Evolution of Reaction Norms
Stephen C. Stearns
OUTLINE
1. Two major features of the genotype-phenotype map
2. Induced responses: Examples of adaptive plasticity
3. Robust traits: Examples of canalization
4. Reaction norms: Phenotypic plasticity and canalization
5. The evolutionary significance of plasticity and canalization
6. The Baldwin effect and genetic assimilation
Because the genetically identical members of clones can develop different phenotypes when they encounter different environments, we infer that one genotype can produce different phenotypes depending on the environment encountered. What difference does this make to the evolutionary process? To help answer that question, evolutionary biologists use the concepts of phenotypic plasticity, reaction norms, and canalization to describe the patterns observed. This chapter describes how those patterns are thought to evolve, what consequences they have for further evolution, whether they can be predicted and whether they are adaptive, nonadaptive, or maladaptive. It concludes with a discussion of the nature, origin, and evolutionary significance of genetic assimilation, one of the consequences of phenotypic plasticity.
GLOSSARY
Canalization. The limitation of phenotypic variation by developmental mechanisms that buffer it against genetic and environmental variation.
Genetic Assimilation. Environmentally induced phenotypes become genetically fixed and no longer dependent on the original environmental stimulus.
Phenotypic Plasticity. Sensitivity of the phenotype to differences in the environment.
Reaction Norm. A property of a genotype and a particular type of phenotype plasticity that arises when a trait is a continuous function of an environmental variable; it describes the mechanism by which development maps the genotype into the phenotype as a function of the environment.
1. TWO MAJOR FEATURES OF THE GENOTYPE-PHENOTYPE MAP
Throughout this chapter, I assume that the phenotype is a fixed property of an individual that develops once in a lifetime and does not change thereafter. This restriction excludes from the discussion both learned behavior and seasonally cyclic morphological changes, which are reversible and more dynamic than the plasticity discussed here.
What is at stake here is how best to build development into our models of the microevolutionary process. This, one of the major projects currently confronting evolutionary biologists, is often expressed as trying to understand the major features of the genotype-phenotype map, the set of rules linking the information contained in the genome to the material stuff of the organism. Understanding those major features—two of which are phenotypic plasticity and canalization—is thought by many to be a key to future breakthroughs in biology.
2. INDUCED RESPONSES: EXAMPLES OF ADAPTIVE PLASTICITY
Induced responses are classical examples of adaptive phenotypic plasticity. They satisfy one definition of an adaptation: a change in a phenotype that occurs in response to a specific environmental signal and improves reproductive success; otherwise, the change does not take place. Some water fleas in the genus Daphnia develop helmets and spines that protect them against predators, but only when they detect predators. Predators feed less effectively on spiny, helmeted Daphnia, but helmets and spines are costly. Individuals that do not produce them have higher reproductive rates than individuals that do produce them; that is why the spines and helmets are not produced when predators are not present.
Similarly, barnacles of the genus Chthamalus react to the presence of a predatory snail, Acanthina, by altering their development. If the snail is present, the barnacles grow into a bent-over form that suffers less from predation but pays for it with a lower reproductive rate. If the snail is not present, the barnacles develop into a typical form with normal reproduction. Tollrian and Harvell review many other examples of induced responses.
3. ROBUST TRAITS: EXAMPLES OF CANALIZATION
Traits that exhibit very little phenotypic variation despite considerable environmental and genetic variation are called canalized because the phenotypic outcome is kept constant, as though development were confined within a canal that allowed no deviations from its course. When the canalization breaks down, for whatever reason, genetic variation for the hidden trait is revealed, demonstrating that the normal state was genetically canalized.
For example, Rendel found that Drosophila melanogaster normally have exactly four scutellar bristles, but in flies homozygous for the mutation scute, the number of bristles is reduced to an average of two with some variation. The mutation both reduces the average number of bristles and allows previously hidden variation for bristle number to be expressed. Because this variation responds to selection for fewer or greater number of bristles, we know it is based on genes other than the scute locus, and can infer that because of developmental buffering, the phenotypic effect of mutations in genes affecting this canalized trait had been suppressed in wild-type flies.
Many developmentally stable features of the phenotype appear to be canalized, including the four limbs of tetrapods, the six legs of insects, the eight legs of spiders, and the seven cervical vertebrae of almost all mammals, from whales to giraffes, none of which respond developmentally to environmental variation or to the genetic variation normally encountered from one generation to the next by a developmental system in sexually reproducing organisms.
Neither plasticity nor canalization is an absolute property. Some traits are more plastic and some more canalized than others; we detect both patterns through comparisons. We will return to canalization after developing the tools needed to analyze plasticity, the most important of which is the concept of a reaction norm.
4. REACTION NORMS: PHENOTYPIC PLASTICITY AND CANALIZATION
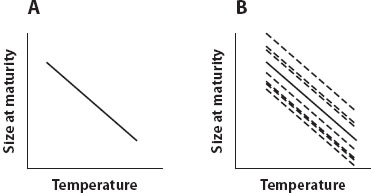
Narrowly defined, a reaction norm is a property of one trait, one genotype, and one environmental factor. We can measure it by raising individuals from one clone at different levels of an environmental factor, measuring the trait at each level, and plotting it as a function of the environmental factor. The resulting line (figure 1A) describes how development maps the genotype into the phenotype as a function of the environment. A population of genotypes can be represented as a bundle of reaction norms (figure 1B); the average reaction of the population to the environmental factor is the population mean reaction norm.

Figure 1. (A) An example of a reaction norm, which is a property of a single genotype: individuals all belonging to a single clone mature at smaller sizes when reared at higher temperatures. (B) An example of a bundle of reaction norms. Each dashed line represents the sensitivity of a single genotype to temperature; the solid line represents the population mean reaction norm.
Depicting trait variation as a bundle of reaction norms does two important things. First, it shows us at a glance how genes and environments interact to determine the trait. Consider three genotypes (G1, G2, and G3) sampled from a population of parthenogenetic lizards, reared as clones, and raised at three population densities of low, medium, and high (figure 2), and two traits, number of digits per foot and fecundity. Figure 2A depicts the reaction norms of the three genotypes for number of digits per foot. In fact, they would lie on top of one another, for every individual in the entire population has exactly five digits per foot at all population densities; in the figure they are separated to show that three genotypes were measured. These are perfectly flat reaction norms. The trait is insensitive to environmental variation, expresses no genetic variation, and cannot respond to selection. It is both genetically and environmentally canalized.
Figure 2. (A) The number of digits in the hand of a lizard is not sensitive to population density and does not vary genetically; it has a flat reaction norm that is both genetically and environmentally canalized. (B) The fecundity of the same three genotypes is sensitive to population density; here, sensitivity to density varies with genotype.
For fecundity, the situation is quite different (figure 2B). All three genotypes reduce their fecundity at higher population densities, but they differ in sensitivity to changes in density. G1 is quite sensitive. It has the highest fecundity at low population density and the lowest fecundity at high population density. G3 is not very sensitive. It has the lowest fecundity at low population densities and the highest fecundity at high population densities. G2 is intermediate.
Second, figure 2B also gives us a starting point for thinking about how natural selection operates on phenotypic plasticity. Phenotypic plasticity, the sensitivity of a trait to a change in an environmental factor, is the slope of the reaction norm. If there is genetic variation in the slopes of reaction norms, selection can change plasticity by selecting genotypes whose reaction norms have larger or smaller slopes, but it will only do so under the particular circumstances discussed next.
Selection on Plasticity: A Matter of Frequency and Quality of Encounters
Plasticity is a second-order effect, defined by the difference in phenotypic response to two or more environments and measured in two or more individuals. The strength of selection on plasticity depends on the frequency with which environments are encountered in space and time and on the reproductive performance of the genotypes in each environment. Environments rarely encountered have little influence on selection; those encountered frequently influence selection more strongly. Frequency of encounter depends on both the frequency of the different environmental types and the size of the population encountering them. Environments in which survival and reproduction are good more strongly influence selection than environments in which survival and reproduction are poor, for subpopulations in good environments contribute more to population growth and recruitment. Thus both the frequency and the quality of an environment encountered determine the degree to which an evolved reaction norm will be adapted to that environment. If an environment is rarely encountered and of poor quality, it makes little difference how the organism responds to it with phenotypic plasticity, for it will not contribute much to future generations. We can expect all portions of a reaction norm to be adjusted by selection to fit the organism to each environment only if all environments are encountered with roughly equal frequency, and if all environments are of roughly equal quality. While that situation is conceivable, it is probably not often the case, suggesting that some parts of reaction norms will usually be better adapted than others.
The Costs of Plasticity: Usually Environment-Specific and Small
If there were no costs or limits to plasticity, the appropriately plastic organism would outcompete all others, for it could adjust to every environment encountered with maximal reproductive performance. For the reasons given in the previous section, such perfect adjustment of plasticity to all environments is unlikely, and the existence of imperfectly plastic phenotypes is not a puzzle, for they can simply be the by-products of environments that are rarely encountered, of poor quality, or both. The issue of costs of plasticity nevertheless remains interesting, for the costs could further limit evolution, particularly if there is a cost of being plastic per se.
The many attempts to measure the costs of plasticity were recently reviewed by Auld, Agrawal, and Relyea. They found it helpful to distinguish two types of costs of plasticity: production costs, specific to the environment in which the phenotype is produced, and maintenance costs, independent of any specific environment and associated with maintaining the general capacity to respond. In more than 200 sets of paired estimates of the costs of plasticity, detected costs were most frequently environment specific and therefore probably production costs rather than costs of maintenance.
Measured costs of plasticity have usually been small, probably for two associated reasons. The first is that fitness costs are not likely to be large in environments frequently encountered, for those are the environments in which selection has had the greatest opportunity to adjust the reaction norms to produce the optimal phenotype. If fitness costs are measured by comparing two frequently encountered environments, it should be no surprise that the estimates will be small. The second reason is that whenever costs are incurred, mutations that reduce costs—compensatory mutations—will be selected. The opportunity to select such mutations depends on the frequency and quality of the environments encountered; their efficacy also depends on the degree to which their expression must be specific to an environment to shape the reaction norm to fit it. There is, however, another type of cost of plasticity: the fitness cost of adjusting a phenotype to an expected environmental state on the basis of a signal that happens at times to mislead. Such costs could be quite high; they are quite relevant to predicting the consequences of global warming, and they have not yet been measured.
Is All Plasticity Adaptive? No, But Often Part of It Is
Selection is thus most likely to shape a plastic response that fits the phenotype to the local environment if that environment is frequent, of high quality, and capable of supporting a large population. Those are general evolutionary conditions. Other conditions are physical and chemical. For example, all chemical reactions proceed more slowly at lower temperatures; there is therefore no reason to invoke a past history of selection to explain the observation of slower development at lower temperatures per se. Only if the observed response differs from that predicted from chemistry unmodified by evolution might we suspect adaptation. Such was found to be the case by Berven, Gill, and Smith-Gill in frogs maintaining populations at high and low altitude. As would be expected from chemistry alone, frogs developed more slowly at lower temperatures at high altitude; however, in addition, frogs that had evolved at high altitude developed more rapidly at those low temperatures than did frogs from low altitude raised at the same low temperatures. This indicates an evolutionary adjustment of developmental rate; it also shows that part of a plastic response can be adapted while another part is the inevitable consequence of chemistry and physics.
Can the Plastic Response Be Predicted? Yes, for Life History Traits
For traits that are direct components of reproductive success, the relationship between a change in the trait and a change in fitness can be calculated, and from that calculation an optimal reaction norm can be predicted. Such a reaction norm is optimal in the sense that any other response would yield lower fitness given the trade-offs assumed. For traits like age and size at maturity, for example, one trade-off usually assumed is that earlier maturation implies less exposure to the risk of death because of a shorter juvenile period, but also because of less time to grow to a large size that would support the production of many offspring (see chapter III.11). Starting with work by Stearns, Crandall, and Koella, models based on such assumptions have often predicted the evolution of optimal reaction norms for age and size at maturity that embody this rule: if growing fast, mature large and young; if growing slow, mature old and small (figure 3).

Figure 3. An optimal reaction norm for age and size at maturity (dark line) with predicted maturation events (Xs) for four different growth trajectories (dotted lines).
The qualitative prediction of figure 3 is often but not always observed; the exceptions suggest ways in which the assumptions of the models may have been violated. Other shapes are predicted if growth rates are correlated with adult or juvenile mortality rates, if growth is determinate rather than indeterminate, if environmental heterogeneity is dominated by spatial structure rather than temporal change, and if explicit account is taken of population dynamics with frequency- and density-dependent effects.
The position and shape of such a reaction norm are seen as genetically determined and shaped by a history of selection. The particular point along the reaction norm at which an individual matures depends on the environment in which that individual has been raised. The reaction norm plot thus reveals how nature and nurture—genes and environment—interact to determine the actual age and size at which an individual matures. That maturation event is thus determined both by the history of selection encountered by the population and by a particular individual’s history of developmental interaction with the environment.
Why Canalization Is Not the Opposite of Plasticity
Canalization, the limitation of phenotypic variation by developmental buffering, can act to buffer environmental or genetic variation, or both. Again, reaction norm plots are a convenient way to visualize some helpful distinctions.
In figure 4, comparison of A and B suggests that both sets of reaction norms are canalized with respect to environmental variation (they are environmentally canalized), because all the reaction norms are flat, but those in A are in a tight bundle, whereas those in B are in a loose bundle. This suggests that those in A may be more genetically canalized (i.e., canalized with respect to genetic variation) than those in B. Comparison of C and D indicates that both sets of reaction norms are not canalized with respect to environmental variation, since all the reaction norms have negative slopes; however, those in C, like those in A, are in a tight bundle, whereas those in D, like those in B, are in a loose bundle, suggesting that those in C may be more genetically canalized than those in D. Thus a trait can be genetically canalized but environmentally plastic; a trait can also be environmentally canalized but genetically free to vary. Care must be taken to specify precisely what pattern is under analysis.
Figure 4. (A) A set of reaction norms suggesting both genetic and environmental canalization when compared with those in B. (C) A set of reaction norms suggesting genetic but not environmental canalization when compared with those in D.
Reaction norm plots can show that traits are not canalized, but at least two reasons can always be given for why they might appear genetically canalized. The first is that they actually are buffered against variation by developmental mechanisms (e.g., the impact of the scute mutation on bristle number in Drosophila); the second is that they have been under strong selection that has depleted the genetic variation in the population. Therefore the description of the pattern must be followed by an analysis of the mechanisms that produced it before a conclusion about the causes driving those mechanisms can be drawn. That search for causes has begun.
Selection for Canalization
Several hypotheses for the selection of canalization have been proposed; while they are not mutually exclusive, and therefore all could act at once, the evidence for some is better than others. Schmalhausen (1986; originally published in English in 1949) suggested that canalization is a result of stabilizing selection, for if there is a single optimal phenotype, then any deviation from it has lower fitness, and canalization would buffer the phenotype from such costly deviations, whether caused genetically—by mutation, recombination, or gene flow—or environmentally. Evidence from genetically and environmentally controlled experiments on fruit flies (Stearns et al. 1995) supports Schmalhausen’s idea, but, as Wagner et al. (1997) point out, the ability of stabilizing selection to shape canalization depends on the amount of genetic variation present in the population and the degree to which canalizing genes have deleterious pleiotropic effects on other characters or direct effects on the same character. Their arguments suggest, given the amount of genetic variation usually present, that the further evolution of canalization will be quite slow on a microevolutionary timescale and that the evolution of canalization through stabilizing selection is quite unlikely ever to achieve complete fixation of a trait.
Another reason for selection of canalization was suggested by Kawecki (2000), who pointed out that in a fluctuating environment, selection tends to produce the phenotypes that work best in the previous environment, not the one currently encountered. (This resembles the complaint that the generals are always fighting the last war.) In such circumstances canalization reduces variation in fitness, increasing geometric mean fitness.
Siegal and Bergman (2002) proposed a strikingly different reason for the existence of canalization. They investigated selection for robustness in genetic networks required to deliver products reliably or the organism would fail to develop properly; they concentrated on selection for developmental stability rather than for stabilizing selection on the phenotype. They discovered that phenotypic canalization was a by-product of selection on developmental stability, whose strength increases with the complexity of the genetic network. Since then, others have established that this conclusion is independent of the details of their model, and that such selection is stronger in larger as well as more complex genetic networks in which more mutations can have an effect. Selection for canalization as a by-product of developmental stability can also be strong even in a small genetic network if it is perturbed by gene flow from other populations, which can have larger effects than single mutations. Their work now motivates further research to establish how much of canalization can be attributed to selection for developmental stability and how much to selection for a specific, optimal phenotype.
Evidence for Canalization
Some of the best evidence for canalization comes from the study of heat-shock proteins. Heat-shock proteins are molecular chaperones that accompany other proteins to the intracellular sites where they function, protecting them on their journey. When the production of one heat-shock protein, HSP 90, is inhibited, development is altered and hidden genetic variation is released in both fruit flies (Drosophila: Rutherford and Lindquist) and wall cress (Arabidopsis: Queitsch, Sangster, and Lindquist). These results establish at least one mechanism that causes canalization and extend Rendel’s classical results, based on mutation in the scute gene, to the molecular level. Note that there is no need to postulate that the effects of HSP 90 evolved because they suppress genetic variation; that consequence could simply be a side effect of its direct chemical function, which is to stabilize proteins in their journey from biosynthesis to cellular action.
Evidence for canalization is also now coming from work inspired by Siegal and Bergman’s ideas on selection for developmental stability supported by robust genetic networks. Duplicating a pathway in such a network can ensure proper development when one pathway is knocked out or perturbed; gene duplications with that effect have been found in flower development (Lenser et al. 2009). And when the inactivation of a gene in such a network has no effect on the phenotype, one suspects some buffering mechanism; such genes are common in yeast. Other genes buffer the effects of such genetic inactivation, and it turns out that the buffering genes also confer robustness to environmental and stochastic changes (Lehner 2010). From the local point of view of a genetic control network, this makes good sense, for at this level of intracellular detail, the source of the perturbation is unknown and irrelevant, but the consequences of the perturbation, no matter what the source, are serious. One therefore expects the buffering mechanisms to be general and not specific to the source, whether genetic or environmental.
5. THE EVOLUTIONARY SIGNIFICANCE OF PLASTICITY AND CANALIZATION
Plasticity has many evolutionary consequences, among the most important of which is that it extends the range of conditions under which organisms can survive and reproduce and thus reduces the frequency at which populations go extinct. Another major consequence, genetic assimilation, is discussed in the next section.
One evolutionary consequence of canalization is that it renders invisible and therefore neutral any mutations that could affect a genetically canalized trait, allowing a greater proportion of them to be stored in the population than would otherwise be the case (see chapter IV.1). If canalization breaks down, this variation is released from buffering and expressed in phenotypes, providing additional material on which selection can act. How frequently this happens in nature is at this point uncertain; in a sample consisting mostly of life history traits, there was no consistent pattern (Hoffmann and Parsons 1991).
Another potential evolutionary consequence of canalization is that it plays a role in the origin of long-term constraint. Once a trait has been canalized and its form and function have been fixed, other traits coevolve with the fixed form of the trait. This can embed the canalized trait in a network of interactions with other traits so that continued successful function depends on the canalized trait remaining canalized; the process selects for further buffering of its canalized state. While this may be one source of developmental constraint, evidence supporting the idea remains scarce, and, as Wagner et al. (1997) point out, the mechanism that leads to complete fixation of a trait is unlikely to be stabilizing selection.
6. THE BALDWIN EFFECT AND GENETIC ASSIMILATION
In a novel environment, a plastic response can help an organism survive where a canalized organism would die. Such a response is not an adaptation to that environment, which has never been encountered before; it is a preadaptation that can allow the adaptive process to continue. One result can be a change in the genetic determination of the trait when it is expressed in the new environment.
This process, or something like it, has been called the Baldwin effect (as described by Baldwin), genetic assimilation (by Waddington), genetic accommodation (by West-Eberhard), and stabilizing selection (by Schmalhausen). Each of the labels emphasizes a different part of a complex process; genetic accommodation is the most inclusive.
Lande analyzed this process by framing it as the evolution of reaction norms in a novel environment. He started from a background condition in which the trait was canalized with minimum genetic and phenotypic variation and there was no correlation between reaction norm elevation and slope. He found that in the first generation in the novel environment, mean fitness drops, and the mean phenotype moves toward the new optimum, a change at that point based only on plasticity. Then adaptation occurs in two phases. First, increased plasticity rapidly evolves, allowing phenotypes to approach the new optimum in the novel environment. Then the new phenotype undergoes slow genetic assimilation that reduces plasticity (the slope of the average reaction norm) but compensates with genetic evolution of the elevation of the reaction norm in the original environment.
Whatever one calls it, such a process has several important consequences. It reduces the probability that a population will go extinct when it encounters novel conditions. And it makes clear that the phenotype has an important role in evolutionary change that has been underappreciated, a role described by West-Eberhard as plasticity and behavior taking the lead in evolution. The generality and significance of this important idea are under continuing examination.
FURTHER READING
Auld, J. R., A. A. Agrawal, and R. A. Relyea. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proceedings of the Royal Society B 277: 503–511.
Hoffmann, A. A., and P. A. Parsons. 1991. Evolutionary Genetics and Environmental Stress. Oxford: Oxford University Press.
Kawecki, T. J. 2000. The evolution of genetic canalization under fluctuating selection. Evolution 54: 1–12.
Lande, R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. Journal of Evolutionary Biology 2: 1435–1446.
Lehner, B. 2010. Genes confer similar robustness to environmental, stochastic, and genetic perturbations in yeast. PLoS ONE 5: e9035. doi:10.1371/journal.pone.0009035.
Lenser, T., G. Theissen, and P. Dittrich. 2009. Developmental robustness by obligate interaction of Class B floral homeotic genes and proteins. PLoS Computational Biology 5: e10000264 doi:10.1371/journal.pdbi.1000264.
Pigliucci, M. 2001. Phenotypic Plasticity: Beyond Nature and Nurture. Baltimore, MD: Johns Hopkins University Press.
Schmalhausen, I. I. 1986. Factors of Evolution: The Theory of Stabilizing Selection. Chicago: University of Chicago Press.
Siegal, M. L., and A. Bergman. 2002. Waddington’s canalization revisited: Developmental stability and evolution. Proceedings of the National Academy of Sciences USA 99: 10528–10532.
Stearns, S. C., and R. E. Crandall. 1984. Plasticity for age and size at sexual maturity: A life-history response to unavoidable stress. In G. Potts and R. J. Wootton, eds., Fish Reproduction. London: Academic.
Stearns, S. C., M. Kaiser, and T. J. Kawecki. 1995. The differential genetic and environmental canalization of fitness components in Drosophila melanogaster. Journal of Evolutionary Biology 8: 539–557.
Stearns, S. C., and J. C. Koella. 1986. The evolution of phenotypic plasticity in life-history traits: Predictions for norms of reaction for age- and size-at-maturity. Evolution 40: 893–913.
Tollrian, R., and C. D. Harvell. 1999. The Ecology and Evolution of Inducible Defenses. Princeton, NJ: Princeton University Press.
Wagner, G. P., G. Booth, and H. Bagheri-chaichian. 1997. A population genetic theory of canalization. Evolution 51: 329–347.
West-Eberhard, M. J. 2003. Developmental Plasticity and Evolution. New York: Oxford University Press.