
Evolutionary Medicine
Paul E. Turner
OUTLINE
1. Evolution and medicine
2. Pathogens
3. Defense mechanisms
4. Trade-offs in human traits
5. Mismatches to modernity
6. Implications of evolutionary medicine
Whereas evolutionary biology concerns the ultimate origins of trait variation within and among populations, human medicine concerns the proximate consequences of individual variation for manifestation of health versus disease. Although knowing how disease symptoms arise is essential for practicing medicine, understanding why these symptoms appear is additionally crucial. The merger of these two disciplines is evolutionary medicine, defined as the use of modern evolutionary methods and theory to better understand human health, with the prospect of improving disease treatment. The central question in evolutionary medicine is, Why has natural selection left our bodies vulnerable to disease? Many possible answers exist, and this chapter focuses on four major ones. First, human evolution is too slow to cope with the coevolutionary arms race involving microbial pathogens. Second, our evolved defense systems against these pathogens may, paradoxically, have harmful effects. Third, there are limitations and constraints on what selection can do, and disease often results from constraints and inevitable trade-offs. Last, human evolution is too slow to cope with novel environments especially of human making, leading to disease resulting from a mismatch to modernity.
GLOSSARY
Coevolutionary Arms Race. The sequence of mutual counteradaptations of two coevolving species, such as a parasite and its host.
Evolutionary Trade-off. A balancing between two traits that occurs when an increase in fitness (reproduction and survival) due to a change in one trait is opposed by a decrease in fitness due to a concomitant change in the second trait.
Germ Theory of Disease. The once-controversial idea that certain diseases are caused by invasion of the body by microbes; research by Louis Pasteur, Joseph Lister, and Robert Koch in the late nineteenth century led to widespread acceptance of the theory.
Hygiene Hypothesis. The idea that a lack of early childhood exposure to infectious agents, symbiotic microorganisms (e.g., gut flora), and parasites increases susceptibility to allergic diseases by modulating development of the immune system.
Mismatch to Modernity. Maladaptation produced by time lags, especially the inability of human adaptation to keep pace with rapid cultural change.
1. EVOLUTION AND MEDICINE
Although disease was not a major focus of Charles Darwin’s 1859 book On the Origin of Species, it described how intimate species interactions select for special traits of host exploitation in parasites; examples included parasitic wasps whose eggs are laid and larvae develop within the bodies of specific host insects, and cuckoos that parasitize other birds by laying eggs in their nests and relying on them to rear their young. Shortly after the book’s publication, Louis Pasteur’s experiments in the 1860s improved our understanding of pathogenesis by confirming that diseases could originate from parasitic microbes, which motivated changes in medical hygiene that saved countless human lives. Given these parallel advances in evolution and medicine, it is ironic that the hybrid discipline of evolutionary medicine did not gain traction until the 1990s, when evolutionary biologist George Williams and physician Randolph Nesse were credited for popularizing the notion that the understanding of human illness may be informed through evolutionary thinking.
The historical separation between the two disciplines may be explained by medicine’s proximate focus on restoring proper functioning of the human body, without considering how evolution shaped humans while causing us to remain susceptible to diseases and other health problems. This evolutionary consideration helps explain why humans become ill and provides knowledge that can be harnessed to suggest revised or novel methods of treatment. In particular, evolutionary thinking can create key insights and save lives when one is prescribing antibiotics, managing virulent diseases, administering vaccines, treating cancer, advising couples with difficulties in conceiving and carrying offspring, elucidating the origins of recent epidemics of obesity and autoimmune diseases, and answering questions relating to aging. The focus of evolutionary medicine is not to insist that an understanding of evolution is an alternative to current medical training; rather, it is to demonstrate that evolutionary biology is a useful basic science that poses new medical questions, raises possible answers or hypotheses, and thereby contributes to research while also improving medical practice.
The field of evolutionary medicine is relatively new, but the following sections provide examples of evolutionary insights that have informed the understanding of medical issues, and instances in which medical treatments have been modified owing to evolutionary thinking.
2. PATHOGENS
Parasitism is perhaps the most common lifestyle on the planet. Throughout history, humans have suffered extensive morbidity and mortality caused by infectious agents including parasites and pathogens. Some of these infections are evident in archaeological remains, such as ancient Egyptian hieroglyphics that depict humans afflicted with limb deformities characteristic of childhood infection by poliovirus (figure 1). Other evidence comes from mummified human remains showing, for example, the typical scarring due to skin lesions associated with the variola virus infection that causes smallpox. Diseases caused by ancient and novel pathogens constitute a substantial fraction of human mortality, perhaps on the order of 25 percent of all deaths per year. One goal of evolutionary medicine is to promote human health by improving therapies to combat infectious disease agents, based on the knowledge of how pathogens evolve in general, and especially in relation to selection pressures imposed by the human immune system and by current therapies.

Figure 1. Ancient Egyptian carving showing a priest with a shriveled leg typical of a recovered case of paralytic poliomyelitis (polio). Polio is an infectious disease caused by polioviruses that can permanently damage parts of the nervous system. In some cases, as seen here, it can cause paralysis. This bas-relief was carved around 1500 BCE. © Photo Researchers.
The evolution of antibiotic resistance in bacterial pathogens is a popular and important example used to illustrate the process of evolution via natural selection. The age of antibiotics began with the discovery of penicillin in the late 1920s, followed by small-scale attempts to use it to treat patients in the 1930s, and its mass-production and application in the 1940s. Unfortunately, penicillin is no longer effective in combating many pathogens, and the pharmaceutical industry is lagging in the race to develop natural and artificial antibiotics that can replace penicillin and other once-effective antibiotics. The explanation for their failure is that the widespread therapeutic use of antibiotics (and popular use of antibiotics as prophylactics in agriculture) has led to a very large “experiment” in which bacteria have been exposed to vast amounts of antibiotics that select for bacterial mutants with greater resistance to the effects of antibiotics. The large population sizes and short generation times of bacteria virtually assure rapid evolution in response to this strong selection. This unfortunate outcome has also been aided by the ability of many bacteria to obtain resistance genes via horizontal gene transfer from conspecifics and other species, thereby speeding the process by which resistance proliferates within and among species of bacteria. The emergence of antibiotic resistance is thus easily understood from an evolutionary perspective, and if any puzzle remains, it is why certain bacterial pathogens have not yet evolved resistance against traditional antibiotics.
The realization that misuse and overuse of antibiotics has selected for bacterial resistance has significantly influenced the practice of dispensing these drugs. Physicians are now encouraged to prescribe an antibiotic only when necessary, avoiding dispensation of a drug in the case of viral illness and when trying to combat bacteria against which an antibiotic is widely known to be ineffective. Realizing this to be a global problem, in 2011 the World Health Organization issued an international call for concerted action to halt the spread of antimicrobial resistance, with specific recommendations on government policies in medicine and agriculture to help control the problem. One major contributing factor highlighted in this report was insufficient measures for preventing the spread of resistant bacteria in hospitals and surrounding communities. Evolution of resistance has led to highly resistant superbugs, such as the methicillin-resistant Staphylococcus aureus (MRSA) that flourish and spread through hospital-acquired infections. The costs and dangers associated with these superbugs have prompted the development of spatially based evolutionary models that predict the spread of resistant bacteria within hospitals, according to factors such as the number of beds per room and placement of immune-compromised patients. This type of evolutionary thinking is an attempt to help reduce the risk of acquiring a life-threatening pathogen as an unfortunate consequence of a routine hospital stay.
Taking a step back in time, bacteria and fungi had evolved antibiotic production and resistance millions of years before the appearance of the human species, and so it is unsurprising that we are challenged to combat a problem (bacterial resistance) that natural selection figured out long ago. A similar problem is the medical challenge of controlling pathogens that have evolved elaborate traits to uniquely exploit humans. Pathogens that cause human disease often have adaptations to promote their growth within specific target cells inside the body, especially when humans constitute the main host. One example is the protist Plasmodium falciparum, which causes malaria. During infection this pathogen enters red blood cells and radically alters the interior of these cells so that it can reproduce. Owing to this cellular intimacy and the huge disease burden in humans caused by malaria, people living in malaria-endemic regions such as sub-Saharan Africa have been strongly selected for resistance, sometimes at an extreme cost. For this reason, malarial parasites have literally shaped the genetic variation of human populations living alongside them. In particular, the high prevalence of malaria in some regions explains why alleles that lead to abnormal hemoglobin and enzyme deficiency have proliferated: they increase resistance to malaria in heterozygotes that carry one copy of the variant allele, despite the burdens of sickle-cell anemia and other disorders suffered by homozygotes that have two copies of the resistance allele. These evolutionary compromises imply that cost-free resistance to malaria parasites is difficult to achieve, and perhaps similar difficulties extend to the largely ineffective efforts to date at generating a malaria vaccine.
Emerging pathogens are defined as those that have recently entered a host population or that are causing disease at increased rates. These pathogens—and future pathogens that fit these criteria—pose a huge medical concern because humans can suffer high rates of mortality when a disease agent jumps into the human population from a nonhuman host, sometimes evolving to become quickly established as a human-specific pathogen. Phylogenetic evidence indicates that simian immunodeficiency viruses (SIV) entered human populations many times in the last century, leading to the emergence and epidemiological spread of human immunodeficiency viruses (HIV) I and II, and high rates of mortality in HIV-I infected individuals. Other emerging pathogens may have a long history of infecting humans, but circumstances may change such that the parasite causes increased mortality. This change may come about owing to pathogen exposure in human populations previously sheltered from the disease and therefore containing little immunity or resistance. For example, European colonization of the Americas brought along the virus that causes smallpox, which contributed to the devastation of Native American populations. Emerging pathogens can thus shape human demographics and alter the course of human history. One goal of evolutionary medicine is to better predict what types of pathogens can emerge in the future and to better prepare the medical community for the inevitable challenges that will be posed by the pathogens of tomorrow that burst on the scene as unexpectedly as the agents for MRSA, HIV, and SARS (severe acute respiratory syndrome) have done in recent memory. At present, the ability to accurately predict which pathogens will next emerge in humans is crude at best. But some patterns seem evident, as disease surveillance data and laboratory evolution studies have shown that generalist parasites that previously evolved to infect multiple hosts appear better able to successfully emerge on novel host species.
Already, evolutionary thinking has revolutionized how we combat influenza virus A, which causes seasonal epidemics in humans and perhaps 500,000 deaths in a typical year. Although flu vaccines can be highly effective in protecting humans against infection, they are strain specific and time-consuming to produce. Thus, a flu vaccine that targets a particular strain must be mass-produced well ahead of the season in which the virus actually circulates in humans. In 1997, Walter Fitch, Robin Bush, and colleagues used evolutionary phylogenetics to analyze influenza virus A isolates, demonstrating that successful lineages of the virus undergo antigenic drift; that is, specific changes in their hemagglutinin proteins promote escape from human immune surveillance, allowing these strains to give rise to viruses that dominate subsequent flu seasons. This approach is now generally used to predict which extant strains will most likely give rise to viruses that will dominate in the coming season, such that vaccines against them are mass-produced before they become problematic. However, this attempt to match a vaccine with a major flu variant sometimes fails, especially when a new strain emerges through antigenic shift (recombination between viruses that creates a variant novel to the immune system). Goals of evolutionary medicine include more accurate forecasting of seasonal flu variants and better predictions of the emergence of new strains capable of spurring global flu pandemics.
The manifestation of an infection can differ markedly, even between closely related pathogens. This harm caused to the host is called virulence. Another goal of evolutionary medicine is to understand how the virulence of a pathogen is shaped by natural selection. If a parasite must rely on direct transmission between hosts, activities of a parasite that increase its opportunity for infectious transmission (e.g., greater within-host production of infectious particles) may greatly weaken the fitness of its current host. These adverse effects may actually reduce the probability of successful transmission; an extreme example would be a parasite that kills its current host faster than a new host is encountered. Thus, if parasite transmission and virulence are tightly coupled, theory predicts that a parasite should evolve an intermediate level of virulence, which balances the costs of harming the current host and the benefits of moving to a new host.
However, virulence is expected to increase when infections move easily between host individuals, as occurs for pathogens spread by insect vectors, via water, or through needles shared by injection-drug users. These situations decouple the success of a pathogen from the viability of its current host, and selection thus pushes the pathogen population to become dominated by more rapidly reproducing variants that exploit the host, leaving it severely debilitated in the process. The situation is often predicted to be similar for a host that is coinfected by multiple pathogen variants, because the advantage goes to the genotype that more quickly replicates, destroying host tissues in the process. Interestingly, the evolution of increased virulence may sometimes be “shortsighted” from the pathogen’s perspective, because selection across multiple generations within the host may cause a virulent genotype to be favored, even though this variant may not be the most successful one at being transmitted to a new host individual. This scenario is not the only possible outcome of evolved virulence under coinfection, however. Certain antagonistic interactions among coinfecting genotypes may cause the overall pathogen load to be reduced and lead to lowered virulence through time. Virulence evolution is an active area of research in evolutionary medicine, one in which mathematical models generate predictions that can be subjected to rigorous tests in the laboratory. Such efforts will help inform whether pathogens are expected to evolve increased or decreased virulence through time, such as in response to pressure exerted by the use of new vaccines that may inadvertently exacerbate some diseases by selecting for especially virulent pathogens that evolve to escape the vaccine-induced host response.
3. DEFENSE MECHANISMS
The history of the human species has involved coevolution with various parasites and pathogens, and their short generation times allow them to evolve much more rapidly than we do. To keep pace in this arms race, we rely on the protection afforded by mechanisms of innate and adaptive immunity, which in essence provide rapid evolution at the cellular and molecular levels during the lifetime of an individual.
However, a drawback to a complex immune system is that it offers myriad possibilities to malfunction. Diseases in which the immune system attacks the body’s own cells, mistaking them for pathogens, are termed autoimmune diseases. Similarly, allergy and atopic diseases such as asthma and anaphylaxis represent situations in which the normal evolved processes of defense are inappropriately or excessively activated, thus causing disease.
Genetic differences among individuals affect the potential to develop autoimmune disease, and these differences are usually associated with certain alleles of the human leukocyte antigen (HLA) system, which is the major histocompatibility complex in humans. One example is HLA-B27, an allele that is associated with increased risk of ankylosing spondylitis, a chronic inflammatory arthritis that is more prevalent in men and can cause fusing of the vertebrae in the spine. Because not all males with HLA-B27 develop the disease, this association suggests the involvement of some additional environmental trigger. Some evidence hints that the disease may involve an aberrant response to Klebsiella bacteria, which usually are benign inhabitants of the human gut.
Yet other autoimmune diseases may be related to a lack of exposure to certain microbes (figure 2A). Historically, the human species has evolved in environments in which individuals were frequently exposed to severe, persistent infections; in particular, most people carried parasitic worms most of the time. In developed countries, by contrast, humans live in more hygienic environments, such that few people have worms, and few adults die from infection. However, in these same settings the prevalence of asthma, allergies, and chronic inflammatory disorders such as Crohn’s disease have increased dramatically (figure 2B). The hygiene hypothesis offers a proposed explanation: the vertebrate adaptive immune system coevolved with commensal and pathogenic microbes, and appropriate exposure to this microbiota early in life may be essential for establishing appropriately regulated immunological pathways. In other words, the lack of exposure may cause improper activation of immune responses, which are then manifested as allergic or autoimmune diseases. Helminth worms are especially implicated as stimulators of proper immune function. This new understanding of the evolutionary role of helminths in modulating proper immunity has led to changes in medical treatment. Some doctors now successfully treat autoimmune diseases, such as Crohn’s disease, by injecting worm eggs or proteins derived from worms, to activate an inhibitory arm of the immune system that is otherwise suppressed in many modern populations.
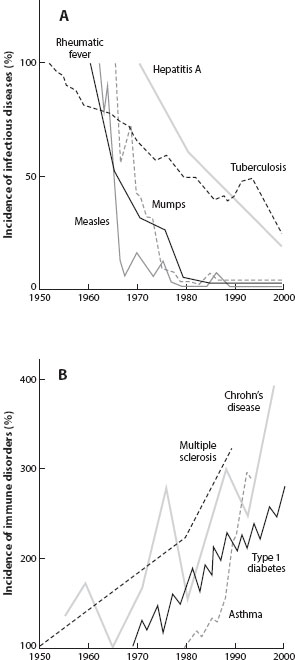
Figure 2. As the incidence of prototypical infectious diseases has fallen in high-income countries (A), the incidence of immune-related disorders has increased (B). (From J. F. Bach. 2002. New England Journal of Medicine 347: 911–920.)
Evolutionary medicine studies are also addressing a separate hypothesis to account for escalating allergy rates. Modern environments may contain specific risk factors that were absent from older environments. For example, modern homes are much warmer and drier than the habitations in which our ancestors lived long ago, typically containing fitted carpets, warm bedding, and central heating. This environment is ideal for mites that inhabit house dust and use sloughed-off human skin for food. The feces of these mites are highly allergenic to susceptible people, and they may contribute to escalating allergy rates.
4. TRADE-OFFS IN HUMAN TRAITS
Perhaps the most important generalization that evolution offers to medicine is to consider the body as a collection of trade-offs. No trait is perfect, and when natural selection improves one trait, it might make another worse. If we produced less stomach acid, then we might be less prone to suffer from ulcers, but the trade-off would be greater vulnerability to gastrointestinal infections. This line of thinking is particularly important as we gain increased ability to alter our bodies. Many of us strive to sleep less because we can cram more activities into 24 hours, but natural selection has had the opportunity to adjust the length of our sleep for many thousands of years, and so the adverse health consequences of sleep deprivation may exceed any benefits. Contraceptive use by many women in postindustrial countries causes them to experience an average of 400 menses per lifetime, whereas women in cultures that experience historically typical birth intervals of two and a half years, and breast-feeding during that time, have about 100 menses per lifetime. This perennial cycling causes prolonged elevated levels of hormones, especially estrogen, which can fuel the growth of cells that cause the tumors often responsible for breast and ovarian cancers. This mechanism may place women at increased risk for certain cancers and might explain why breast cancer rates are often much higher for women in postindustrial societies. Contraceptives need not induce a monthly period, and so perhaps a new solution can be found that allows women to experience a level of estrogen sufficient to maintain bone strength and avoid osteoporosis while avoiding increased risk of cancer.
Every trait can be analyzed in terms of its associated costs and benefits. Trade-offs may limit the extent to which fitness can be improved because, in the limit, an improvement in one trait will compromise some other trait. These compromises can emerge as unpleasant and costly surprises when interventions are made in ignorance of their potential trade-offs. From that perspective, consider that the human life history strategy involves deploying resources toward peak reproductive performance but trading off that investment in reproduction with a reduced investment in reparative functions that might sustain health in the postreproductive years. The life span in most societies has markedly improved owing to large reductions in extrinsic mortality following improvements in public health and, to a lesser extent, in medical care. But one consequence of this longer postreproductive life is an increase in chronic noncommunicable diseases of middle to older age that may reflect insufficient investment in self-repair including, for example, of cellular damage from oxidative stress. The outcomes may include atherosclerosis, arthritis, osteoporosis, cognitive decline, neurodegeneration, and increased susceptibility to infection. An evolutionary perspective might help by developing predictive tests to identify those individuals most at risk of suffering the adverse effects of these trade-offs, which might then allow novel therapies based on a better understanding of this variability.
5. MISMATCHES TO MODERNITY
Evolutionary change is a slow process, especially for organisms with generations as long as our own species. By contrast, our social and physical environments have changed very rapidly owing to our cultural evolution and the impact of technologies on the environments in which we live. Thus, our species has largely evolved under circumstances very different from the present environments that constitute our living conditions. Some of the examples discussed earlier likely reflect the medical consequences of living in an environment that is evolutionarily novel and in which an individual’s capacity to adapt physiologically is exceeded. This type of disconnect has been called a mismatch to modernity.
It is ironic that mismatches to modernity may result in diseases that stem from humans having access to excess resources. The modern lifestyle allows many people to experience an excess of energy intake relative to energy expenditure (figure 3), especially given technologies that limit the need for physical labor. The body’s inability to cope with this metabolic mismatch may well explain the increasing global incidence of obesity, type 2 diabetes, stroke, and cardiovascular disease. Lifestyle changes can combat this problem, but such changes are difficult to achieve because the pleasures of the present often outweigh considerations of the distant future; after all, few of our ancestors lived such long lives that the distant future offset their present needs. Also, our evolutionary history of living with limited resources may have selected for a physiology that benefited from taking on extra nutrients when they were available. Aside from public health approaches that aim to adjust eating behaviors in adults, recent evidence suggests that early-life interventions may be useful. In particular, individuals of lower birth weight are at higher risk of becoming obese and developing metabolic disorders, including hypertension and diabetes. Early nutritional stress is a signal whose evolved response sets the individual on a special developmental course with a physiology effective for conserving energy but ill prepared for abundant food. This finding suggests that in utero cues about nutrition may affect the development of metabolic priorities later in life. In particular, the mismatch between early- and late-life nutritional status may be contributing to increasing obesity rates, rendering those born in poverty and growing into plenty especially vulnerable. A better understanding of the global epidemic of metabolic diseases will require consideration of these early cues and their effects on development, in addition to the interaction between our changing lifestyle and the physiological predilections we inherited from ancestors who lived under very different conditions.
Figure 3. Total daily energy expenditure (TDEE; kcal/d) versus body weight (kg) for adult men and women of industrial and subsistence-level populations. Individuals in subsistence-level groups have systematically higher levels of energy expenditure at a given body weight. (Figure 20.2 from S. C. Stearns and J. C. Koella, eds. Evolution in Health and Disease. 2nd ed. Oxford: Oxford University Press. Reprinted with permission.)
Diseases caused by the mismatch between our bodies and environments can arise from deficiencies as well as excesses. For example, iodine was often routinely consumed by humans who ate seafood and plants grown in iodine-rich soils; however, endemic goiter and cretinism (a specific syndrome of mental retardation) occur when humans live in noncoastal environments deficient in iodine, such as certain mountainous regions. In developed countries, salt is iodized to reduce the risk of both goiter and cretinism. Similarly, the annals of sea exploration contain many stories of scurvy outbreaks caused by a lack of dietary vitamin C, for which fruits and some vegetables are the only natural source. Humans cannot synthesize vitamin C, unlike most other species, and so we are entirely dependent on an environmental source. Our ancestors evolved in environments where they had constant access to fruit, and the mutation that caused our inability to synthesize vitamin C was apparently neutral in that environment. But when a person is exposed to a novel environment—say, a long sea voyage—where a dietary source of vitamin C is lacking, scurvy is the result.
6. IMPLICATIONS OF EVOLUTIONARY MEDICINE
Evolutionary medicine should be of interest and use to practicing physicians as well as to biomedical researchers. We continue to be locked into a coevolutionary arms race with pathogens. These professions can therefore benefit from viewing infection from the pathogen’s perspective and from anticipating how pathogens may respond evolutionarily to treatments such as antibiotics and vaccines. Evolutionary thinking should also help clinicians and researchers who deal with cancer, reproductive medicine, metabolic disorders, and autoimmune diseases consider how our bodies might be affected by their mismatch with modernity and the inability of human adaptation to keep pace with cultural changes. More generally, medical researchers gain from evolutionary thinking because it brings new perspectives both to posing new questions and addressing old questions in new ways, including tough biomedical problems for which new insights can save lives.
The promise of evolutionary medicine is that this cross disciplinary science will ultimately yield new or improved methods of treatment. However, concrete examples of modified treatments are fairly limited, because the field is relatively young, and it usually takes a long time for basic-research findings to be translated to changes in medical practice. Still, some changes in medical treatment have clearly resulted from an influx of evolutionary thinking. Examples include the aforementioned changes in the ways that antibiotics are used and prescribed, and the use of products derived from helminths to treat certain autoimmune diseases. Also, due to the link between HLA and pathogen resistance, evidence suggests that an HLA mismatch between parents may increase the risk of miscarriage because the embryo is attacked by the immune system; this understanding has informed the practice of sperm donation to reduce the potential risk of spontaneous abortion. Another example is the increasing recognition that the genetic variations in human populations can affect the response of individuals and groups to drug treatments and can increase the likelihood of developing disorders such as alcoholism; this understanding informs the new field of evolutionary pharmacogenomics and may eventually lead to personalized medicine for optimizing a patient’s preventive and therapeutic care.
An important educational goal relevant to the growth of evolutionary medicine is to incorporate instruction in evolutionary biology into premedical and medical school education. Currently, few medical schools have evolutionary biologists on their faculties, and none teach evolutionary biology as a basic medical science. Physicians and medical researchers may learn something about evolution before medical school, but few have anywhere near the level of knowledge demanded for other basic sciences. An evolutionary view would correct mistaken notions of the body as a designed machine and would provide physicians with a better sense for the organism and what constitutes disease. A recent change in the Medical College Admissions Test (MCAT) requires competency in evolutionary biology and will probably improve understanding of evolutionary issues among clinicians more than any other potential measure. But in addition to changes in the MCAT, every undergraduate institution could offer courses in evolutionary medicine as part of its premedical curriculum. Specific renovations of the medical curriculum would also infuse evolutionary thinking into medicine. But it is likely that new national policies would be needed to educate physicians, allowing them to make full use of evolution as a crucial basic science for medicine.
Evolutionary medicine is a young field, and as more discoveries demonstrate how evolution-based thinking can improve the understanding and treatment of medical disorders, it should become clear that fundamental knowledge of evolution belongs in the medical toolkit.
See also chapter III.8, chapter III.11, chapter VI.7, chapter VIII.2, chapter VIII.3, chapter VIII.10, and chapter VIII.11.
FURTHER READING
Diamond, J. 1997. Guns, Germs, and Steel: The Fates of Human Societies. New York: W. W. Norton. A Pulitzer Prize–winning book about Eurasian colonization of other societies, sometimes aided by introducing infectious diseases that decimated native populations.
Ewald, P. W. 1980. Evolutionary biology and the treatment of signs and symptoms of infectious disease. Journal of Theoretical Biology 86: 169–176. A perspective on the need to understand whether disease symptoms, such as fever and diarrhea, are beneficial for the host, the pathogen, or neither organism.
Gluckman, P., A. Beedle, and M. Hanson. 2009. Principles of Evolutionary Medicine. New York: Oxford University Press. A thorough account of evolutionary medicine geared to a medical audience that includes fundamentals of evolutionary biology.
Merlo, L. M. F., J. W. Pepper, B. J. Reid, and C. C. Maley. 2006. Cancer as an evolutionary and ecological process. Nature Reviews Cancer 924–935. A review that uses concepts in ecology and evolution to explain why cancer evolves, and why it is often difficult to treat.
Stearns, S. C., and J. C. Koella, eds. 2008. Evolution in Health and Disease. 2nd ed. New York: Oxford University Press. Research advances in evolutionary medicine, mostly from the perspective of evolutionary biologists.
Trevathan, W. R., E. O. Smith, and J. McKenna. 2007. Evolutionary Medicine and Health: New Perspectives. New York: Oxford University Press. Collected essays on new treatments of mostly noninfectious diseases that have been informed by the field of evolutionary medicine.
Turner, P. E., N. M. Morales, B. W. Alto, and S. K. Remold. 2010. Role of evolved host breadth in the initial emergence of an RNA virus. Evolution 64: 3273–3286. A laboratory test of the general hypothesis that pathogens that historically evolved on multiple hosts should be more likely to infect a novel host.
Williams, G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11: 398–411. A classic paper examining the evolution of aging from an evolutionary perspective.
Williams, G. C., and R. M. Nesse. 1991. The dawn of Darwinian medicine. Quarterly Review of Biology 66: 1–22. A review of the power of evolutionary medicine to improve understanding of a wide variety of medical disorders.