CHAPTER 14
Neuromuscular Anatomy and Physiology
Introduction
One of the key activities in conducting a general anesthetic for surgery can be to provide muscle relaxation (paralysis) in order to facilitate placement of an endotracheal tube in the trachea or to provide optimum operating conditions for the surgeon with quiet, soft muscles. This is accomplished with medications that interfere with normal functioning of the junction between nerves and muscles. Knowledge of the anatomy and physiology of the neuromuscular system helps the anesthesia technician understand how drugs affect the neuromuscular junction (NMJ) and how nerve stimulators are used to monitor the neuromuscular system (discussed in Chapter 32, Neurologic Monitoring).
Anatomy of the Neuromuscular System
The neuromuscular system, as implied by the name, is composed of nerves and muscles. The peripheral nerves exit from the spinal cord and then act upon the muscles of the body. There are three different types of muscle: cardiac muscle in the heart, smooth muscle, and skeletal muscle. Although similar, each of the different types of muscles has slightly different properties. Both cardiac and smooth muscle contraction and relaxation are involuntary, that is, they occur independent of conscious control. Smooth muscle is controlled by the autonomic nervous system (see Chapter 13, Autonomic Nervous System). Examples of smooth muscle include the walls of blood vessels, lymphatic vessels, bronchioles in the lungs, urinary bladder, and the reproductive tract of males and females. Contraction or relaxation of these muscles occurs in response to the activity or needs of the organ. Cardiac muscle is also a type of involuntary muscle.
Skeletal muscles are the voluntary muscles of the body that allow us to move. Skeletal muscles attach to bones by way of tendons. Skeletal muscle is composed of many muscle fibers, which are held together by protein and fibrous tissue (Fig. 14.1). These muscle fibers in turn are composed of multiple myofibrils, the individual muscle cells. Within each myofibril are abundant cell structures specialized for processing energy as well as for contraction.

FIGURE 14.1. Muscle anatomy.
The main contractile unit in skeletal muscle cells is called the sarcomere. There are many sarcomeres within one myofibril. When a sarcomere is further examined under an electron microscope, one observes repeating sections of light and dark areas. The light areas are the thin filaments, composed of the protein actin. The dark areas of the sarcomere are the thick filaments, composed of the protein myosin. During contraction, the myosin binds to actin in a process that requires calcium and energy. This binding and overlap process results in shortening of the sarcomere, which is anatomically recognized as muscle contraction. The molecular basis of muscle contraction is explained by the “sliding filament theory.” This theory states that in the presence of calcium and energy, actin and myosin slide past each other and the sarcomere shortens. The protein filaments themselves do not shorten, but overlap. The sliding and overlap are what causes the sarcomere to shorten.
The Neuromuscular Junction
The neuromuscular junction (NMJ) is the meeting place of the nerve and the muscle, composed of the terminal portion of a motor neuron axon and the motor end plate of a muscle fiber. The function of the NMJ is to allow the muscle to contract based on messages originating in the brain. Once the motor cortex of the brain sends the message, the message is delivered by way of action potentials (electrical signals) along the axons of motor neurons. When the action potential is sent down the axon, it eventually reaches the NMJ (Fig. 14.2).
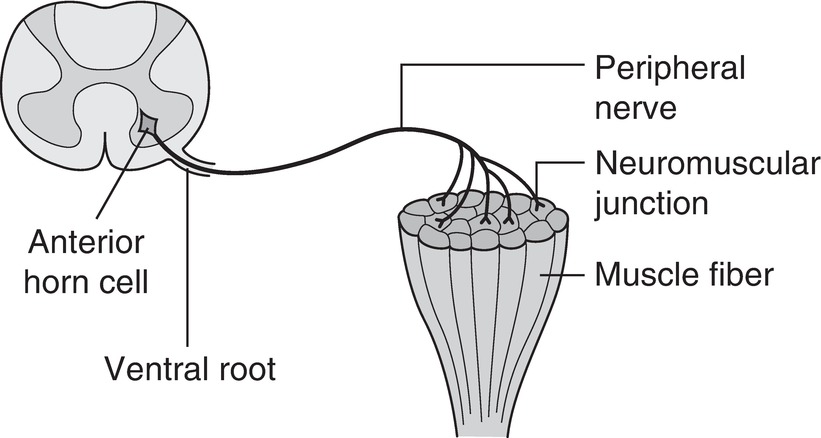
FIGURE 14.2. A motor nerve axon terminating at the junction between the nerve and muscle.
Just as in the nervous system, the junction between the end of the axon of the nerve cell and the receiving end plate of the muscle is called a synapse. The terminal portion of the nerve where the signal originated is the “presynaptic” nerve. Once the nerve action potential arrives at the terminal portion of the presynaptic nerve, it causes acetylcholine (ACh) to be released from the axon terminal into the synaptic cleft. The ACh then travels across this area to attach to nicotinic ACh receptors on the motor end plate of the muscle (Fig. 14.3).
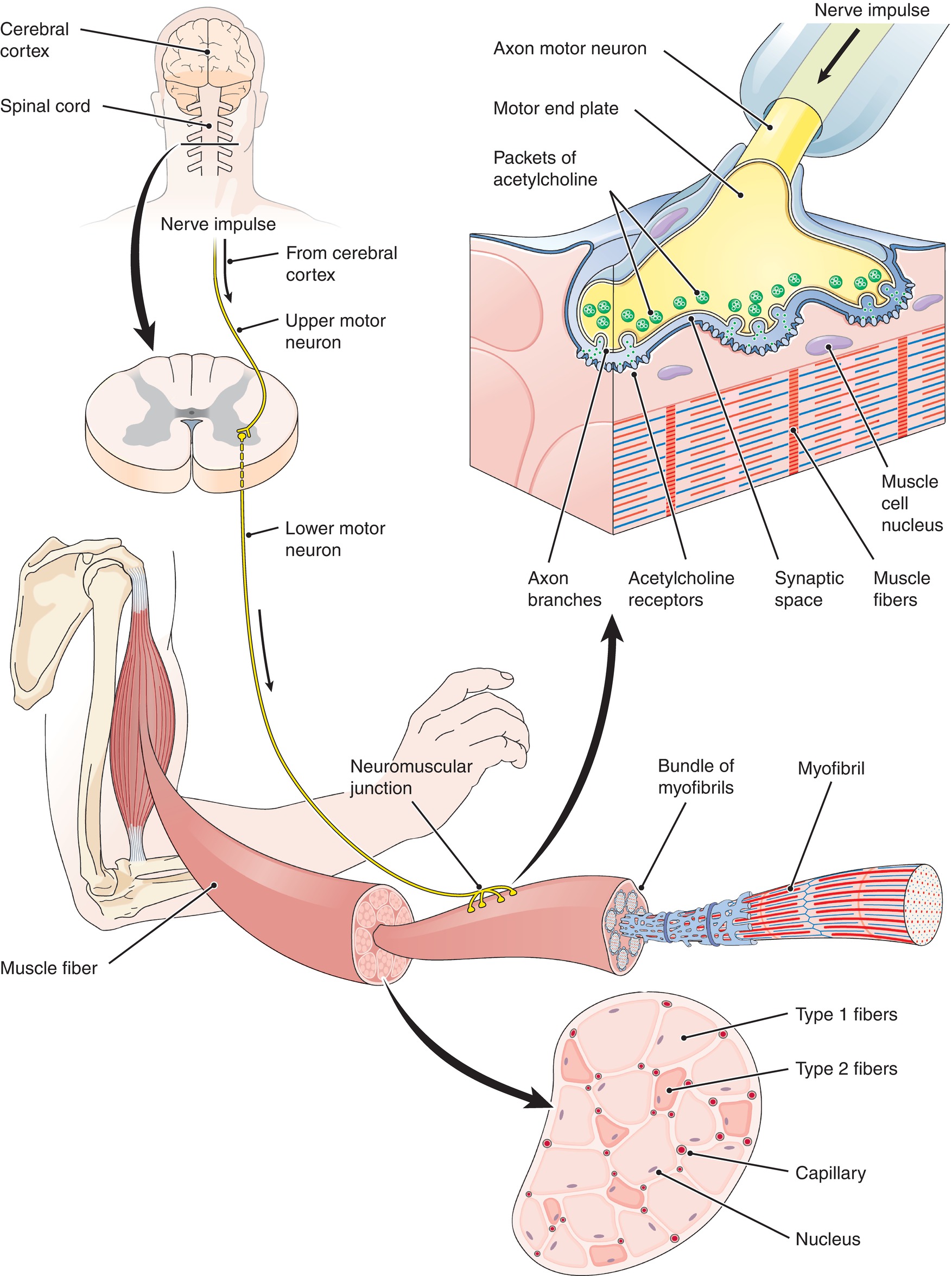
FIGURE 14.3. The neuromuscular junction with release of presynaptic acetylcholine binding to postsynaptic nicotinic receptors.
Binding of ACh to these receptors causes sodium and potassium channels to open in the muscle cell membrane. Sodium and potassium travel opposite each other; sodium travels into the cell, and potassium trickles outward. Because sodium rushes into the motor cell much more quickly than potassium leaves the cell, the cell becomes more positively charged and depolarizes (a change in the charge of the interior of the cell membrane compared to the outside). Depolarization of the muscle cell then causes the release of calcium from sarcoplasmic reticulum in the muscle cell. Excess calcium in the muscle cell binds to the thin filament in the sarcomere, triggering a cascade of events resulting in muscle contraction.
But how does our brain send a message to the many muscles of the body to contract or relax? Let us use the example of an anesthesia technician carrying an arterial line setup to an operating room. When the anesthesia technician wants to lift his or her arm, the biceps muscle must contract. A message is sent from the brain to tell the biceps muscle that it must shorten to accommodate the weight of the arterial line setup. The message originates in upper motor neurons in the motor area of the brain. The upper motor neuron crosses to the opposite side at the level of the brainstem and then synapses with a lower motor neuron in the anterior portion of the spinal cord, just before exiting the spinal canal. Thus, movement of the right side of your body comes from a message that originates in the left side of your brain. Because the movement of the arm happens almost instantaneously, the signal must travel very quickly from the brain to the muscles. This is why motor neurons are myelinated nerve fibers, to speed conduction (see Chapter 12, Peripheral Nervous System). Recall that myelin is an insulating material that covers nerve axons to increase the speed of signal transmission.
Diseases That Alter Neuromuscular Function
Normal function of the complex transmission of information from the brain to muscle through the NMJ is essential for normal movement. Disease processes can interrupt normal function at any step along the way. Most proximal to the NMJ would be a defect in the motor cortex of the brain. For example, a stroke (an obstruction in a blood vessel or a ruptured blood vessel) in the motor cortex can prevent movement on the opposite side of the body. This type of injury would present with upper motor neuron signs and symptoms (described below). Leaving the cortex, motor signals are sent out to the body through motor neurons, and disease of these upper motor neurons can affect transmission of the signal; two common central nervous system diseases affecting motor neurons include multiple sclerosis and Parkinson disease. A spinal cord injury can interfere with upper motor neurons. Because the nerves have crossed over to the opposite side of the body at the level of the brainstem, a spinal cord injury will cause upper motor neuron signs and symptoms on the same side as the injury. Upper motor nerve signs and symptoms common to all these problems evolve over time (weeks to months) and include increased intensity of reflexes (e.g., knee jerk reflex) and muscle spasticity.
Disease or injury can also affect lower motor neurons. Diseases that directly affect lower motor neurons include Lou Gehrig disease, Charcot-Marie-Tooth disease, and Guillain-Barré syndrome. Trauma is another common source of injury to lower motor neurons after they leave the spinal canal. This can even happen in the operating room. For example, poor positioning of a patient on the operating room table can cause prolonged compression of a nerve, resulting in permanent injury (see Chapter 20, Patient Positioning). Patients with lower motor neuron injuries exhibit decreased reflexes and muscle wasting. Disorders of either upper or lower motor neurons can cause proliferation of ACh receptors on the muscle membrane.
Finally, disease can strike the NMJ itself. There are two main diseases of the NMJ: Lambert-Eaton syndrome and myasthenia gravis. In Lambert-Eaton syndrome, patients have antibodies to the mechanism that causes release of calcium within the presynaptic nerve cell. Patients with Lambert-Eaton syndrome often have concurrent lung cancer, and the best treatment is to treat their underlying cancer. In myasthenia gravis, patients have antibodies to nicotinic receptors on the postsynaptic side of the NMJ (muscle motor end plate). Efforts to treat patients with myasthenia gravis focus on preventing antibody attack of the nicotinic receptors, which ultimately improves transmission of nerve signals from the brain and strength/endurance. Patients with myasthenia gravis are also treated with drugs that limit the breakdown of ACh (acetylcholinesterase inhibitors) in the synaptic cleft, which allows for a higher concentration of ACh and an increased ability to bind to the undamaged nicotinic receptors.
Pharmacologic NMJ Blockade
In the clinical setting, muscle relaxation can prevent patient movement, can improve operating conditions for the surgeons, and can facilitate ventilation and intubation with paralysis. The agents used to achieve paralysis are referred to as neuromuscular blocking agents, since they have their direct effect on the communication between nerves and muscle, rather than having any direct effect on muscle fibers. Anesthesia providers must understand the anatomy and physiology of the NMJ in order to determine the proper way to block neuromuscular transmission. Insufficient paralysis may make intubation difficult, if not impossible, or a patient may move at an inopportune time during surgery and sustain an unintended injury. If too much neuromuscular blocking agent is given, the patient may have weak breathing muscles and respiratory insufficiency after surgery.
There are two basic ways to block neuromuscular transmission: presynaptic inhibition of ACh release or postsynaptic blocking of the ACh nicotinic receptors on the muscle end plate. Although not used clinically in the operating room, two examples of drugs that cause presynaptic inhibition of ACh release are botulinum toxin (Botox) and aminoglycoside antibiotics. Botox is so potent that it is used only in tiny doses in very localized injections; aminoglycoside antibiotics, on the other hand, only rarely cause significant depression of ACH release, but anesthesia providers must be aware of their ability to cause weakness in patients with kidney failure, myasthenia gravis, or other diseases.
In the operating room, anesthesiologists administer NMJ-blocking drugs that work primarily on the postsynaptic side of the NMJ. Postsynaptic NMJ blockade falls into two main categories, with each achieving blockade by a different mechanism: depolarizing and nondepolarizing NMJ blockers.
Depolarizing agents (currently, this is only succinylcholine) bind to the nicotinic ACh receptor and cause a depolarization of the motor end plate. This causes the muscle to quickly contract and then relax. During contraction it is normal for potassium to be released from the cell into the surrounding tissue and blood. Continued presence of succinylcholine in the synaptic cleft with binding to nicotinic receptors prevents further stimulation of the muscle, resulting in paralysis. Whereas ACh is metabolized quickly to allow the muscle to quickly recover, metabolism of succinylcholine is much slower, which results in more prolonged (5-10 minutes) depolarization of the affected muscle cell and transient paralysis. Because succinylcholine causes depolarization of a large number of muscle cells and the associated leakage of potassium out of the cell, its administration is associated with a small (0.7-1.0 mEq/L) increase in serum potassium level. This increase is generally not clinically significant; however, there are several groups of patients who are at risk for much greater increases in serum potassium. Any condition that results in a greater density of nicotinic receptors on the postsynaptic membrane is at risk for much greater rises in serum potassium after succinylcholine administration. Examples of patient groups with higher densities of nicotinic receptors include those with upper or lower motor neuron injury, prolonged periods of bed rest, or burns. In these patients, succinylcholine is contraindicated, as serum potassium may increase to levels high enough to cause cardiac arrest.
Today in anesthesia, nondepolarizing agents are most commonly used to achieve NMJ blockade. Nondepolarizing NMJ blockers are often also called competitive blockers due to their mechanism of action. Competitive NMJ blockers compete against ACh for the ACh nicotinic receptor sites on the postsynaptic membrane. Nondepolarizing agents prevent the binding of ACh with nicotinic receptors; thus, depolarization does not occur and the muscle will remain in the noncontracted state. Various medications fall into the nondepolarizing category, including rocuronium, vecuronium, and cisatracurium. Nondepolarizing agents are longer acting than depolarizing agents due to their slower metabolism. They also have a slower onset time. For example, succinylcholine has an onset time of 30 seconds and will last for 7-10 minutes, whereas rocuronium has an onset time of 2-3 minutes and will last from 30 to 60 minutes. Both the onset time and the duration of action of nondepolarizing drugs are affected by the dose of the drug; the higher the dose, the quicker the onset and also the longer the duration of action. In addition, the duration of action of nondepolarizing agents can be prolonged even further in patients with certain medical conditions including kidney disease, liver disease, respiratory acidosis, metabolic alkalosis, hypothermia, presence of calcium channel blockers in the blood, increased magnesium in the blood, and decreased potassium in the blood.
Due to the longer duration of action of nondepolarizing agents, it is usually necessary to antagonize (“reverse”) the agent’s action. In order for the action of the NMJ-blocking agent to be antagonized, the patient must be given two different types of medications: acetylcholinesterase inhibitors (e.g., neostigmine) and anticholinergic agents (e.g., atropine or glycopyrrolate). The acetylcholinesterase inhibitor prevents the metabolism of ACh in the synaptic cleft, which increases the amount of ACh in the synapse. The large amounts of ACh are better able to compete with the NMJ-blocking drug for the nicotinic receptor and initiate muscular contraction. Acetylcholinesterase inhibitors cannot fully reverse very deep levels of NMJ block. Unfortunately, acetylcholinesterase inhibitors also increase ACh concentration at many sites in the body, not just at the NMJ. Due to binding with ACh receptors throughout the body (both nicotinic and muscarinic), when used alone, acetylcholinesterase inhibitors are associated with severe reductions in heart rate, bronchospasm (wheezing), miosis (very small pupils), and gastrointestinal cramping referred to as a cholinergic crisis (see Chapter 13, Autonomic Nervous System, for locations of cholinergic receptors in the body), due to their effects on muscarinic Ach receptors. In fact, anticholinesterases are commonly used as poisons. To prevent a cholinergic crisis, anticholinergic drugs that preferentially block muscarinic receptors for ACh (e.g., glycopyrrolate or atropine) are administered simultaneously with the administration of an acetylcholinesterase inhibitor.
Sugammadex is a new drug on the market, which is used to reverse neuromuscular blockade produced by either rocuronium or vecuronium, which are referred to as amino-steroid agents. Sugammadex (unlike the acetylcholinesterase inhibitors) can be used to reverse either moderate or deep NMJ blockade. Sugammadex inactivates rocuronium or vecuronium by encapsulation (chelating) the drug and, by doing so, causes inactivation. Fortunately, the interaction between sugammadex and either rocuronium or vecuronium is very intense, which allows the drugs to be removed from the tissue and ultimately excreted in urine.
Monitoring Neuromuscular Blockade
The degree of neuromuscular blockade may be monitored by observing clinical signs or by objective assessment with a nerve stimulator. The use of objective means of measurement helps the anesthesia provider determine if the proper amount of blockade has been reached or to ensure that the blockade has been sufficiently reversed. As discussed earlier, the assessment of the degree of neuromuscular blockade is clinically important to prevent complications from insufficient blockade or inadequate reversal at the end of a case.
Clinical signs can be used to estimate neuromuscular recovery. These signs include a head lift greater than 5 seconds, leg lift greater than 5 seconds, the patient holding a tongue depressor in clenched teeth while the anesthesia provider tries to pull it out, and, lastly, a maximum inspiratory pressure of greater or equal to 50 cm H2O. They are often used as a measure of adequate reversal of neuromuscular blockade. Unfortunately, these signs require a cooperative patient and cannot be used during a general anesthetic to monitor the depth of neuromuscular blockade.
The use of a nerve stimulator overcomes this limitation. These devices help to assess the function of the NMJ by stimulating a peripheral nerve and monitoring the effect on muscle innervated by the nerve (e.g., stimulation of the ulnar nerve and observation of the effect on the adductor pollicis muscle). After placing the stimulating electrodes near the peripheral nerve, the anesthesia provider will apply a train of four stimulations to the nerve. These stimuli are four equal impulses administered 0.5 seconds apart. The reaction of the muscle is used to determine the intensity of the block. The response of the muscle depends upon whether the neuromuscular agent administered was a nondepolarizing or depolarizing drug.
Nondepolarizing agents are characterized by a response called fade; the muscle response to the stimulation decreases with repetitive nerve stimulation. With a train of four stimulations, the strength of each muscular contraction will diminish if there is residual neuromuscular blockade. Another characteristic of nondepolarizing agents is called posttetanic potentiation. If a tetanic stimulation is applied to a muscle and a single stimulus is immediately applied right afterward, the muscle twitch response to the single stimulus is greater than the previous tetanic muscle response. This response occurs because of an increase in presynaptic ACh release in addition to more ACh being present at the motor end plate.
Depolarizing agents do not exhibit posttetanic potentiation or fade in response to a train of four stimuli. With depolarizing agents, the muscle response to all four of the nerve stimuli will be exactly the same (even if that response is very weak).
It is important to note that complete return of train of four to baseline, without detectable fade, does not guarantee that the patient will be able to breathe adequately or protect the airway from obstruction or aspiration. Indeed, patients can still have as many as 75% of their nicotinic receptors occupied by NMJ-blocking drug (e.g., rocuronium) with a complete return of train of four. Not surprisingly, weakness due to residual nondepolarizing NMJ-blocking drugs is a common reason for complications in the postanesthesia care unit (PACU). Thus, most anesthesia providers still elect to administer a reversal/antagonism drug in any patient receiving a nondepolarizing NMB, even when patients demonstrate no fade to a train of four stimulus or tetanic stimulation.
Summary
Neuromuscular blockers have significant benefits and risks and are fundamental to anesthetic practice. The anesthesia technician should understand the physiologic events leading to muscle contraction, what happens during neuromuscular blockade, the agents used to create the neuromuscular blockade, and how to measure the blockade during surgery. This will prepare anesthesia technicians to foresee what will be asked of them during routine and emergency anesthetic circumstances as well as surgical procedures.
Review Questions
1. Which of the following is the CORRECT order of events for muscle contractions?
A) The motor cortex of the brain sends a message→action potential sent down the axon→ACh attaches to the nicotinic ACh receptors on the motor end plate.
B) ACh from the axon terminal goes to the synaptic cleft→action potential sent down the axon→muscle contraction.
C) ACh attaches to the nicotinic ACh receptors on the motor end plate→action potential sent down the axon→muscle contraction.
D) The motor cortex of the brain sends a message→ACh attaches to the nicotinic ACh receptors on the motor end plate→action potential reaches the presynaptic terminal.
E) None of the above.
Answer: A
The correct order of events is as follows: the motor cortex of the brain sends a message→action potential sent down the axon→action potential reaches the presynaptic terminal→ACh attaches to the nicotinic ACh receptors on the motor end plate→muscle contraction.
2. If a patient were to have a stroke on the right side of the motor cortex, which area of the body would be affected and what would be the resulting symptoms?
A) Right side of the body, lower motor neuron signs
B) Right side of the body, upper motor neuron signs
C) Left side of the body, upper motor neuron signs
D) Left side of the body, lower motor neuron signs
E) None of the above
Answer: C
Due to crossover of nerve fibers, the motor cortex of the brain affects the opposite (contralateral) side of the body. Therefore, a stroke in the right motor cortex will affect the left side of the body. Upper motor neurons are in the brain. Lower motor neurons are in the nerves that leave the spinal cord.
3. What are the two basic ways to block neuromuscular transmission at the neuromuscular junction?
A) Presynaptically block receptor release; postsynaptically block ACh nicotinic receptors on the muscle end plate
B) Presynaptically block ACh release; postsynaptically block ACh muscarinic receptors on the muscle end plate
C) Presynaptically block ACh release; postsynaptically block ACh nicotinic receptors on the muscle end plate
D) All of the above
E) None of the above
Answer: C
The presynaptic neuron releases ACh as the neurotransmitter, which then binds to nicotinic ACh receptors on the motor end plate of the muscle. Muscarinic ACh receptors are present in other parts of the body. Receptors are not released; they are present on cell membranes.
4. Which of the following statements are FALSE regarding nondepolarizing agents?
A) Examples of nondepolarizing agents include rocuronium, vecuronium, and cisatracurium.
B) Nondepolarizing NMJ blockers are also called competitive blockers and are most commonly used in the operating room.
C) Nondepolarizing agents are shorter acting and have a faster onset than depolarizing agents.
D) Nondepolarizing agents can be reversed with anticholinesterases.
E) All of the above are TRUE.
Answer: C
The agent with the fastest onset and shortest duration of action is succinylcholine, which is a depolarizing agent. All of the other statements are true regarding nondepolarizing neuromuscular blockers. These agents are competitive antagonists for the ACh receptor on the motor end plate. To reverse the effects of these agents, the administration of an anticholinesterase will prevent the breakdown of ACh and allow it to outcompete the nondepolarizing agent for the ACh receptor.
5. Which of the following statement is TRUE when monitoring neuromuscular blockade on a twitch monitor?
A) Nondepolarizing agents are monitored by placing the twitch monitor on the facial nerve only, while depolarizing agents are monitored by placing the twitch monitor on the ulnar nerve only.
B) Nondepolarizing agents demonstrate a response called “fade” where the twitch response decreases with repetitive nerve stimulation.
C) Depolarizing agents always cause “fade.”
D) Neuromuscular blockade caused by nondepolarizing agents cannot be monitored with a nerve stimulator.
E) None of the above.
Answer: B
Nondepolarizing agents cause the muscle response (twitch) to a stimulus applied to a nerve innervating the muscle to “fade.” The twitch strength will decrease with repetitive stimuli. This is a major method of monitoring the intensity of neuromuscular blockade caused by nondepolarizing agents. The nerve stimulator may be placed over a variety of nerves including the ulnar and facial nerves. Depolarizing agents, in most cases, do not cause “fade.”
6. An intubating dose of succinylcholine can be reversed by:
A) Succinylcholine is not reversed
B) Neostigmine alone
C) Neostigmine and glycopyrrolate
D) Sugammadex
Answer: A
Succinylcholine is a depolarizing neuromuscular blocker. Its paralysis is noncompetitive, brief, and temporarily irreversible. Nondepolarizing neuromuscular blockers are competitive inhibitors and are reversed by increasing the concentration of acetylcholine at the neuromuscular junction; this is done with an acetylcholinesterase inhibitor, neostigmine. Glycopyrrolate prevents neostigmine (and its associated acetylcholine) from having muscarinic toxicity at autonomic nerve terminals, particularly the heart. Sugammadex chelates some nondepolarizing neuromuscular blockers, particularly rocuronium.
SUGGESTED READINGS
Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015.
Chandler JT, Brown LE. Conditioning for Strength and Human Performance. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
Fuchs-Buder T. Neuromuscular Monitoring in Clinical Practice and Research. Heidelberg, Germany: Springer; 2010.
Neal MJ. Medical Pharmacology at a Glance. 5th ed. West Sussex, UK: Wiley-Blackwell; 2005.