Origin and Early Evolution of Animals
Paulyn Cartwright
OUTLINE
1. The Cambrian explosion and the origin of animal phyla
2. Animal phylogeny
3. Multicellularity and the origin of sponges (phylum Porifera)
4. The origin of the nervous system and the evolution of sensory structures in Cnidaria
5. The origins of Bilateria and the phylogenetic placement of Ctenophora, Acoela, Myxozoa, and Placozoa
6. Animal diversity
Around 600 million years ago, members of the animal clade were present but distinct from animals seen on earth today. The origin of most living animal lineages occurred relatively suddenly during the Cambrian period (543–510 Ma). The rapid appearance of animal phyla in the fossil record is referred to as the Cambrian explosion. Understanding the evolutionary relationships by reconstructing the animal tree of life is fundamental for unraveling key transitions in animal evolution. While much progress has been made, the phylogenetic position of many major animal lineages remains uncertain. Through the study of animals’ closest living relatives, the choanoflagellates, and early diverging animal lineages, such as sponges and cnidarians, we can begin to understand how major innovations such as the evolution of multicellularity, the nervous system and sense organs, and bilateral symmetry evolved. Future investigation into the phylogenetic placement of several enigmatic taxa will increase our understanding of when and how major transitions in animal evolution occurred. Whole genome sequencing of early diverging animals has revealed that the ancestral animal genome was remarkably complex and that the ancestral animals had a molecular toolkit permitting the development of diverse and complex animal body plans.
GLOSSARY
Bilateria. A clade of animals that display bilateral symmetry and are traditionally divided into two major groups, the protostomes and the deuterostomes. Includes most major animal phyla except sponges, cnidarians, and ctenophores.
Cambrian Explosion. Refers to an interval in the history of life from 543 to 510 million years ago when most animal phyla suddenly appeared in the fossil record.
Choanoflagellates. A clade of single-celled or colonial eukaryotes that are the closest living relatives to animals.
Early Diverging Animals. An informal term for animals that are not members of Bilateria. Includes sponges, cnidarians, and ctenophores. (Also sometimes called “basal animals” or “basal metazoans”; see chapter II.1 for a critique of such terms.)
Metazoa. Another name for multicellular animals; eukaryotic organisms that are generally motile and possess an embryonic stage that undergoes gastrulation.
Molecular Toolkit. The set of genes and gene pathways present in a genome that can be co-opted to facilitate body plan evolution. Usually refers to signaling molecules and transcriptional factors that are important for regulating and specifying key aspects of animal development.
1. THE CAMBRIAN EXPLOSION AND THE ORIGIN OF ANIMAL PHYLA
The origin and diversification of major animal lineages represents a key episode in the history of life. To understand these critical events, we must turn to the fossil record, which provides the only tangible evidence for the origin of animal life. The earliest fossils thought to be animals are a diverse assemblage of macroscopic fossils known as the Ediacaran fauna, appearing in the Vendian period (610–550 Ma). Many of these fossils bear a superficial resemblance to jellyfish, crustaceans, and worms, but none of the fossils can be definitively assigned to modern groups of animals. Many scientists dispute their affinity to animals at all. For example, paleontologist Adolf Seilacher claims that ediacarans may have been an independent experiment in multicellular life that subsequently went extinct.
The next glimpse at possible animal life is found in the Doushantuo fossil formation in China, which dates back to 580 million years ago. This formation holds a diverse set of microscopic fossils that bear a striking resemblance to embryos of cnidarians (jellyfish and sea anemones) and embryos of bilaterian animals such as worms. Detailed cellular structure of these microscopic fossils has been preserved by a rare form of preservation known as phosphatic fossilization. As with the Ediacaran fossils, assignment to modern animal lineages is controversial.
Most animal phyla bearing unmistakable characteristics of modern-day animals made their first appearance in the early Cambrian period, between 510 and 543 million years ago. During a window of slightly more than 30 million years, most modern animal phyla, including mollusks, annelids, arthropods, and chordates, suddenly appeared in the fossil record. This time period, while long in human terms, is incredibly brief compared to the entire history of life, which spans more than 3.5 billion years. As a result, this key event is often referred to as the Cambrian explosion.
Two famous fossil formations, the Chenjiang in the Yunning Province of China (525 Ma) and the Burgess Shale in British Columbia, Canada (500 Ma), provide a remarkable glimpse of Cambrian animal diversity. These fossil formations contain preservations of animals that lack hard parts, providing detailed preservations of soft-bodied forms in addition to a diversity of skeletonized fossils. In these fossil formations we find representatives from sponge, ctenophore, arthropod, annelid, priapulid, and chordate lineages. Another fossil formation similar to the Burgess Shale, the Marjum Formation in Utah (500 Ma), has yielded a remarkable diversity of jellyfish fossils (phylum Cnidaria). Table 1 provides names and dates of some of the earliest animal fossil representatives of major phyla.
Differing explanations have been given for the Cambrian explosion. Some argue that a long hidden history of animal evolution dates well into the Precambrian, with glimpses of this diversity provided by the Doushantuo phosphatized embryos, but that Precambrian conditions were not conducive to preservation of macroscopic animals. Others have argued that the Cambrian radiation represents a true explosion of animal diversification. The timing of this explosion could be due to changes in the abiotic environment, including fragmentation of a supercontinent that provided more opportunities for geographic isolation and speciation. Studies of trilobites show that at the time of their first appearance in the fossil record 525 million years ago, they already showed significant biogeographic differentiation; their origins must therefore have occurred well before the Cambrian (Lieberman 2003). In addition, a warming climate with reduced ice cover and increasing levels of atmospheric oxygen provided conditions more conducive to the survival of macroscopic animals. Changes in ocean chemistry could have facilitated biomineralization used to build skeletons as protection from predators. The evolution of skeletal armor and hardened feeding structures (e.g., teeth) may have triggered an arms race that further increased diversification. Another explanation is that the genetic toolkit present in single-celled ancestors happened to have features that enabled rapid diversification of metazoan body plans. No single hypothesis can adequately explain this significant event in the history of life. Evidence from fossils, phylogenies, biogeography, paleoecology, genomics, and developmental biology, when interpreted together, provide the best explanation for the Cambrian explosion.
Table 1. Earliest animal fossil representatives of some major phyla
Phylum |
Name |
Date in millions of years (fossil formation) |
Porifera (sponges) |
Palaeophragmodictya |
560 (Ediacara) |
Ctenophora (comb jellies) |
Maotianoascus |
540 (Meishucun) |
Arthropoda |
Anomalocaris |
530 (Burgess Shale) |
Chordate |
Yunnanozoon, Haikouichthys |
525 (Chengjiang) |
Brachiopoda |
Many |
525 (Chengjiang) |
Urochordate |
Shankouclava |
525 (Chengjiang |
Mollusca |
Fordilla |
514 (Greenwich) |
Cnidaria (jellyfish) |
Unnamed |
500 (Marjum) |
2. ANIMAL PHYLOGENY
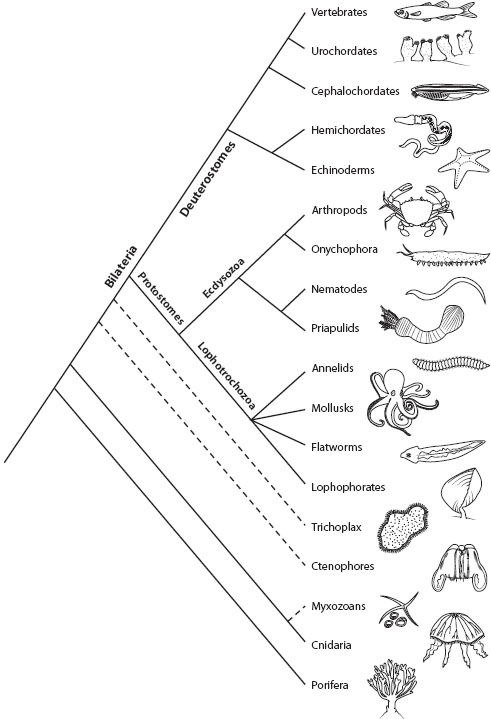
Reconstructing the animal tree of life remains one of the grand challenges in evolutionary biology and is essential to understanding key transitions in animal evolution. The challenges arise in part because of the rapid divergence and ancient origin of the major animal lineages, which date back more than 500 million years, as discussed above. Most of our current understanding of animal relationships comes from recent studies using large data sets of DNA sequences. Figure 1 summarizes our current understanding of the evolutionary relationships of most major animal phyla.

Figure 1. Evolutionary relationships of major animal phyla.
The closest living relatives to animals are the choanoflagellates. Choanoflagellates exist as single cells or small colonies. The name choanoflagellate comes from a distinct collar structure, made up of closely packed microvilli, that surrounds their flagellum. The phylum Porifera (sponges), which is outside the major clade that includes all other known lineages of animals, also possess cells, called choanocytes, with flagellated collar cells. Sponges display a diversity of forms in their adult stage, but have long been thought to be united by the presence of choanocytes and a distinctive body plan: benthic filter feeders with chambers that circulate water and filter food; however, recent molecular phylogenetic studies suggest that Porifera may not possess a single common ancestor and may instead comprise two to four separate lineages (Calcarea, Demospongiae, Hexactinellida, and Homoscleromorpha). Separate sponge lineages imply that a sessile, filter-feeding existence is not a unique feature uniting sponges but was, instead, present in the last common ancestor of all animals.
The cnidarians, which include corals, sea anemones, and jellyfish, diverged from the main animal lineage following the divergence of sponges. All cnidarians possess stinging cells that house complex intracellular, venom-containing structures called nematocysts. Cnidarians are radially or biradially symmetrical, comprise just a few cell types, are diploblastic, meaning they lack organs and consist of an outer ectodermal cell layer and an inner gastrodermal cell layer, but lack a third layer, the mesoderm. Despite this simple construction, cnidarians comprise a huge diversity of forms and habitats, including, for example, the simple freshwater Hydra polyp, intertidal anemones, expansive coral reefs, benthic colonial hydrozoans, and pelagic jellyfish.
Ctenophores are commonly referred to as comb jellies because they have ciliated rows, called combs, arranged longitudinally along their bodies that are used for locomotion. Ctenophores superficially resemble jellyfish, and traditionally ctenophores and cnidarians were classified in a group called Coelenterata; however, morphological and molecular data do not support this hypothesis, although the exact phylogenetic placement of ctenophores remains controversial (see below).
The remaining animal lineages belong to the clade Bilateria. Bilaterian animals, as the name implies, display bilateral symmetry, in conjunction with triploblasty: possession of a third, middle cell layer, the mesoderm. Given their bisymmetrical organization, early wormlike bilaterians could move in a forward motion with their sensory and feeding structures located at the anterior ends. With the acquisition of a mesoderm, they could also form organs and organ systems. The simplest bilaterian organization is exemplified by the small wormlike animals in Acoelomorpha (acoels and nematodermatids). These small worms lack a through-gut and instead have one opening for eating and excreting, similar to cnidarians. They also lack a body cavity called a coelom that is found in almost all other bilaterians. Although it has long been thought that Acoelomorpha are an early diverging relative to the main group of bilaterians, their phylogenetic placement has recently come into question (see below).
The majority of bilaterian animals are divided into two major groups, Protostomia and Deuterostomia. These clades are named based on the fate of the blastopore (an opening in the early embryo); in protostomes, the blastopore generally develops into a mouth, whereas in deuterostomes, it forms an anus; however, in many animals the blastopore is an ephemeral feature and the opening to the gut develops anew. Thus, although Protostomia and Deuterostomia are indeed separate lineages, as evidenced by molecular data, the actual names should not be overinterpreted.
Recent work has revealed that Protostomia comprises two main clades, the Lophotrochozoa and the Ecdysozoa (Halanych 2004). Members of Lophotrochozoa include annelids, mollusks, bryozoans, phoronids, and brachiopods and are characterized by either a trochophore larva (annelids and mollusks) or a lophophore feeding structure in the adult (bryozoans, phoronids, and brachiopods). Spiral cleavage, a particular pattern of cell division in early embryogenesis, was likely ancestral for Lophotrochozoa but subsequently modified in the lophophore-bearing taxa. Relationships between major lophotrochozoan lineages remain uncertain.
Ecdysozoa are a group of animals united by having a cuticular exoskeleton that is shed through molting. Ecdysozoa includes arthropods, onychophorans, nematodes, nematomorphs, kinorhynchs, priapulids, and tardigrades. Relationships between major ecdysozoan lineages, especially with respect to the placement of tardigrades, remain uncertain.
Deuterostomia is a large group of animals supported by numerous molecular phylogenetic analyses. Morphological characters that unite deuterostomes are the development of the coelom through pinching off of the gut (enterocoely), and a coelom divided into three separate sections (tripartite). Deuterostomes comprise two main clades, Ambulacraria, which includes xenoturbellids, echinoderms, and hemichordates, and Chordata, which includes tunicates, cephalochordates, and craniates (vertebrates and their relatives). Molecular phylogenetic studies have revealed the inclusion of the small worm Xenoturbella in Deuterostomia, the placement of hemichordates as the closest relative to echinoderms, and the possible placement of tunicates, and not cephalochordates, as the closest relatives to vertebrates. These findings have required the reevaluation of the evolution of many deuterostome-specific characters. For example, xenoturbellids lack all of the traditional, diagnostic deuterostome traits, in addition to lacking a through-gut and a coelom, suggesting extensive loss of characters in this lineage. Likewise, hemichordates and chordates have gill slits, which implies that gill slits might be ancestral for deuterostomes, with subsequent losses in echinoderms and xenoturbellids. The placement of tunicates relative to craniates is controversial. Classically, tunicates have been considered the closest relative to craniates + cephalochordates, and this hypothesis has been supported by some molecular phylogenetic studies with ribosomal RNA genes (rDNA). Other molecular phylogenetic studies combining morphology with rDNA genes, or employing multiple protein markers, place tunicates as the closest relative to craniates, with cephalochordates falling outside of tunicates + craniates. This latter hypothesis is supported by the presence of migratory neural crest cells found in vertebrates and also reported present in tunicates but not cephalochordates. This resolution also implies that metameric segmentation, classically used to unite craniates + cephalochordates, is ancestral for Chordata and was secondarily lost in tunicates.
3. MULTICELLULARITY AND THE ORIGIN OF SPONGES (PHYLUM PORIFERA)
Animals evolved from a single-celled eukaryotic ancestor; the evolution of multicellularity is thus a key event in the evolution of animals. Choanoflagellates represent the closest living relative to animals. They exist as free-living single cells or as small colonies. As noted above, choanoflagellates display a distinctive morphology that includes a collar-shaped structure surrounding their flagellum. Sponges possess a cell type, the choanocyte, that is similar to an individual choanoflagellate. In sponges, the choanocytes line interior cavities and function to circulate water and filter food particles. Given that sponges represent either a grade (i.e., distinct lineages united by ancestral characters) or a clade sister to all other animals, and choanoflagellates are the closest relatives to animals, it is likely that the ancestor of animals resembled a modern-day choanoflagellate. Insights into the transition from a single-celled organism to a complex multicellular animal can therefore be gleaned from the study of choanoflagellates.
Two primary functions are required for multicellularity in animals: the ability of cells to adhere to one another, and the ability of cells to communicate to one another. These functions are necessary precursors for cellular specialization and coordination required for proper animal development and function. Recent research has shown that single-celled choanoflagellates possess a large array of genes that in animals function in cell signaling and cell adhesion, including C-type lectins, cadherins, components of the extracellular matrix, and participants in protein kinase signaling pathways (King 2004). Given the surprising complexity of the choanoflagellate genome, it is certain that many of the molecules necessary for multicellularity were already present in the animal ancestor. Although the role of these genes in choanoflagellates has not been sorted out, it is thought they may function in adhering cells to surfaces, catching prey, mating, and responding to environmental cues. Later in evolution, these same gene families were likely co-opted for cell-to-cell communication in multicellular animals.
Some choanoflagellates form small colonies, providing a glimpse of how the first multicellular animal may have been organized. Colonies of the choanoflagellate Proterospongia, for example, show signs of limited functional specialization and cellular differentiation. Proterospongia has two types of cells; the outer flagellated collar cells propel the colony through the water, whereas the inner amoeboid cells divide to enlarge the colony. The cell signaling pathways likely function in the coordination among cell types within these small colonies.
Further cellular specialization and coordination between cells is evident in sponges. As noted above, sponges likely do not form a single lineage but instead represent a grade. They are multicellular, but lack organized tissues, muscles, nerves, or a gut. The adult sponge is organized around chambers that circulate water and filter food, which is ingested by specialized amoeboid cells. Although adult sponges are markedly different from all other animals, sponge embryos possess some of the hallmarks of animal development. Sponges undergo a process akin to gastrulation (reorganization of cells into layers), although sponges do not form true epithelia as found in other animals. This is followed by a ciliated larval stage. Gastrulation, which is lacking on choanoflagellates, is critical for setting up the adult body plan in animals, in that it provides spatial organization for the differentiation of specific cell types. It certainly evolved in the ancestor of all animals and marks the beginning of the evolution of animal body plans.
4. THE ORIGIN OF THE NERVOUS SYSTEM AND THE EVOLUTION OF SENSORY STRUCTURES IN CNIDARIA
Sponges lack nerve cells; however, they do possess the ability to sense and respond to their environment. Recent whole-genome sequencing of the sponge Amphimedon queenslandia revealed that sponges have genes that code for components of the nervous system, even though they lack nerve cells. Although their functions are unknown, these protoneuronal components may have served as the building blocks for the first nerve cell to evolve in animals.
Cnidarians display clear signs of organized tissues, including muscle cells and nerve cells. Comparative genome studies reveal that cnidarians possess a large array of genes involved in neural development and signaling that is nearly as complex as seen in bilaterians, despite the lack of a centralized nervous system or brain in Cnidaria. Instead, cnidarians possess a diffuse nerve net, dispersed among epithelial cells. Cnidarians also possess a type of neural cell not found in other phyla: stinging cells called nematocysts, which possess mechanosensory capabilities. This type of decentralized nervous system likely represents an ancestral condition for animals. Even among cnidarians, there are various degrees of complexity and specialization of the nervous system. Although they lack a central nervous system, many cnidarians have areas where nerve cells form a plexus or longitudinal track.
The nerve cells of cnidarians express various types of neural peptides that often show spatially structured expression patterns. For example, RFamide-positive neurons are expressed around the mouth in hydrozoan polyps and near epithelial muscles in hydromedusae. The pelagic colonial hydrozoan Aglantha digitale has elaborate nerve-ring systems that control complex behavior among the different polyps and medusae in the colony, including directional swimming and food capture. These nerve cell complexes and nerve rings are signs of the centralization and coordination of a nervous system as needed for complex behaviors.
Cnidarians possess the ability to sense and respond to light, chemicals, and touch. These abilities are derived from nerve cells, which can communicate with non-neural cells to elicit behavioral responses through signaling mechanisms. For example, cnidarians possess seven-pass transmembrane G protein–coupled receptors (GPCRs) that can respond to mechanical or chemical stimuli through ion channel signaling. The most developed sense organs occur in medusozoan cnidarians, which include Scyphozoa, the true jellyfish, Cubozoa, the box jellyfish, and Hydrozoa, which includes colonial hydroids and hydromedusae. Scyphozoans and cubozoans organize their sense organs in structures called rhopalia, located on the bell of the medusa. Rhopalia house statocysts that serve as balance organs, and eyes for responding to visual cues. The cubozoans possess complex eyes that include lenses, retinas, and corneas. Cubozoans express the developmental regulatory gene PaxB in their developing rhopalia (Kozmik et al. 2003). This gene is a homolog to Pax genes in bilateria that are involved in eye and ear development. Likewise, a number of regulatory genes involved in sensory structures in bilaterians are also expressed in the sensory structures of the jellyfish Aurelia. The genomic and developmental evidence suggest that the molecular and cellular precursors to complex centralized nervous systems and sense organs were present in the common ancestor of cnidarians and Bilateria (with some present before the divergence of sponges) and were modified and elaborated in individual animal lineages.
5. THE ORIGINS OF BILATERIA AND THE PHYLOGENETIC PLACEMENT OF CTENOPHORA, ACOELA, MYXOZOA, AND PLACOZOA
The phylogenetic placement of four enigmatic animal groups—ctenophores, acoels, placozoans (Trichoplax), and myxozoans—has been controversial. Understanding their position in the animal tree of life is critical for elucidating the evolutionary transitions that occurred between major animal lineages, as many of these taxa display traits that might be seen as intermediate forms. Key evolutionary innovations such as the origin of mesoderm, a through-gut, bilateral symmetry, and a centralized nervous system all occurred sometime between the divergence of cnidarians and the last common ancestor of Bilateria. Many classic and recent molecular studies have attached the four enigmatic animal groups to the stem lineage of Bilateria; however, other studies have placed ctenophores and placozoans at the base of Metazoa (diverging before Cnidaria), myxozoans within Cnidaria, and acoels as derived deuterostomes. Given the important implications of these phylogenetic hypotheses, they are worth exploring in more detail.
Recent work has provided evidence that ctenophores have some degree of bilateral symmetry, a developed nervous system, muscles, and evidence of mesodermal tissue, suggesting they are closely allied to bilaterians; however a phylogenetic analysis using a large molecular data set positioned ctenophores as sister to all other animals, suggesting that the group’s bilaterian features evolved independently in the ctenophore lineage (Dunn et al. 2008). This study, however, has been questioned, and subsequent studies have refuted this claim. Resolution of the phylogenetic placement of Ctenophora has important implications for our understanding of the evolution of bilaterian symmetry and various organ systems.
Acoels are small wormlike animals that, like cnidarians, lack a coelom and a through-gut; however, unlike the diploblastic cnidarians, they are triploblasts, possessing a mesoderm. Hence it appears, at least superficially, that acoels represent a transitional form between diploblasts like cnidarians and bilaterians. Although originally placed in the phylum Platyhelminthes, which is now known to be part of the lophotrochozoan clade in Protostomia, numerous molecular phylogenetic studies have supported the placement of acoels as sister to other Bilateria, consistent with morphological evidence that acoels possess some but not all bilaterian features. A more recent but still controversial study has suggested that acoels are not early diverging bilaterians but are derived deuterostomes (Philippe et al. 2011); specifically, this study placed acoels as the closest relatives of xenoturbellids (in a clade with echinoderms). This phylogenetic placement, if corroborated, would suggest that acoels are not an example of a transition to bilaterians but instead resulted from secondary losses of derived bilaterian structures. Clarification of the position of acoels in the animal tree of life awaits further study.
Myxozoans are a diverse group of minute freshwater and marine organisms that alternate their parasite life cycle between invertebrate and fish hosts. Historically, myxozoans were thought to be protists; however, a number of characteristics led many to consider the possibility that they might be animals, including a multicellular stage in their life cycle and the possession of intracellular polar capsules used to attach to a host. These polar capsules bear remarkable similarity to nematocysts in the stinging cells of cnidarians, leading to the suggestion that myxozoans could be animals related to cnidarians. Molecular phylogenetic studies using nuclear ribosomal genes have placed myxozoans within bilateria, but outside its major subclades (Protostomia and Deuterostomia); however, other studies, combining nuclear ribosomal genes with morphology or using protein-coding genes, place myxozoans with cnidarians. The failure to come to a consensus in the placement of myxozoans with molecular data is likely due to their highly aberrant DNA sequences (Evans et al. 2010); however, the remarkable similarities between polar capsules and nematocysts of cnidarians, along with some molecular support, suggest that myxozoans are probably cnidarians and that the polar capsules and nematocysts represent a single evolutionary origin.
Placozoans are microscopic animals that lack a mouth, gut, organs, nervous system, bilateral symmetry, or even an anterior-posterior axis. The only described species in this phylum is Trichoplax adhaerens. These organisms have just a dorsal and ventral cell layer; they digest food particles intracellularly with their ventral cells. The phylogenetic position of placozoans is controversial. Their simple construction suggests they could be the sister group to all other animals. This hypothesis is supported by the analysis of the structure of the Trichoplax mitochondrial genome, which is more protist-like than animal-like (Dellaporta et al. 2006); however, the presence of cell junctions, which are lacking in sponges, suggests that placozoans are more derived, and analyses of rDNA support a divergence from other animals after sponges but before cnidarians. This conclusion has been confirmed by analysis of multiple genes, although other hypotheses of relationships have also been reported. Although it is tempting to view the simplicity of the Trichoplax as an ancestral condition to all other animals, this conclusion should perhaps be viewed as tentative, pending further studies of the animal tree of life.
6. ANIMAL DIVERSITY
The ecological, molecular, and evolutionary processes that occurred at the dawn of animal evolution were fundamental for setting the stage for the diversity of animal life that we see on earth today. All major animal lineages evolved in the oceans, but subsequent diversification has resulted in numerous groups that have invaded freshwater. In addition, after land was colonized by plants (see chapter II.14), multiple animal lineages invaded land, most famously the tetrapods (Vertebrata) and insects (Arthopoda), but also including arachnids (Arthropoda), isopods (Arthropoda), gastropod snails (Mollusca), and earthworms (Annelida). In addition to these major ecological transitions, animal diversification on both land and water has been accompanied by shifts into different ecological niches. In these transitions, animals had to overcome major challenges associated with respiration, reproduction, and osmoregulation. Animals have evolved parasitic (e.g., tapeworms), mutualistic (e.g., anemones and anemone fish), and highly cooperative (e.g., bee colonies) lifestyles. Through the course of evolution, animals have generally increased in overall complexity, although animals have also re-evolved simpler body plans. The most extreme example of the latter, the reevolution of a single-celled organism, is found in the canine transmissible venereal tumor (CTVT), whose ancestor was a dog. The past 500 million years have been witness to evolutionary processes that have resulted in the amazing diversity of animals we observe on earth today, which display an unimaginable diversity of forms, ecological habitats, and lifestyles.
FURTHER READING
Cartwright, P., S. L. Halgedahl, J. R. Hendricks, R. D. Jarrard, A. C. Marques, A. G. Collins, and B. S. Lieberman. 2007. Exceptionally preserved jellyfishes from the Middle Cambrian. PLoS ONE 2(10): e1121, 1–9. This study documents the first exquisitely preserved true jellyfish from the Cambrian.
Dellaporta, S. L., A. Xu, S. Sagasser, W. Jakob, M. A. Moreno, L. W. Buss, and B. Schierwater. 2006. Mitochondrial genome of Trichoplax adhaerens supports Placozoa as the basal lower metazoan phylum. Proceedings of the National Academy of Sciences, USA 103(23): 8751–8756. Provides molecular evidence that the enigmatic invertebrate Trichoplax was an early diverging animal.
Dunn, C. W., A. Hejnol, D. Q. Matus, K. Pang, W. E. Browne, S. A. Smith, E. Seaver, et al. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452: 745–749. Provides a hypothesis about the relationship of major animal phyla using sequences from multiple protein-coding genes.
Evans, N. M., M. T. Holder, M. S. Barbeitos, B. Okamura, and P. Cartwright. 2010. The phylogenetic position of Myxozoa: Exploring conflicting signals in phylogenomic and ribosomal datasets. Molecular Biology Evolution 50(3): 456–472. Discusses the controversies surrounding the phylogenetic placement of Myxozoa.
Gould, S. J. 1989. Wonderful Life: The Burgess Shale and the Nature of History. New York: W. W. Norton. The book that introduced the Burgess Shale fossil fauna to a broader audience.
Halanych, K. M. 2004. A new view of animal phylogeny. Annual Review of Ecology and Systematics 35: 229–256. A comprehensive review of major issues surrounding animal phylogenetics.
King, N. 2004. The unicellular ancestry of animal development. Developmental Cell 7: 313–325. Provides evidence that choanoflagellates share a similar genetic toolkit with animals.
Kozmik, Z., M. Daube, E. Frei, B. Norman, L. Kos, L. J. Dishaw, M. Noll, and J. Piatigorsky. 2003. Role of Pax genes in eye evolution: A cnidarian PaxB gene uniting Pax2 and Pax6 functions. Developmental Cell 5: 773–785. Provides molecular evidence uniting light-sensing organs in all animals.
Lieberman, B. S. 2003. Taking the pulse of the Cambrian radiation. Journal of Integrative and Comparative Biology 43: 229–237. Documents the role played by geological change in the Cambrian radiation.
Minelli, A. 2009. Perspectives in Animal Phylogeny and Evolution. New York: Oxford University Press. Provides a comprehensive overview of major features considered important in animal evolution.
Philippe, H., H. Brinkmann, R. R. Copley, L. L. Moroz, H. Nakano, A. J. Poustka, A. Wallberg, K. J. Peterson, and M. J. Telford. 2011. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470: 255–258. Provides molecular evidence that acoelomophs are deuterstomes.