Evolution of Parasite Virulence
Dieter Ebert
OUTLINE
1. Defining virulence
2. The phase model of virulence
3. The trade-off model
4. Vertically transmitted parasites
5. How well do optimality models predict virulence?
Diseases caused by parasite or pathogen infections impair normal functioning in organisms. These impairments can include very diverse symptoms leading to the organism’s morbidity and mortality. Studies of the evolution of the virulence of infectious diseases strive to understand the expression of these symptoms as the result of the evolutionary process. This approach is primarily focused on the evolution of parasites (here used to include pathogens), but it may also consider the coevolution of hosts and parasites.
Until about 30 years ago, it was widely accepted that the harmful symptoms of infectious diseases were the side effects of poorly adapted parasites, and that over time virulence would therefore generally evolve toward avirulence. The underlying logic was that a well-adapted parasite should not harm its host, as doing so would deplete its own resource. This view was challenged in the 1980s by Roy Anderson, Robert May, and Paul Ewald, who argued that virulence is often a necessary consequence when parasites exploit their hosts, and depending on the specific conditions, the optimal level of virulence (i.e., the level that maximizes parasite fitness) may range from low to high virulence. Virulence is now understood as a trait whose evolution can be analyzed within the general framework of evolutionary biology, thus considering the roles of history, chance, and natural selection.
The evolution of virulence is not only of academic interest; its conceptual framework also has implications in various applied fields, such as human and veterinary medicine and agriculture. In particular, public health workers can benefit from considering population-biological aspects of the evolution of virulence.
GLOSSARY
Basic Reproductive Number, R0. The average number of secondary infections resulting from a primary infection in a population of susceptible hosts.
Horizontal Transmission. The passing of a parasite between two hosts that are not related in direct line, for example, vector-borne transmission and sexual transmission.
Kin Selection. Selection on organisms that share genes by common descent. Fitness is said to be inclusive because it considers the fitness of related individuals.
Myxoma Virus. A DNA virus from the family Poxviridae. This virus causes myxomatosis and has been used to control rabbit pests in Australia and Europe. Its virulence evolved rapidly after its release.
Parasite. An infective agent transmitted among hosts and growing or replicating within hosts. Pathogens are included in this definition.
Reproductive Manipulator. Parasites with maternal (vertical) transmission that manipulate host reproduction to increase their representation in the offspring of the next host generation, for example, by killing or feminizing male offspring.
Vertical Transmission. The passing of a parasite from a parent (usually the mother) to an offspring.
Virulence. Parasite-induced morbidity and mortality of a host. More precise definitions, such as parasite-induced host death rate or host fecundity reduction, are used for specific situations.
1. DEFINING VIRULENCE
Virulence is simply defined here as the parasite-induced morbidity and mortality of the host. This definition includes any fitness effect the parasite has on the host, whether that effect is an incidental by-product of the infection or an adaptive trait for the parasite. This definition does not, however, explain how virulence evolves, because it does not specify the link between parasite fitness and virulence. Most attempts to understand the evolution of virulence are based on models of parasite evolution and therefore consider only aspects of virulence that are important for parasite fitness. For an exclusively horizontally transmitted parasite, host mortality is important, as the parasite might die with the host, whereas reduced host fertility or sexual attractiveness—which are important for host fitness—may be of little concern for the parasite. For example, congenital rubella syndrome is a serious illness of babies born to mothers who became infected with the rubella virus during the first trimester of pregnancy. It is a big concern for the human host but is unlikely to have an impact on the evolution of the rubella virus. This picture changes, however, for parasites that rely on vertical transmission. For them, host fecundity becomes an important part of the virulence definition. Thus, understanding the evolution of virulence requires detailed knowledge about the host-parasite system in question. In the application of models of optimal virulence to actual diseases, the key factors to consider are the mode of parasite transmission and the trade-offs among parasite fitness components.
Virulence may be further categorized by distinguishing between effects directly beneficial for the parasite (e.g., when host death is required for parasite transmission, as in many parasites of invertebrates) and those effects that are costly for both the host and the parasite (e.g., host death when infections are transmitted among living hosts). Most models of the evolution of virulence consider the latter scenario. Examples of effects with a direct benefit for the parasite include parasitic castration (which liberates resources for parasite reproduction), impaired host mobility (which may increase access to the host by vectors that transmit the parasite further), and parasite-induced changes in host behavior (which may increase chances of transmission).
2. THE PHASE MODEL OF VIRULENCE
The once-dominant view that only novel diseases are highly virulent and that well-adapted parasites are less virulent is based on two related ideas: first, that virulence is a result of a new interaction between a host and a parasite, and second, that virulence changes as the parasite adapts to the new host. A refined version of this two-stage scenario tries to combine the different aspects of virulence evolution and expression into a unified framework (Ebert and Bull 2008). This model distinguishes three successive phases of disease evolution. Phase 1 is the first contact of a parasite with a host that it usually does not infect, often called accidental infection. In phase 2 the parasite has only recently established itself in a new host species, at which point the parasite’s virulence is not yet the result of adaptive evolution. In phase 3 the parasite has evolved for some time in a particular host and has adapted to the specific conditions of this host population.
The phase model emphasizes that not all aspects of parasite virulence can be understood as a result of adaptive evolution. Consider, for example, the following diseases of humans. The highly virulent Ebola virus does not circulate long enough in humans to evolve an optimal level of virulence. Transmission chains are short, so it remains in phase 1. The human immune deficiency virus (HIV) entered the human population several decades ago. It is clearly able to persist in humans, but it may not have had time to reach an optimal level of virulence. Thus, it can be considered to be in phase 2. Much older human diseases, such as tuberculosis and leprosy, are likely to be in phase 3.
Phase 1: Accidental Infections
Many terrifying human diseases are caused by accidental infections including, for example, Ebola, bird flu, SARS, anthrax, Lyme disease, Legionnaires’ disease, West Nile virus, and echinococcosis. For some of these diseases, untreated infections can approach 100 percent mortality rates. Transmission chains are short, and epidemics do not persist, so the parasites have little opportunity to adapt to their new hosts. At first glance, this case might seem to support the view that novel diseases are highly virulent. However, the most virulent accidental infections are most likely to be recognized, whereas avirulent accidental infections will often go unnoticed, thus producing a strong sampling bias. In fact, avirulent accidental infections may outnumber virulent infections, as experimental transspecies infection trials suggest. Although a few novel infections are highly virulent, most are avirulent. Thus, highly virulent novel diseases are the exception, not the rule, although they may have profound impact on humans and natural populations.
Accidental infections have played and continue to play an important role in applied fields such as medicine and agriculture. In medicine, vaccine development has taken advantage of transspecies host shifts, both by using parasites of closely related species (Jenner’s pox vaccine derived from accidental infections with cowpox) and by evolving attenuated parasite lines as vaccines. In agriculture, highly virulent novel infections have been used in pest control, as, for example, to control the very dense populations of European rabbits in Australia and the United Kingdom with the Myxoma virus, which is derived from a virus of a related host species.
Phase 2: Evolution of Virulence Following Successful Invasion
After a parasite infects a novel host species, its transmission success will determine whether it will persist and spread in this species. Initial spread is typically epidemic, and the level of virulence of the nonadapted parasite is unlikely to be close to optimum. Selection typically shapes a parasite’s life history and virulence during the epidemic and especially over the following period, as it becomes endemic. Every endemic infectious disease has made the successful transition from phase 1 to 2 at some stage in its evolutionary history, but the number of observed cases is very low, despite many more examples of accidental infections (more than 1000 human diseases, so-called zoonoses). Phase 2 can also help us understand the emergence and spread of novel variants (mutants) of a parasite in an established host-parasite association. These mutants may have extreme effects and may spread rapidly, causing an epidemic and being unaffected by existing host defenses. If they occur frequently, a parasite population may never reach an optimum virulence and thus will remain in phase 2 (Bull and Ebert 2008). In this case, the fitness consequences of having suboptimal virulence are likely to be minor relative to the fitness gains the mutants achieve by evading host immunity and other defenses.
Studying parasites in phase 2 illuminates how the tempo and mode of virulence evolution proceeds in real time. The best example is the Myxoma virus. A highly virulent strain of this virus was introduced to Australia to control European rabbits, an invasive species that was causing extensive damage. Within a few years the average virulence of the parasite had changed drastically, attaining a new level far below the virulence of the original strain (Fenner and Kerr 1994). Apparently, the original virulence had been far above the presumed optimum, although not so high that it had prevented the spread of the virus. The Myxoma example leaves many questions open, as multiple factors changed simultaneously (e.g., host density, host genetic composition). But it does demonstrate that virulence can evolve rapidly, that virulence evolution does not necessarily lead to complete avirulence, and that virulence coevolves with the host (Fenner and Kerr 1994).
Laboratory experiments that follow parasite evolution after a change of environmental conditions or a host shift are in effect creating phase 2 situations. This situation is particularly true for serial passage experiments, which played an important role in vaccine development, and also for the understanding of virulence evolution. In these experiments parasites are passaged in novel hosts with transmission controlled by the researcher (in some cases, the parasite might go extinct without this intervention). The evolution of the parasite is then monitored over many generations. Evolution typically results in a strong increase in virulence in the host in which the passages take place. At the same time, the parasites display reduced virulence in their former hosts. This attenuation of virulence makes these parasites good candidates for vaccines, as the immune response of the former host still recognizes the parasite, but its low virulence prevents disease or limits its severity. The increased virulence in the novel host is linked to an increase in the parasite’s within-host multiplication rate. This increase is likely driven by within-host competition among parasite variants, with the most prolific variants having the highest chance to be transmitted during the next passage.
Phase 3: The Evolution of Optimal Virulence
Parasites that persist for some time in a host are expected to evolve an optimal level of virulence, that is, the level of virulence at which parasite fitness is maximal. It is widely thought that this optimum is characterized by trade-offs among different parasite fitness components. Thus, a key difference between phase 2 and phase 3 is that in phase 2, the parasite is not yet subject to the constraint imposed by the trade-offs. The Myxoma virus example was the first to show the existence of a trade-off, in this case between the rate at which rabbits clear the infection (host-induced parasite death) and the rate at which the parasite kills the host (and itself). Highly virulent Myxoma strains kill the host too quickly, while strains with low virulence are quickly cleared by the host’s immune response. The optimal balance between these two parasite fitness components was shown by Anderson and May (1982) to maximize the parasite’s spread in the host population. A mathematical model, latter called the trade-off model, and the observed data agreed well.
3. THE TRADE-OFF MODEL
When considering models of optimal virulence, it is essential to define virulence precisely. In the mathematical model first applied to analyze the Myxoma data, virulence is defined as the parasite-induced host death rate. Other detrimental effects the parasite may have on the host, such as reduced mating success and fecundity, are not considered because they are not fitness components of the parasite. This simplification is acceptable under the assumption that parasite-induced host death rate is positively correlated with the various expressions of morbidity. These positive correlations also justify the use of surrogate measures of virulence in empirical studies, such as host fatigue, sensitivity to stress, fever, or other physiological parameters. However, it is important to keep in mind that correlations between parasite-induced host death rate and other disease-related traits may be weak or may have a negative sign. For example, many parasites of invertebrates castrate their hosts, which eliminates host fecundity but allows the parasite to keep its host alive for much longer than a noncastrator would be able to. In the following discussion of optimal virulence, parasite-induced host mortality is used as a definition for virulence.
The first and still most used model for the evolution of virulence is the trade-off model (Anderson and May 1982). This model is a powerful starting point for analyzing the evolution of virulence, although its simplicity implies certain assumptions and makes it vulnerable to various criticisms. The model stresses the importance of trade-offs between parasite-induced host death and other parasite fitness components. In its simplest form, parasite fitness is estimated as the number of secondary infections that result from a primary infection, R0:
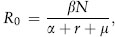
where β is the transmission rate in a susceptible host population of density N, α is virulence (parasite-induced host death rate), r is the rate at which hosts clear infections, and μ is the host background (parasite-independent) mortality rate. Thus, α, r, and μ are all components of the parasite’s overall death rate, while βN indicates the production of new infections. Without trade-offs, parasite evolution would maximize β and minimize the total parasite death rate (α + r + μ), thus driving virulence to zero. A positive correlation between β and α, or a negative one between α and r (as in the case of the Myxoma example), constrains the evolution of virulence. If the increase in β is leveling off relative to the increase in α (i.e., the relationship is asymptotic), intermediate levels of virulence are predicted. This simple trade-off model relies entirely on the assumption that between-host transmission is the quantity maximized by parasite evolution. Although 30 years old, the hypothesis that between-host transmission is crucial for the evolution of virulence is still not strongly supported by empirical evidence. A critical test will be to show that transmission success has the assumed hump-shaped relationship with virulence. Empirical studies that tested this prediction were conducted with unicellular parasites that infect mice, butterflies, and water fleas (Daphnia).
Some of the major limitations of the simple trade-off model are that it ignores the role of multiple infections and within-host evolution of the parasites, kin structure of parasites, and host genetic variation. The model also ignores changes over time in the density of susceptible hosts, including those changes that may occur as the parasite itself evolves.
Multiple Infections, Inclusive Fitness, and Virulence
In the 1990s the trade-off model was extended to include within-host competition. Within-host competition describes scenarios in which different variants of parasites compete within hosts, which strongly influences both their likelihood of transmission to the next host and the level of virulence expressed in the multiply infected hosts. Parasite variants that replicate more quickly are assumed to be superior in within-host competition, even if this reduces host survival and thus shortens the period for transmission to take place. Because higher replication rate is associated with higher virulence, average virulence is expected to be higher in populations with frequent multiple infections than in those with single infections, all else being equal. As a consequence, more virulent parasite variants may dominate although they do not maximize the R0 as predicted by the simple trade-off model. However, more complex models incorporating additional factors that might occur with multiple infections have been developed that predict the opposite result under specific circumstances. In particular, cooperation among parasites to exploit the host or parasite strategies to exploit one another may lead to the evolution of lower virulence.
Empirical tests on the role of multiple infections have generally supported the key assumption and prediction of the basic multiple infection model. Thus, higher within-host multiplication rates have been found to be associated with both superior competitive ability and higher virulence. Interestingly, it has also been found that some parasites increase their virulence facultatively when the host has been multiply infected, which implies that the parasites are able to sense, either directly or indirectly, the presence of competing parasite variants.
Multiple infection virulence models make a number of other predictions as well. Ecological and demographic factors such as higher host density, longer parasite survival outside the host, longer host life span (e.g., low, parasite-independent mortality), and less spatial structure of the host population (more mixing) are predicted to lead to increased virulence, because these factors should increase the incidence of infections by multiple parasite variants. These predictions have been explained using inclusive fitness theory, which considers the kin structure of the parasite population. Inclusive fitness is a crucial factor in the evolution of virulence, because within-host competition cannot be considered independently from the genetic relatedness of the competing parasites (Frank 1996). More closely related parasites have more common reproductive interests and thus gain less from competition. Using inclusive fitness theory, one can generalize findings that link ecological features with virulence and place the role of transmission mode into a unified context. For example, in well-mixed host populations, multiple infections result mostly from unrelated parasites, which maximizes competition and thus also virulence. In contrast, in viscose host populations, multiple infections more often arise through infections from the same parasite lineage. In these latter cases, lower levels of virulence are expected to evolve because of kin selection in the parasite population. As will be discussed further, kin selection also plays an important role in the evolution of virulence of vertically transmitted parasites.
Host Genetic Variation
Empirical studies have shown that virulence is not only the product of parasite evolution but also the result of the coevolutionary interaction between hosts and the parasites. Some models of virulence evolution have incorporated certain aspects of host genetic variation, but the complexity of the interactions that influence the expression of virulence makes it unlikely that general predictions can be made. Nevertheless, models and empirical data agree that parasite virulence should be lower in genetically diverse host populations. This effect is based on the observation that greater host diversity slows the spread of parasites (parasites spread faster in host monocultures), and this diversity thus reduces multiple infections. Trade-offs in the performance of parasite genotypes across different host genotypes or host species suggest that parasite fitness is compromised (and virulence reduced) in diverse host populations. Finally, host evolution that counters the effects of infections may lead to a reduction in parasite virulence. Incorporating the various effects of host genetic variation into models of disease virulence remains one of the big future challenges for understanding the evolution of virulence.
4. VERTICALLY TRANSMITTED PARASITES
The discussion thus far has focused on those parasites that engage exclusively in horizontal transmission, that is, transmission among hosts unrelated in direct line. Many parasites, however, are entirely or partially vertically transmitted, usually from mothers to offspring. In this case, the parasite’s fitness depends on host reproductive success, and this dependency must be included in models that seek to explain the evolution of virulence. It is best to begin this discussion by focusing on those parasites that are transmitted exclusively from mothers to offspring. Such parasites must either evolve to manipulate host reproduction to their own benefit or evolve to complete avirulence. This necessity can readily be understood when one realizes that a parasite that is transmitted only vertically, and that harms its host’s reproductive success without promoting its own transmission, will go extinct as parasite-free hosts outcompete those that are infected.
Some vertically transmitted parasites, such as the bacterium Wolbachia and some microsporidians, have evolved mechanisms to manipulate host reproduction in ways that increase their presence in future generations, despite being virulent. These mechanisms include the killing of host sons, feminizing males to become functional females, and inducing cytoplasmic incompatibility. In some of these cases, only kin selection can explain the observed virulence, because the individual parasites that produce the virulent effects (e.g., killing sons, inducing cytoplasmic incompatibility), in essence, commit suicide because they preclude their own propagation. Other individual parasites are genetically identical or nearly so, and they benefit from these behaviors. Parasites that manipulate host reproduction are very rarely transmitted horizontally.
Parasites with exclusive maternal transmission that do not manipulate their hosts must evolve avirulence, because the parasite’s reproductive success is perfectly linked to the reproductive success of its host. In these cases, there is no conflict of interest between host and parasite. Some of the best empirical support for virulence theory has come from the experimental evolution of parasites in diverse systems being propagated under contrasting conditions of vertical and horizontal transmission, with the result that the parasites evolve toward avirulence when they are restricted to exclusive vertical transmission. However, complete avirulence no longer fulfills the definition of a parasite, so that the resulting entities might better be described as symbionts.
Many parasites are transmitted both vertically and horizontally. In some cases, the population structure imposes a strict trade-off between the two modes of transmission, such that more frequent horizontal transmission leads to less vertical transmission. Observations and experiments under such conditions have shown that the more horizontal transmission takes place, the more virulent the parasite will be. However, this prediction does not generally hold when the host population is well mixed. In such cases, vertical and horizontal transmission may be positively correlated, making general predictions difficult. Thus, without detailed knowledge about transmission trade-offs and host population structure, the finding that a particular parasite is partially vertically transmitted cannot be used as a predictor of low virulence.
5. HOW WELL DO OPTIMALITY MODELS PREDICT VIRULENCE?
Models of the evolution of virulence are deceptive in their simplicity and power to make testable predictions. Unfortunately, the evidence in support of these models is still rather thin, although many of the key assumptions have been supported experimentally in several systems (e.g., trade-offs have been observed, and multiply infected hosts typically suffer from higher virulence). For example, the field lacks compelling examples of evolutionary changes in virulence associated with trade-offs. Even the frequently cited Myxoma–rabbit case leaves open some alternative explanations. Those experimental studies that produced the clearest outcomes also had to use rather extreme conditions (e.g., 100 percent vertical versus 100 percent horizontal transmission) that may limit the ability to generalize their findings. Comparative studies employing data from many different host-parasite systems do not explain much of the variation in virulence, suggesting that other effects may overrule general patterns. For example, the type of host tissue affected by the parasite seems to explain more of the variation in virulence than do either trade-offs or transmission dynamics. Next-generation models that take into account host diversity and multidimensional trade-offs might be able to make more accurate predictions, although they are likely also to suffer in terms of generality.
Despite these limitations, models of optimal virulence are important because they provide a starting point for formulating testable predictions. In those cases in which a single environmental factor changes, it may indeed be possible to predict the associated change in virulence. However, changes in one factor often go hand in hand with changes in other factors, which may exert opposing selection on virulence. For example, the trade-off model predicts that an increase in host life span favors low virulence, but this same change may increase the frequency of multiple infections, which favors higher virulence. Therefore, careful evaluations of epidemiological circumstances and host demographic conditions are likely to be necessary before predictions can be made with confidence. It is currently not possible to make simple and robust recommendations for pest management that will favor the evolution of less virulent parasites. Proposals to manage virulence by changing environmental conditions must therefore be evaluated with appropriate care before they are put into practice.
See also chapter III.4, chapter VI.7, chapter VIII.1, and chapter VIII.4.
FURTHER READING
Alizon, S., A. Hurford, N. Mideo, and M. Van Baalen. 2009. Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. Journal of Evolutionary Biology 22: 245–259. A conceptual review of the trade-off model, mainly from a theoretical perspective.
Anderson, R. M., and R. M. May. 1982. Coevolution of hosts and parasites. Parasitology 85 (pt. 2): 411–426. The classic reference on this topic and still readable.
Bull, J. J., and D. Ebert. 2008. Invasion thresholds and the evolution of nonequilibrium virulence. Evolutionary Applications 1: 172–182.
Ebert, D., and J. J. Bull. 2008. The evolution of virulence. In S. C. Stearns and J. K. Koella, eds., Evolution in Health and Disease. 2nd ed. Oxford: Oxford University Press. The phase model for virulence is here worked out in more detail.
Fenner, F., and P. J. Kerr. 1994. Evolution of poxviruses, including the coevolution of virus and host in myxomatosis. In S. S. Morse, ed., The Evolutionary Biology of Viruses. New York: Raven. Much about the biological background for the rabbit–myxoma case.
Frank, S. A. 1996. Models of parasite virulence. Quarterly Review of Biology 71: 37–78.