrology. Paleoceanography. 1994; 9:529–543.
Gibbs, R. J. The geochemistry of the Amazon River system: Part I, The factors that control the salinity and composition and concentration of suspended solids. Geol. Soc. Am. Bull. 1967; 78:1203–1232.
Gilkes, R. J., Suddhiprakarn, A., Armitage, T. M. Scanning electron microscope morphology of deeply weathered granite. Clays Clay Miner. 1980; 28:29–34.
Goldich, S. S. A study in rock weathering. J. Geol. 1938; 46:17–58.
Gravenor, C. P. Erosion by continental ice sheets. Am. J. Sci. 1975; 275:594–604.
Harden, J. W., Sundquist, E. T., Stallard, R. F., Mark, R. K. Dynamics of soil carbon during deglaciation of the Laurentide Ice Sheet. Science. 1992; 258:1921–1924.
Harmon, R. S., White, W. B., Drake, J. J., Hess, J. W. Regional hydrochemistry of North American carbonate terrains. Water Resour. Res. 1975; 11:963–967.
Heimsath, A. M., Dietrich, W. E., Nishiizumi, K., Finkel, R. C. The soil production function and landscape equilibrium. Nature. 1997; 388:358–361.
Herbillon, A. J., Nahon, D., Laterites and laterization processesStucki J. W., Goodman B. A., Schertmann U., eds. Iron in Soils and Clay Minerals. NATO ASI Series C: Mathematical and Physical Sciences; 217. Kluwer Academic Publishers, Princeton, New Jersey, 1988. 267–308.
Hochella, M. F., Jr., Banfield, J. F., Chemical weathering of silicates in nature: A microscopic perspective with theoretical considerationsWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Dordrecht, The Netherlands, 1995. 353–406.
Holland, H. D., Marine evaporites and the composition of sea water during the PhanerozoicHay W. W., ed. Studies in Paleo-oceanography. Special Publication; 20. Society of Economic Paleontologists and Mineralogists, Washington, DC, 1974. 187–192.
Holland, H. D., Kirsipu, T. V., Huebner, J. S., Oxburgh, U. N. On some aspects of the chemical evolution of cave waters. J. Geol. 1964; 72:36–67.
Hooke, R. L. On the efficacy of humans as geomorphic agents. GSA Today 1994; 4:218. Hooke, R. L., On the efficacy of humans as geomorphic agents. GSA Today. 1994; 4:224–225.
Hughes, T. J. The great Cenozoic ice sheet. Palaeogeogr. Palaeoclim. Palaeoecol. 1985; 50:9–43.
Irion, G. Mineralogisch-geochemische Unterschungen an der pelitischen Fraktion amazonischer Oberboden und Sedimente. Biogeographica. 1976; 7:7–25.
Irion, G. Sedimentation and sediments of Amazonian rivers and evolution of the Amazonian landscape since Pliocene times. In: Sioli H., ed. The Amazon, Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Tulsa, Oklahoma: Dr. W. Junk Publishers; 1984:201–214.
James, P. E. The geomorphology of eastern Brazil as interpreted by Lester C. King. Geogr. Rev. 1959; 49:240–246.
Johnsson, M. J., Stallard, R. F. Physiographic controls on the composition of sediments derived from volcanic and sedimentary terrains on Barro Colorado Island, Panama. J. Sediment. Petrol. 1989; 59:768–781.
Johnsson, M. J., Stallard, R. F., Lundberg, N. Controls on the composition of fluvial sands from a tropical weathering environment: Sands of the Orinoco River drainage basin, Venezuela and Colombia. Geol. Soc. Am. Bull. 1991; 103:1622–1647.
Johnsson, M. J., Stallard, R. F., Meade, R. H. First-cycle quartz arenites in the Orinoco River basin, Venezuela and Colombia. J. Geol. 1988; 96:263–277.
King, L. C. Canons of landscape evolution. Geol. Soc. Am. Bull. 1953; 64:721–751.
King, L. C. A geomorfologia do Brasil oriental. Rev. Brasil. Geograf. 1956; 18:147–265.
King, L. C., The Morphology of the Earth. Oliver and Boyd, The Hague, The Netherlands, 1967. 762.
Klammer, G. The relief of the extra-Andean Amazon basin. In: Sioli H., ed. The Amazon, Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Edinburgh: Dr. W. Junk Publishers; 1984:47–83.
Koehnken, L. The composition of fine-grained weathering products in a large tropical river system, and the transport of metals in fine-grained sediments in a temperate estuary, Ph. D. thesis. Dordrecht, The Netherlands: Princeton University, Department of Geological and Geophysical Sciences; 1990.
Krook, L. Sediment petrographical studies in northern Surinam, Ph. D. thesis. Free University; 1979.
Laine, E. P. New evidence from beneath the western North Atlantic for the depth of glacial erosion in Greenland and North America. Quatern. Res. 1980; 14:188–198.
Langmuir, D. The geochemistry of some carbonate ground waters in central Pennsylvania. Geochim. Cosmochim. Acta. 1971; 35:1023–1045.
Larsen, M. C., Simon, A. A rainfall intensity-duration threshold for landslides in a humidtropical environment, Puerto Rico. Geograf. Annal. 1993; 75A:13–23.
Larsen, M. C., Torres Sánchez, A. J. The frequency and distribution of recent landslides in three montane tropical regions of Puerto Rico. Geomorphology. 1998; 24:309–331.
Lasaga, A. C., Fundamental approaches in describing mineral dissolution and precipitation ratesWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, 1995. 23–86.
Lewis, W. M., Jr., Hamilton, S. K., Jones, S. L., Runnels, D. D. Major element chemistry, weathering, and element yields for the Caura River drainage, Venezuela. Biogeochemistry. 1987; 4:159–181.
Li, Y. H. Denudation of Taiwan Island since the Pliocene Epoch. Geology. 1976; 4:105–107.
Likens, G. E., Bormann, F. H., Pierce, R. S., Eaton, S., Johnson, N. M. Biogeochemistry of a Forested Ecosystem. Washington, DC: Springer-Verlag; 1977.
Manias, W. G., Covey, M., Stallard, R. F. The effects of provenance and diagenesis on clay content and crystallinity in Miocene through Pleistocene deposits, southwestern Taiwan. Petrol. Geol. Taiwan. 1985; * 173–185.
Mast, M. A., Drever, J. I., Baron, J. Chemical weathering in the Loch Vale watershed, Rocky Mountain National Park, Colorado. Water Resour. Res. 1990; 26:2971–2978.
McConnell, R. B. Planation surfaces in Guyana. Geogr. J. 1968; 134:506–520.
McNabb, D. H., Swanson, F. J. Effects of fire on soil erosion. In: Walstad J. D., Radosevich S. L., Sandberg D. V., eds. Natural and Prescribed Fire in the Pacific Northwest. New York: Oregon State University Press; 1990:159–176.
The Geology of North America, O-1 Meade, R. H., Yuzyk, T. R., Day, T. J. Movement and storage of sediment in rivers of the United States and Canada. In: Wolman M. G., Riggs H. C., eds. Surface Water Hydrology. Corvallis, Oregon: Geological Society of America; 1990:255–280.
Menendez, A., Sarmentearo, A., Geology of the Los Pijiguaos bauxite deposits, VenezuelaJacob L., Jr., eds. Bauxite, Proceedings of the 1984 Bauxite Symposium. Feb. 27–March 1, 1984. American Institute of Mining, Metallurgical, and Petroleum Engineers, Boulder, Colorado, 1984. 387–406.
Meybeck, M. Concentrations des eaux fluviales en éléments majeurs et apports en solution aux océans. Rev. Géol. Dynam. Géogr. Phys. 1979; 21:215–246.
Meybeck, M. Global chemical weathering of superficial rocks estimated from river dissolved loads. Am. J. Sci. 1987; 287:401–428.
Meyer, G. A., Wells, S. G., Jull, A. J. T. Fire and alluvial chronology in Yellowstone National Park: Climatic and intrinsic controls on Holocene geomorphic processes. Geol. Soc. Am. Bull. 1995; 107:1211–1230.
Milliman, J. D., Meade, R. H. World-wide delivery of river sediment to the oceans. J. Geol. 1983; 91:1–21.
Milliman, J. D., Syvitski, J. P. M. Geomorphic/tectonic control of sediment discharge to the ocean: The importance of small mountainous rivers. J. Geol. 1992; 100:525–544.
Morgan, W. J. Hotspot tracks and the early rifting of the Atlantic. Tectonophysics. 1983; 94:123–139.
Murnane, R. J., Stallard, R. F. Germanium and silicon in rivers of the Orinoco drainage basin, Venezuela and Colombia. Nature. 1990; 344:749–752.
Nagy, K. L., Dissolution and precipitation kinetics of sheet silicatesWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, New York, 1995. 173–233.
Pain, C. F. Characteristics and geomorphic effects of earthquake initiated landslides in the Albert Range of Papua New Guinea. Eng. Geol. 1972; 6:261–274.
Paolini, J. Transporte de carbono y minerales en el río Caroní. Interciencia. 1986; 11:295–297.
(MARAVEN) Petroleos de Venezuela, S. A., Mosaico de Imágenes de Radar de Visión Lateral. Ministereo de Energía y Minas, Dirección General Sectoral de Geología y Minas, Caracas, Venezuela Scale 1:2,500,000, Sheet No. NB19-8, 1977.
Pitman, W. C., III. Relationship between eustacy and stratigraphic sequences of passive margins. Geol. Soc. Am. Bull. 1978; 89:1389–1403.
Potter, P. E. Petrology and chemistry of modern big river sands. J. Geol. 1978; 86:423–449.
Potter, P. E. Modern sands of South America: composition, provenance and global significance. Geol. Rundschau. 1994; 83:212–232.
Ronov, A. B., Migdisov, A. A., Barskaya, N. V. Tectonic cycles and regularities in the development of sedimentary rocks and paleogeographic environments of sedimentation of the Russian Platform (an approach to a quantitative study). Sedimentology. 1969; 13:179–212.
Schubert, C., Briceño, H. O., Fritz, P. Paleoenvironmental aspects of the Caroní-Paragua river basin (southeastern Venezuela). Interciencia. 1986; 11:278–289.
Scott, G. A. J., Relationships between vegetation cover and soil avalanching in Hawaii. Proc. Assoc. Am. Geogr. ; 7. 1975:208–212.
Scott, G. A. J. Soil profile changes resulting from the conversion of forest to grassland in the montaña of Peru. Great Plains-Rocky Mount. Geogr. J. 1975; 4:124–130.
Scott, G. A. J., Street, J. M. The role of chemical weathering in the formation of Hawaiian Amphitheatre-headed Valleys. Z. Geomorph. N. F. 1976; 20:171–189.
Simon, A., Larsen, M. C., Hupp, C. R. The role of soil processes in determining mechanisms of slope failure and hillslope development in a humidtropical forest, eastern Puerto Rico. Geomorphology. 1990; 3:263–286.
Sloss, L. L. Sequences on the cratonic interior of North America. Geol. Soc. Am. Bull. 1963; 74:93–114.
Sloss, L. L., Global sea level changes: A view from the cratonWatkins J. S., Montardert L., Dickerson P. W., eds. Geological and Geophysical Investigations of Continental Margins. American Association of Petroleum Geologists Memoir; 29. American Association of Petroleum Geologists, Washington, DC, 1979.
Sloss, L. L., Speed, R. C., Relationships of cratonic and continental-margin tectonic episodesDickenson W. R., ed. Tectonics and Sedimentation. SEPM Special Publication; 22. Society of Economic Paleontologists and Mineralogists, Tulsa, Oklahoma, 1974. 98–119.
Soares, P. C., Landim, P. M. B., Fulfaro, V. J. Tectonic cycles and sedimentary sequences in the Brazilian intracratonic basins. Geol. Soc. Am. Bull. 1978; 89:181–191.
WHOI-80-29 Stallard, R. F. Major element geochemistry of the Amazon River system. Ph. D. Dissertation, Massachusetts Institute of Technology/Woods Hole Oceanographic Institution, Joint Program in Oceanography, Woods Hole Oceanographic Institution, Woods Hole, MA. 1980.
Stallard, R. F., River chemistry, geology, geomorphology, and soils in the Amazon and Orinoco basinsDrever J. I., ed. The Chemistry of Weathering. NATO ASI Series C: Mathematical and Physical Sciences; 149. D. Reidel Publishing Co., Tulsa, Oklahoma, 1985. 293–316.
Stallard, R. F., Weathering and erosion in the humid tropicsLerman A., Meybeck M., eds. Physical and Chemical Weathering in Geochemical Cycles. NATO ASI Series C: Mathematical and Physical Sciences; 251. Kluwer Academic Publishers, Dordrecht, Holland, 1988. 225–246.
Stallard, R. F., Relating chemical and physical erosionWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Dordrecht, Holland, 1995. 543–564.
Stallard, R. F. Tectonic, environmental, and human aspects of weathering and erosion: A global review using a steady-state perspective. Ann. Rev. Earth Plan. Sci. 1995; 12:11–39.
Stallard, R. F. Terrestrial sedimentation and the carbon cycle: Coupling weathering and erosion to carbon burial. Glob. Biogeochem. Cycles. 1998; 12:231–252.
microfiche supplement Stallard, R. F., Edmond, J. M. Geochemistry of the Amazon 2: The influence of the geology and weathering environment on the dissolved load. J. Geophys. Res. 1983; 88:9671–9688.
Stallard, R. F., Edmond, J. M. Geochemistry of the Amazon 3. Weathering chemistry and limits to dissolved inputs. J. Geophys. Res. 1987; 92:8293–8302.
Stallard, R. F., Koehnken, L., Johnsson, M. J. Weathering processes and the composition of inorganic material transported through the Orinoco River system, Venezuela and Colombia. Geoderma. 1991; 51:133–165.
Sugden, D. E. A case against deep erosion of shields by ice sheets. Geology. 1976; 4:580–582.
Sugden, D. E. Glacial erosion by the Laurentide Ice Sheet. J. Glaciol. 1978; 20:367–391.
Suppe, J. Mechanics of mountain building in Taiwan. Geol. Soc. China Mem. 1981; * 67–89.
Sverdrup, H., Warfvinge, P., Estimating field weathering rates using laboratory kineticsWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Washington, DC, 1995. 485–541.
Szczerban, E. Cavernas y simas en areniscas precámbricas del Territorio Federal Amazonas y Estado Bolívar. Venezolana Direc. Geol. Bol. Geol. Public. Esp. 1976; 7:1055–1072.
Tardy, Y., Bocquier, G., Paquet, H., Millot, G. Formation of clay from granite and its distribution in relation to climate and topography. Geoderma. 1973; 10:271–284.
Trolard, F., Tardy, Y. An ideal solid solution model for calculating solubility of clay minerals. Clay Miner. 1989; 24:1–21.
Urbani, P. F. Notas sobre el origen de las cavidades en rocas cuarcíferas precámbricas del Grupo Roraima, Venezuela. Interciencia. 1986; 11:298–300.
Vail, P. R., Herdenbol, J. Sea-level changes during the Tertiary. Oceanus. 1979; 22:71–79.
Vail, P. R., Mitchum, R. M., Todd, R. G., Widmier, J. M., Thompson, S., III., Scngree, J. B., Bubb, J. N., Hatlelid, W. G., Seismic stratigraphy and global changes of sea-level. Seismic-stratigraphy Applications to Hydrocarbon Exploration. American Association of Petroleum Geologists Memoir; 26. American Association of Petroleum Geologists, Washington, DC, 1977.
van Breeman, N., Effects of seasonal redox processes involving iron on the chemistry of periodically reduced soilsStucki J. W., Goodman B. A., Schwertmann U., eds. Iron in Soils and Clay Minerals. NATO ASI Series C: Mathematical and Physical Sciences; 217. Kluwer Academic Publishers, Tulsa, Oklahoma, 1988.
van Breeman, N., Long-term chemical, mineralogical, and morphological effects of iron-redox processes in periodically flooded soilsStucki J. W., Goodman B. A., Schwertmann U., eds. Iron in Soils and Clay Minerals. NATO ASI Series C: Mathematical and Physical Sciences; 217. Kluwer Academic Publishers, Dordrecht, The Netherlands, 1988.
Vitousek, P. M. The regulation of element concentrations in mountain streams in the northeastern United States. Ecol. Monogr. 1977; 47:65–87.
Wentworth, C. K. Soil avalanches on Oahu, Hawaii. Geol. Soc. Am. Bull. 1943; 54:53–64.
White, A. F. Surface chemistry and dissolution kinetics of glassy rocks at 25°. Geochim. Cosmochim. Acta. 1983; 47:805–815.
White, A. F., Chemical weathering rates of silicate minerals in soilsWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Dordrecht, The Netherlands, 1995. 407–461.
White, A. F., Blum, A. E. Effects of climate on chemical weathering in watersheds. Geochim. Cosmochim. Acta. 1995; 59:1729–1747.
White, A. F., Brantley, S. L., Chemical weathering rates of silicate minerals: An overviewWhite A. F., Brantley S. L., eds. Chemical Weathering Rates of Silicate Minerals. Reviews in Mineralogy; 31. Mineralogical Society of America, Washington, DC, 1995. 1–22.
White, W. A. Deep erosion by continental ice sheets. Geol. Soc. Am. Bull. 1972; 83:1037–1096.
Zonneveld, J. I. S. Preliminary remarks on summit levels and the evolution of the relief in Surinam (S. America). Verhand. Kon. Ned. Geol. Mijnbouwk. Gen. 1969; 27:53–60.