CHAPTER 13
Autonomic Nervous System
The autonomic nervous system (ANS) is part of the peripheral nervous system. It acts “autonomously” to control the visceral (internal) functions of the body; its actions happen involuntarily, that is, without conscious thought. The ANS is separated into two divisions: the sympathetic system and the parasympathetic system. The sympathetic nervous system works to prepare the body for stressful situations. Thus, the sympathetic nervous system is also referred to as the “fight-or-flight” system. The parasympathetic nervous system counteracts the sympathetic nervous system and works to return the body to normal after a stressful situation, helping to maintain homeostasis. It is sometimes referred to as the “rest-and-restore” system.
The ANS differs from the somatic nervous system in several ways. A brief review of the somatic system reveals a one-motor neuron system with a synaptic cleft. Acetylcholine is the primary neurotransmitter that allows for propagation of an impulse across the synaptic cleft. The effector site for the somatic system is skeletal muscle. In contrast, the ANS is a two-motor neuron system comprised of a preganglionic neuron and a postganglionic neuron. Located between the two neurons is a ganglion, and it is within this ganglion that the neuron cell bodies are located, and where the synapse occurs. The postganglionic neurotransmitters (at the effector site) used in the ANS consist of acetylcholine and norepinephrine. The effector sites of the ANS are smooth muscle, cardiac muscle, and secretory glands.
Sympathetic ganglia are also called paravertebral ganglia. The sympathetic paravertebral ganglia branch off the spinal nerves anterior to the ventral roots. The paravertebral ganglia are connected vertically, forming a chain lateral to the spinal cord. This chain is referred to as the sympathetic chain or trunk. Parasympathetic ganglia, by contrast, are located near the organs they serve.
In the sympathetic nervous system, the first-order neurons of the ANS arise from the central nervous system (CNS). The preganglionic fibers deliver impulses to second-order neurons or the paravertebral ganglia. These ganglia contain the cell bodies of the postganglionic fibers responsible for delivering the impulse to the effector organs. The preganglionic fibers of the ANS are myelinated, and the postganglionic fibers of the ANS are nonmyelinated. Postganglionic fibers of the sympathetic division are long and are spread throughout the body. They produce a rapid, generalized, mass response. The postganglionic fibers of the parasympathetic division are short, with their terminal ganglia near the effected organ. Their effect is more localized.
Anatomy of the Autonomic Nervous System
The sympathetic nervous system preganglionic fibers originate in the thoracic (T1-T12) segments and the first three lumbar (L1-L3) segments of the spinal cord (Fig. 13.1). For this reason, it is sometimes referred to as the thoracolumbar division. The myelinated effector nerves leave the spinal cord and enter the ganglia. After reaching the ganglia, the impulse may travel in one of three ways: (1) directly across the ganglion to synapse with cell bodies of the postganglionic fibers, (2) cephalad or caudad to synapse with a higher or lower postganglionic neuron, or (3) through the sympathetic chain without synapsing. Some preganglionic fibers exit the sympathetic chain and synapse with outlying ganglia such as the celiac ganglia or the superior and inferior mesenteric ganglia. Synapses with these outlying ganglia, sometimes also referred to as collateral ganglia, innervate the visceral organs below the diaphragm. Innervation of the adrenal medulla is unique in that the secretory cells are considered modified postganglionic neurons. Therefore, preganglionic fibers do not synapse prior to reaching the adrenal gland. Because the preganglionic fibers are myelinated, the signal speed is quick, causing a rapid release of norepinephrine and epinephrine into the bloodstream from cells within the adrenal medulla. These are the same norepinephrine and epinephrine that are neurotransmitters at the synapse; they are also the same norepinephrine and epinephrine that are used as vasoactive drugs regularly in the operating room (see Chapter 6, Cardiovascular Pharmacology).

FIGURE 13.1. Spinal cord with medulla pons and midbrain, connecting parasympathetic and sympathetic nerves to the ganglion to the affected organs, glands.
The parasympathetic nervous system cell bodies stem from cranial nerves III, VII, IX, and X, which are the oculomotor, facial, glossopharyngeal, and vagus nerves, respectively, and the sacral segment of the spinal cord (Fig. 13.1). It is thus sometimes referred to as the craniosacral division. The preganglionic fibers of the parasympathetic system differ from those of the sympathetic system in that they travel uninterrupted to their effector organ before synapsing at the ganglion with a short postganglionic fiber. Parasympathetic stimulation arising from the cranial nerves innervate viscera of the head, thorax, and abdomen. A large percentage of parasympathetic innervation to the thorax and abdomen stems from the vagus (X) nerve. This includes parasympathetic stimulation to the heart, lungs, stomach, small intestine, liver, gallbladder, and pancreas.
The eye receives parasympathetic stimulation via the oculomotor (III) nerve, and the lacrimal and salivary glands are stimulated through fibers from the facial (VII) nerve. Additionally, salivary glands also receive parasympathetic stimulation through the glossopharyngeal (IX) nerve. Parasympathetic nervous system fibers arising from the sacral portion of the spinal cord innervate the large intestine, rectum, and bladder.
Physiology of the Autonomic Nervous System
The primary neurotransmitters released in the ANS are acetylcholine and norepinephrine. The preganglionic fibers of the sympathetic division and the preganglionic and postganglionic fibers of the parasympathetic division all release acetylcholine. Most of the postganglionic fibers of the sympathetic division release norepinephrine. There are a few exceptions, such as the postganglionic fibers of the sympathetic nervous system that stimulate the sweat glands. These fibers release acetylcholine. Acetylcholine exerts its effect on cholinergic receptors found in the ganglia or in the effector organs. There are two types of cholinergic receptors: nicotinic and muscarinic. Nicotinic receptors are almost always excitatory. Acetylcholine released from preganglionic fibers act on nicotinic receptors found in the ganglia on the postganglionic fibers in both the sympathetic and parasympathetic nervous systems.
Acetylcholine that is released from postganglionic fibers in the parasympathetic nervous system exerts its systemic effects by acting on muscarinic receptors, which can exhibit excitatory or inhibitory properties. Muscarinic activation in the heart causes decreased heart rate and contractility. Muscarinic activation also causes bronchoconstriction (e.g., wheezing), increased secretion by salivary glands, and intestinal and bladder contraction with release of their sphincter tone (often resulting in urination and defecation).
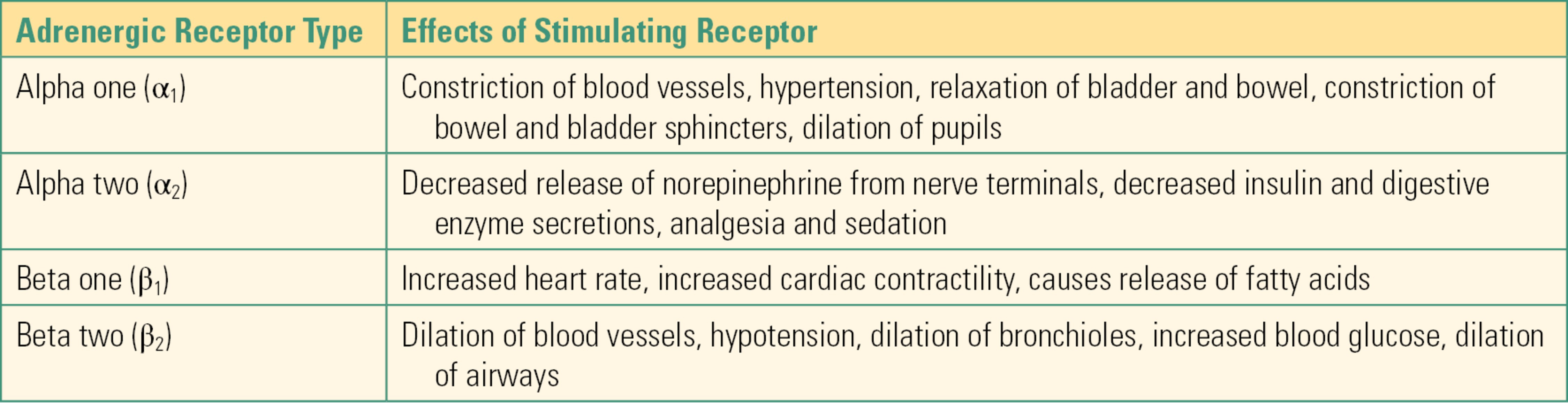
Within the sympathetic nervous system, norepinephrine released from postganglionic nerves acts on adrenergic receptors. The two major classes of adrenergic receptors are alpha (α) receptors and beta (β) receptors. These receptors are further subclassified as α1 and α2 and β1 and β2 (Table 13.1). In general, stimulation of α1 receptors that exist outside of the CNS results in constriction of blood vessels (hypertension) and relaxation of bladder and bowel while at the same time causing constriction of the sphincters of the bladder and bowel. Stimulation of α2 receptors results in decreased release of norepinephrine from nerve terminals. Stimulation of β1 receptors causes increased heart rate and increased cardiac contractility, whereas stimulation of β2 receptors causes dilation of blood vessels (hypotension), dilation of bronchioles, and increased blood glucose (from glycogenolysis and gluconeogenesis).
Table 13.1. Adrenergic Receptor Types and Receptor Stimulation Effects
Effects of Autonomic Nervous System Stimulation
Unlike innervation of skeletal muscle, which is all excitatory, visceral organs receive both excitatory and inhibitory innervation. The two divisions of the ANS are responsible for this antagonistic innervation. As noted previously, the sympathetic system is usually excitatory and the parasympathetic system inhibitory. Stimulation of the sympathetic division of the ANS produces a physiologic response characterized by increased heart rate, increased force of contraction of the heart, increased blood pressure through constriction of blood vessels (vasoconstriction), increased blood sugar through release of glucose from the liver, inhibition of digestion, dilation of respiratory passageways, increased blood flow to skeletal muscle, and dilation of pupils (Table 13.2). The individual receptors involved in this effect of the sympathetic nervous system are outlined above. These functions are the classic “fight-or-flight” response. Although the stimulation of these organ systems can be helpful to escape a bear attack, they can also be detrimental. For example, surgical stimulation produces a pronounced sympathetic response. The increases in heart rate could increase myocardial oxygen demand, and the patient could suffer a heart attack if there is limitation in oxygen supply (e.g., if the coronary arteries are partially occluded).
Table 13.2. Characteristics of the Autonomic Nervous System
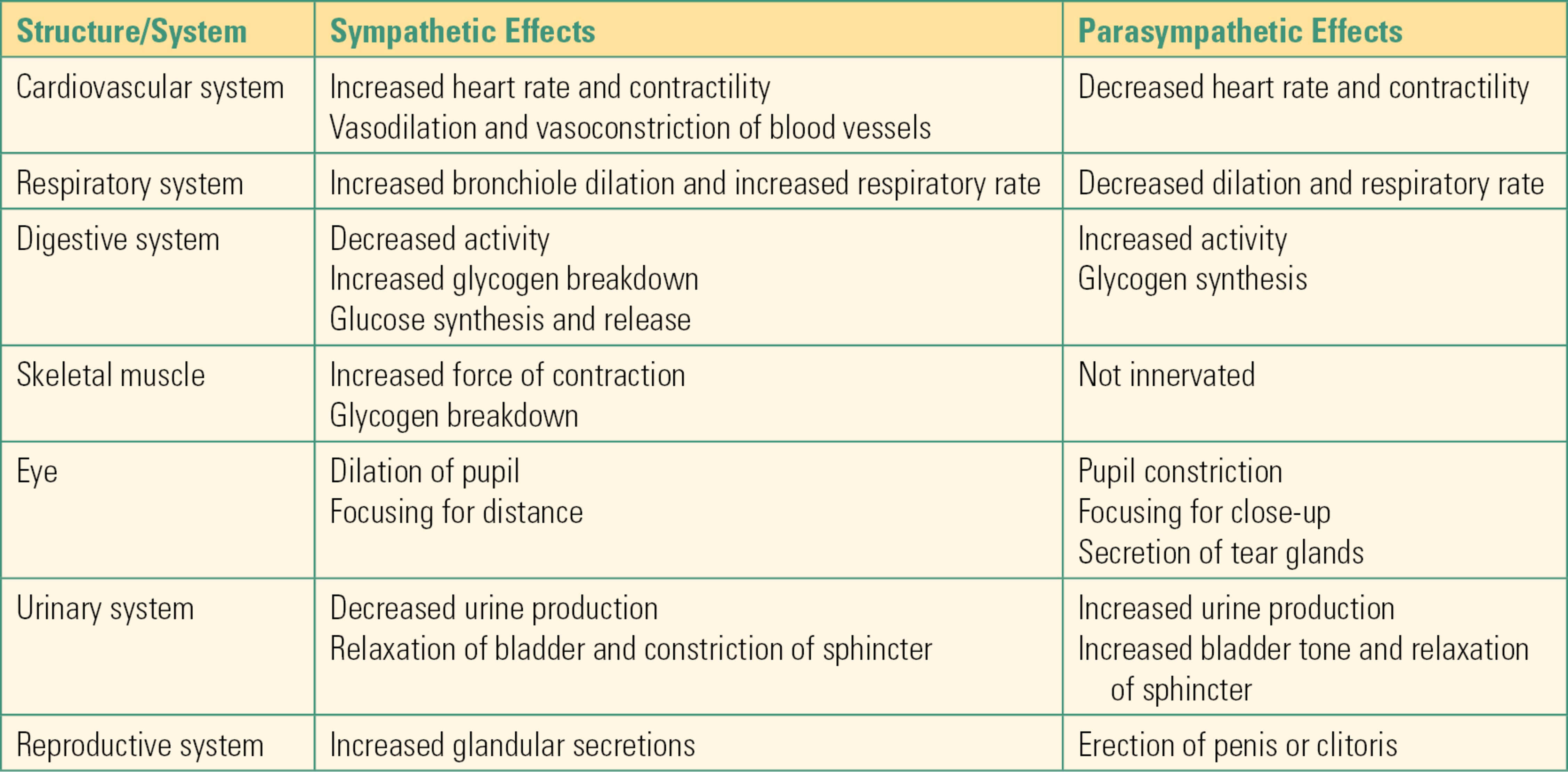
Activation of the parasympathetic nervous system generally counterbalances the sympathetic system. Stimulation of the parasympathetic system decreases heart rate and increases blood flow to the digestive tract, increasing peristalsis. The parasympathetic nervous system is also responsible for relaxation of sphincters, allowing for urination and defecation (Table 13.2).
Anesthetics and the Autonomic Nervous System
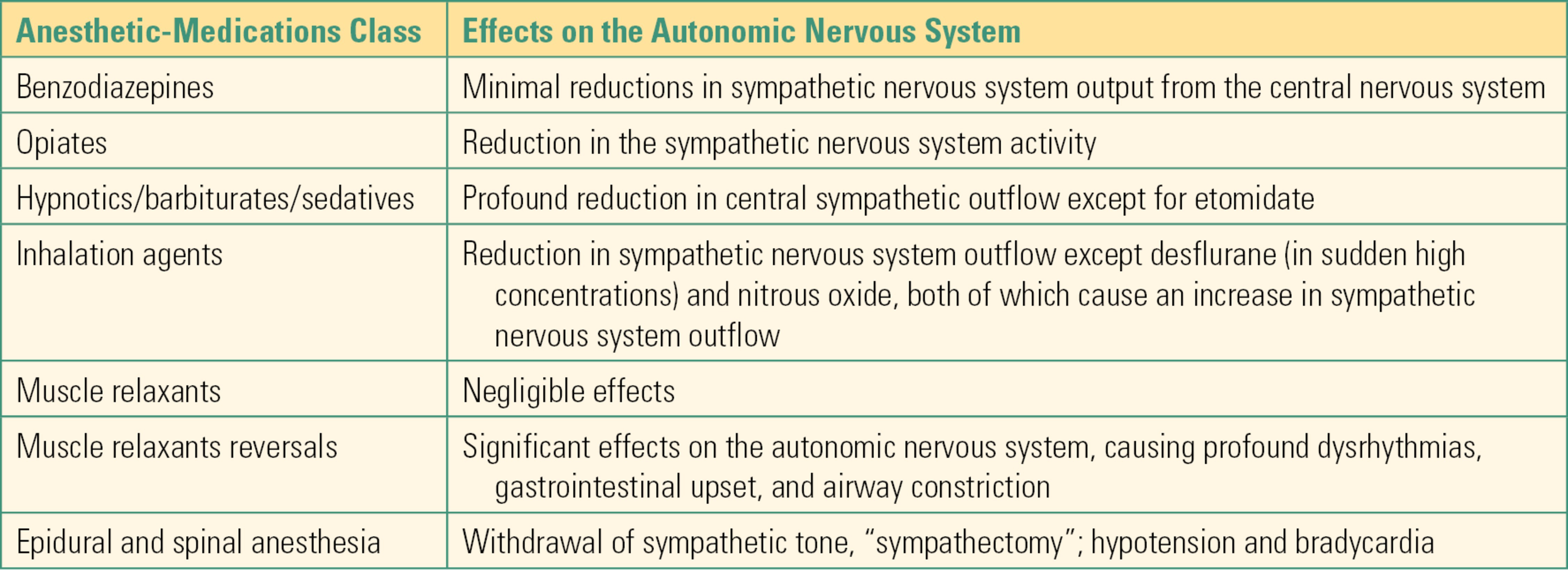
Most of the medications that are administered during anesthesia affect the ANS. This is especially true during general and neuraxial anesthesia (spinal and epidural anesthesia). Anesthetic interventions can directly reduce autonomic signals from the CNS (including the spinal cord), act at the ganglia, or act on the end-organ receptors for the neurotransmitters of the ANS. For example, the induction of general anesthesia often results in a generalized reduction in sympathetic outflow from the CNS. This results in hemodynamic changes such as a decrease in blood pressure. In this section of the chapter, we describe effects on the ANS of the different classes of medications that are utilized during general or neuraxial anesthesia.
General Anesthesia
Benzodiazepines
These medications (e.g., midazolam, diazepam) are often utilized in the preoperative area and during sedation cases. At low doses, they produce minimal reductions in sympathetic nervous system output from the CNS. When they are combined with narcotic medications, the reduction in sympathetic outflow can be more pronounced and can result in a significant decrease in blood pressure. The calming effects of the benzodiazepines—termed anxiolysis—may cause the heart rate to decrease and the blood pressure to drop as a result of decreased catecholamine production.
Opiates
Fentanyl, hydromorphone, and morphine are opioids and are some of the most commonly administered perioperative medications. Most commonly, opiate analgesics will produce a reduction in the sympathetic nervous system activity. And as noted above, when opiates are administered together with benzodiazepines, they cause a more pronounced reduction in the sympathetic outflow and corresponding decrease in blood pressure. Opioids can also dramatically drop blood pressure for patients who are in pain: these patients’ sympathetic nervous system has been highly activated by pain, supporting blood pressure and heart rate; when pain is relieved, sympathetic outflow can drop rapidly, and this can unmask an underlying problem with the circulation.
Hypnotics/Barbiturates/Sedatives
Propofol, methohexital, and etomidate are classified as hypnotics and sedatives. They are potent depressants of the CNS and are used in anesthesia in low doses to produce sedation and in higher doses to produce unconsciousness. With the exception of etomidate, hypnotics usually produce a profound reduction in central sympathetic outflow, which can result in significant decreases in blood pressure, even in healthy patients. The reduction in sympathetic outflow reduces cardiac output and causes peripheral vasodilation. It is important to utilize these drugs judiciously, particularly in patients who may be more dependent on their sympathetic system to maintain blood pressure (e.g., a trauma patient) or in patients who may not be able to tolerate a significant decrease in blood pressure. Etomidate is the only hypnotic/sedative that does not result in significant attenuation of sympathetic outflow, and as a result, it is often chosen to induce anesthesia in hemodynamically fragile patients. Ketamine is a hypnotic anesthetic that is associated with sympathetic system stimulation while at the same time causing direct myocardial depression. Thus, when given to normal patients, ketamine administration can be associated with hypertension and tachycardia. However, when administered to patients who have been in the intensive care unit for an extended period of time, ketamine causes direct myocardial depression and hypotension because these patients have often depleted their catecholamine stores.
Inhalation Agents
Of all the currently utilized volatile inhalation agents, desflurane is the only agent that will produce an increase in the sympathetic outflow, causing tachycardia. However, this increase occurs only when there is a sudden elevated level of desflurane. Otherwise, all volatile inhalation agents will produce varying degrees of cardiovascular depression due to direct dilation of systemic blood vessels and reduced sympathetic nervous system activity. The result is often a drop in blood pressure that is antagonized with intravenous administration of a vasopressor drug (e.g., ephedrine or phenylephrine) or by release of endogenous catecholamines from painful stimuli such as surgical incision.
Nitrous oxide is a nonvolatile inhalation anesthetic agent and is commonly associated with centrally propagated increase in sympathetic outflow, particularly when it is administered without other anesthetic agents. During noxious stimulation (e.g., tracheal intubation), patients who have nitrous oxide as part of their anesthetic have higher concentrations of blood catecholamines (particularly norepinephrine) due to direct effects of this drug on the sympathetic nervous system but attenuated cardiovascular responses due to the anesthetic effect on the cardiovascular system.
Neuromuscular Junction Blocking Agents (Muscle Relaxants)
In general, muscle relaxants do not exhibit significant effects on the ANS. That is especially true with rocuronium and vecuronium, two of the more commonly used muscle relaxants during anesthesia and surgery. Pancuronium, an older long-acting muscle relaxant, inhibits postganglionic muscarinic receptors in the heart and produces tachycardia. Succinylcholine, a depolarizing muscle relaxant, can produce dysrhythmias manifested as bradycardia, junctional rhythms, or ventricular dysrhythmias. As a depolarizing neuromuscular blocking agent, succinylcholine causes stimulation of both types of cholinergic receptors, nicotinic ganglionic receptors, and postsynaptic muscarinic receptors. This stimulation of both sympathetic and parasympathetic nerves and muscarinic receptors in the sinus node of the heart means that the cardiovascular effects are unpredictable but often result in bradycardia and cardiac arrhythmias.
Muscle-Relaxant Reversals
Neostigmine is one of the most commonly used medications in anesthesia to reverse the effects of the nondepolarizing muscle relaxants (such as rocuronium, vecuronium, and pancuronium) (see Chapter 14, Neuromuscular Anatomy and Physiology). This medication, which belongs to a class of drugs called anticholinesterases, has significant effects on the ANS, as it increases acetylcholine at all parasympathetic ganglia. Its undesirable symptoms include salivation, bronchoconstriction, defecation, and bradycardia. These effects are counteracted by the simultaneous administration of anticholinergic medications such as atropine or glycopyrrolate, which reliably mitigate these (i.e., they prevent the heart from stopping!). The anticholinergics, in turn, cause tachycardia and drying of secretions in order to oppose the effects of the anticholinesterases. These undesirable autonomic effects are the result of the intersection of acetylcholine as the neurotransmitter for both neuromuscular and parasympathetic transmission.
Regional Anesthesia
Epidural and spinal blocks involve injection of local anesthetics in close proximity to the CNS (spinal cord). These blocks are commonly used to inhibit sensory and motor transmission to various parts of the body. However, another important property of these procedures is the interruption of sympathetic (in the thoracic and lumbar region) and parasympathetic (in the sacral region) signals. When the level of anesthesia for a spinal or epidural anesthetic is allowed to ascend to the high thoracic region, the patient may experience a “sympathectomy” because the spinal/epidural anesthetic will inhibit the entire sympathetic outflow, since it originates in the thoracic and lumbar regions of the spinal cord. In this situation, the only part of the ANS that is functional is the parasympathetic system (cranial division). The patient is likely to exhibit bradycardia and hypotension from unopposed parasympathetic activity, even during noxious stimulation above the spinal/epidural level.
On the other hand, paravertebral blocks may not inhibit sympathetic activity, as the sympathetic chain is anterior to the target for this block. Even if the local anesthetic administered at a specific paravertebral level diffuses anteriorly and anesthetizes the sympathetic ganglion at that specific level, sympathetic fibers from other levels above and below in the thoracolumbar chain will continue to be functional, as the block will not reach multiple ganglia.
Blockade of sympathetic ganglia outside of the sympathetic chain does have clear effects on target organs. For example, in celiac plexus block, sympathetic innervations to intra-abdominal organs are anesthetized to alleviate pain for patients who have cancer in these organs. It is important to recognize that, anatomically, celiac ganglia are also mixed with sensory nerves that originate in these organs.
Table 13.3 in this chapter summarizes the commonly used medications in anesthesia and their effects on the ANS.
Table 13.3. Common Anesthesia Medications and Effects on ANS
In summary, the ANS is divided into two subdivisions. The sympathetic division arises from preganglionic fibers originating from the thoracic and lumbar segments of the spinal cord. The sympathetic division is activated during times of crises (“fight or flight”) and works to produce increased alertness, increased cardiovascular and respiratory function, and increased energy. The parasympathetic division arises from the preganglionic fibers originating in the cranial nerves of the brainstem and sacral segments of the spinal cord. Parasympathetic stimulation produces depression of cardiovascular function and promotes digestive function (“rest and restore”). These two divisions are antagonistic and work to counterbalance one another to regulate cardiovascular, respiratory, digestive, excretory, and reproductive functions. Most of the medications that are administered during anesthesia will affect the ANS.
Every day, the educated and observant anesthesia technician in the operating room can see the effects of the ANS: some of many examples include a rise in the blood pressure after tracheal intubation as the sympathetic nervous system is activated; atropine given in the regional anesthesia area after a patient complains of nausea and his heart rate drops into the 40’s. A clear understanding of the ANS will also help the anesthesia technician understand the effects of medications, anticipate providers’ needs, and participate fully in the anesthetic management of patients.
Review Questions
1. The primary neurotransmitter released by the preganglionic fibers of the sympathetic nervous system and the preganglionic and postganglionic fibers of the parasympathetic nervous system is:
A) Epinephrine
B) Acetylcholine
C) Norepinephrine
D) Dopamine
E) None of the above
Answer: B
Acetylcholine is the primary neurotransmitter for the preganglionic fibers of the sympathetic nervous system and the preganglionic and postganglionic fibers of the parasympathetic nervous system. Norepinephrine is released by most of the postganglionic fibers of the sympathetic nervous system. Both norepinephrine and epinephrine are released from the adrenal medulla after direct stimulation from preganglionic fibers.
2. Acetylcholine exerts its effect on which of the following receptors?
A) Cholinergic
B) Muscarinic
C) Nicotinic
D) Adrenergic
E) A, B, and C
Answer: E
Acetylcholine exerts its effect on cholinergic receptors of which there are two types: nicotinic and muscarinic. Nicotinic receptors are almost always excitatory. Muscarinic receptors exhibit both excitatory and inhibitory effects.
3. Which of the following effects is elicited by stimulation of the α1 receptors?
A) Hypertension
B) Hypotension
C) Increased heart rate
D) Dilation of bronchioles
E) None of the above
Answer: A
Hypertension and relaxation of bladder and bowel are effects caused by stimulation of the α1 receptor. Hypotension and dilation of the bronchioles is caused by stimulation of the β2 receptors. Increased heart rate is an effect of stimulating the β1 receptors.
4. A patient with poor cardiac function presents for surgery and requires a general anesthetic. The anesthesia provider prepares several medications. Which of the following is an anesthesia induction agent that is commonly used in this scenario?
A) Atropine
B) Etomidate
C) Norepinephrine
D) Propofol
E) None of the above
Answer: B
Etomidate is the only hypnotic/sedative that does not result in significant decrease in sympathetic outflow, and as a result, it is often chosen to induce anesthesia in patients with poor cardiac function. Propofol is an anesthesia induction agent that produces sympathetic attenuation, a drop in venous and arterial vascular tone, and a drop in cardiac contractility; it would not be a common choice for a patient with very poor cardiac function, though certainly a possibility if combined with a sympathetic stimulant. The other drugs are not anesthesia induction agents. Atropine is a muscarinic antagonist and norepinephrine is a sympathetic agonist.
5. Which of the following inhalation anesthesia agents is considered to be the only inhalation agent that will produce an increase in the sympathetic outflow, causing tachycardia, when it is suddenly increased to high levels?
A) Isoflurane
B) Sevoflurane
C) Desflurane
D) Halothane
E) None of the above
Answer: C
Desflurane is the only agent that will produce an increase in the sympathetic outflow, causing tachycardia. Thus, it is not usually used as an induction agent.
6. A severe asthmatic patient is emerging from anesthesia. The anesthesia provider will likely use a medication to reduce the potential of severe airway constriction and spasm during extubation. Which adrenergic receptor do you anticipate this medication will likely stimulate?
A) β1
B) β2
C) α1
D) α2
Answer: B
A beta two (β2) agonist medication is most appropriate in this situation. Stimulating the beta two (β2) adrenergic receptor will cause the airway and bronchioles to dilate and thus reducing the potential for airway complications during emergence.
7. A patient has received a “high spinal” and “sympathectomy” during the administration of spinal anesthesia when a high dose and concentration local anesthetic was inadvertently injected. The patient quickly becomes unconscious and vital signs reveal severe hypotension and bradycardia. The anesthesia provider provides bag-mask ventilation and then prepares for emergent intubation of the unresponsive patient. You expect this patient to have the following symptoms as a result of the noxious stimulation from emergency intubation:
A) Severe hypertension due to unopposed sympathetic response.
B) Severe tachycardia due to a surge in catecholamine release from the adrenal glands.
C) Both hypertension and tachycardia as commonly occur during every intubation.
D) No change in vital signs.
Answer: C
Patients often experience a surge in sympathetic response during intubation yielding high blood pressure and possibly tachycardia. However, in this patient, the sympathetic response is obliterated and the parasympathetic response is unopposed and the patient will likely continue to suffer from severe hypotension and bradycardia. Very occasionally patients have bradycardia during laryngoscopy due to stimulation of cranial nerves IX and X; these parasympathetic nerves are unopposed in this patient, and it is actually possible the patient could have further bradycardia.
SUGGESTED READINGS
Boron WF, Boulpaep EL. Medical Physiology. Philadelphia, PA: Elsevier Science; 2003.
Huether SE, McCance KL. Understanding Pathophysiology. 2nd ed. St. Louis, MO: Mosby; 2006.
Martini FH, Bartholomew EF. Essentials of Anatomy & Physiology. 5th ed. San Francisco, CA: Benjamin Cummings; 2010.
Morgan GE, Mikhail MS, Murray MJ. Clinical Anesthesiology. 4th ed. New York, NY: McGraw-Hill; 2006.
Shier D, Lewis R, Butler J. Hole’s Human Anatomy & Physiology. 9th ed. New York, NY: McGraw-Hill; 2002.
Stoelting RK, Hillier SC. Pharmacology & Physiology in Anesthetic Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.