CHAPTER 12
Peripheral Nervous System
The nervous system coordinates all information, sensation, thought, and voluntary and involuntary action in the human body. The nervous system has two major divisions: the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord and was covered in Chapter 11. The PNS is the information highway between the CNS and the rest of the body and consists of the nerves and their supporting structures extending beyond the brain and spinal cord. It is subdivided into the autonomic and sensory-somatic nervous systems. The autonomic nervous system (ANS) governs the actions of glands and involuntary muscles (such as smooth and cardiac muscle) and will be covered in detail in Chapter 13. The sensory-somatic nervous system relays sensory information from the periphery to the brain and spinal cord and controls the actions of voluntary muscles such as skeletal muscle, certain organs, and reflex movements. This chapter focuses on the anatomy, physiology, and pharmacology related to the sensory-somatic nervous system.
The Sensory-Somatic Nervous System
The sensory-somatic nervous system consists of 12 pairs of cranial nerves and 31 pairs of spinal nerves. Cranial nerves I and II originate in the brain, while the other 10 cranial nerve pairs originate from the brainstem. All 31 pairs of spinal nerves arise from the spinal cord.
Spinal Cord Organization
Longitudinally, the spinal cord is divided into five regions. From the brainstem downward, these are the cervical, thoracic, lumbar, sacral, and coccygeal regions. The cord has a total of 31 segments. Each segment roughly corresponds to a region of bone, muscles, and skin in the body and to a segment of the bony vertebral column. Spinal nerves essentially act as telephone lines at each spinal cord segment, connecting the area of the spinal cord to its respective region of skin and muscles and carrying messages between the body and the spinal cord to control sensation and movement.
There are 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal spinal nerves, emerging in a pair, one on each side of the spinal cord. The nerve roots emerge from both the front and back of the spinal cord at each horizontal segment; these merge to form a spinal nerve (Fig. 12.1). The front root is known as the ventral root and carries motor (movement) commands. The back root, known as the dorsal root, carries sensory information from the body. Once the ventral and dorsal roots fuse into a spinal nerve, they travel along the left and right side of the spinal canal beside the spinal cord until they reach their exit hole, the intervertebral foramen, in the space between each vertebra.

FIGURE 12.1. Peripheral distribution of spinal nerves. Dorsal ramus (to skeletal muscles, smooth muscles, and glands, etc., of back; from receptors of back); ventral ramus (to skeletal muscles, smooth muscles, and glands, etc., of body wall, limbs; from receptors of body wall, limbs); and sympathetic nerve (to smooth muscles, glands, and visceral organs in the thoracic and abdominopelvic cavity; from receptors of organs). (From Stedman’s Medical Dictionary. 27th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000, with permission.)
Spinal nerves are named according to the spinal cord segment where they emerge. In the cervical spine, spinal nerves exit the vertebral column above their respective vertebrae. Thus, C1 arises above the first cervical vertebra, and this pattern continues through C7. Note that there are only seven cervical vertebrae, but there are eight cervical spinal nerves. The eighth cervical spinal nerve (C8) courses below the seventh cervical vertebrae (and above the first thoracic vertebra; thus, there is a C8 nerve root, but no C8 vertebra). All thoracic, lumbar, sacral, and coccygeal spinal nerves (i.e., nerves in most of the spinal column) course below their corresponding vertebrae (Fig. 12.2).

FIGURE 12.2. Relationship of vertebral column, spinal cord, and spinal nerves. Note the relation of the spinal cord segments and spinal nerves to the vertebral column. (From Moore KL, Agur AM, Dalley AF. Essential Clinical Anatomy. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014, with permission.)
Each spinal segment corresponds roughly to a horizontal area of the body. Hence, spinal nerves innervate (provide nerves for) specific regions of bone, muscle, and skin that correspond to the individual spinal cord segments they each come from. A myotome is a group of muscles innervated by a single spinal nerve root. A dermatome is an area of skin innervated by a single nerve root (Fig. 12.3). Nerve blocks can provide complete anesthesia when a surgery is restricted to a small set of dermatomes and myotomes, which is often the case for surgery on an arm or leg. A detailed knowledge of peripheral nerve anatomy is essential to the safe and successful practice of regional anesthesia (see Chapter 16, Regional Anesthesia).
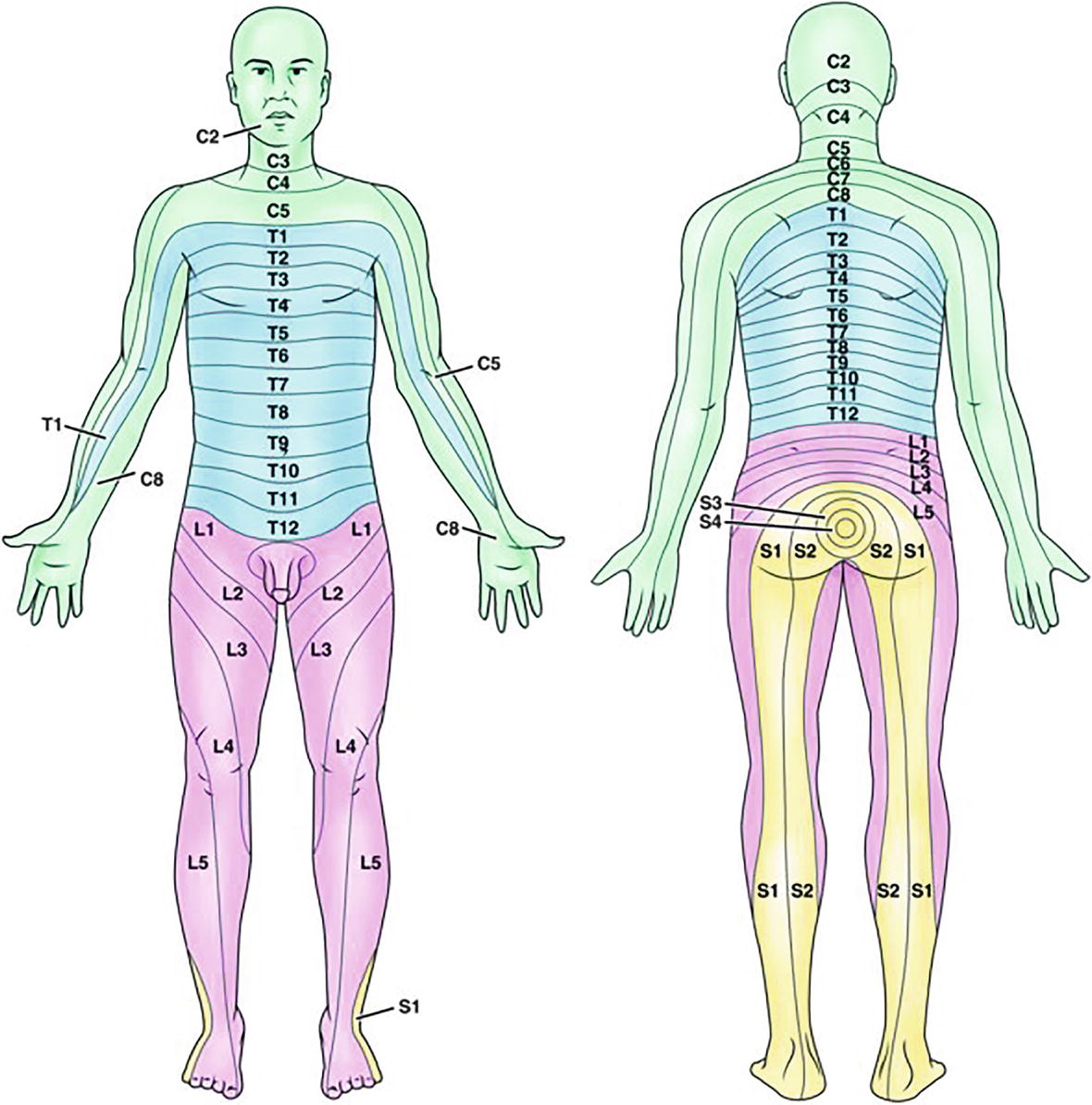
FIGURE 12.3. Dermatome distribution. (From Farrell M. Smeltzer & Bares Textbook of Medical-Surgical Nursing. 4th ed. Philadelphia, PA: Wolters Kluwer; 2016, with permission.)
Afferent and Efferent Nerves
Peripheral nerves carry information both to and from the CNS. Sensory nerve fibers “sense” signals from all different parts of the body and carry these signals from the periphery to the brain and spinal cord. Another name for a sensory nerve or nerve fiber that transmits information from the periphery to the CNS is an afferent nerve. The term afferent can be remembered by the notion that afferent nerves transmit signals that approach the CNS. Afferent nerves transmit a variety of sensation signals to the CNS, including information about position, pressure, pain, and vibration. Motor nerves, on the other hand, carry command signals from the brain and spinal cord to peripheral structures involved in voluntary movement, mainly skeletal muscle. Motor nerves are called efferent nerves because they carry signals away from the brain and spinal cord to the periphery. The term efferent can be remembered by the notion that efferent nerves carry signals exiting the CNS. The brain and spinal cord regulate and control all bodily functions and actions, whether voluntary or involuntary, through efferent nerves.
Anatomy: Peripheral Nerves
Nerves provide structured pathways for electrochemical signals to be sent between the CNS and all other tissues in the body. Peripheral nerves can be one-way or two-way pathways carrying afferent-only nerve fibers, efferent-only nerve fibers, or both afferent and efferent nerve fibers. Typically, ventral and dorsal roots meet to form two-way peripheral nerves carrying both afferent and efferent nerve fibers to and from the CNS. Given the vast amount of information constantly being relayed between all different areas of the body and the CNS, and the precise coordination and control of muscles it takes to make any sort of movement, it is not surprising that peripheral nerves are highly organized, detailed structures.
Knowledge of nerve anatomy is essential to safely perform any regional anesthetic, and an understanding of peripheral nerve anatomy will help the anesthesia technician appropriately assist with regional anesthesia (see Chapter 16). Successful regional anesthesia depends on knowing exactly where anesthesia will be produced with an injection of local anesthetic. Conversely, safe regional anesthesia depends on understanding where not to place needles or drugs. Injection of local anesthetic directly into a nerve has the potential to cause severe injury and life-altering complications.
A nerve is a cable-like structure made up of multiple groupings of myelinated and unmyelinated nerve cell axons, glial cells, blood vessels, and layers of connective tissue. Each distinct nerve fiber axon is enveloped by a loose layer of connective tissue called the endoneurium. Endoneurium-surrounded axons are grouped together in bundles called fascicles. Each fascicle is surrounded by a connective tissue layer called the perineurium. The perineurium is surrounded fascicles, and small blood vessels coursing between them are further encased by a dense connective tissue sheath called the epineurium and form a nerve (Fig. 12.4).
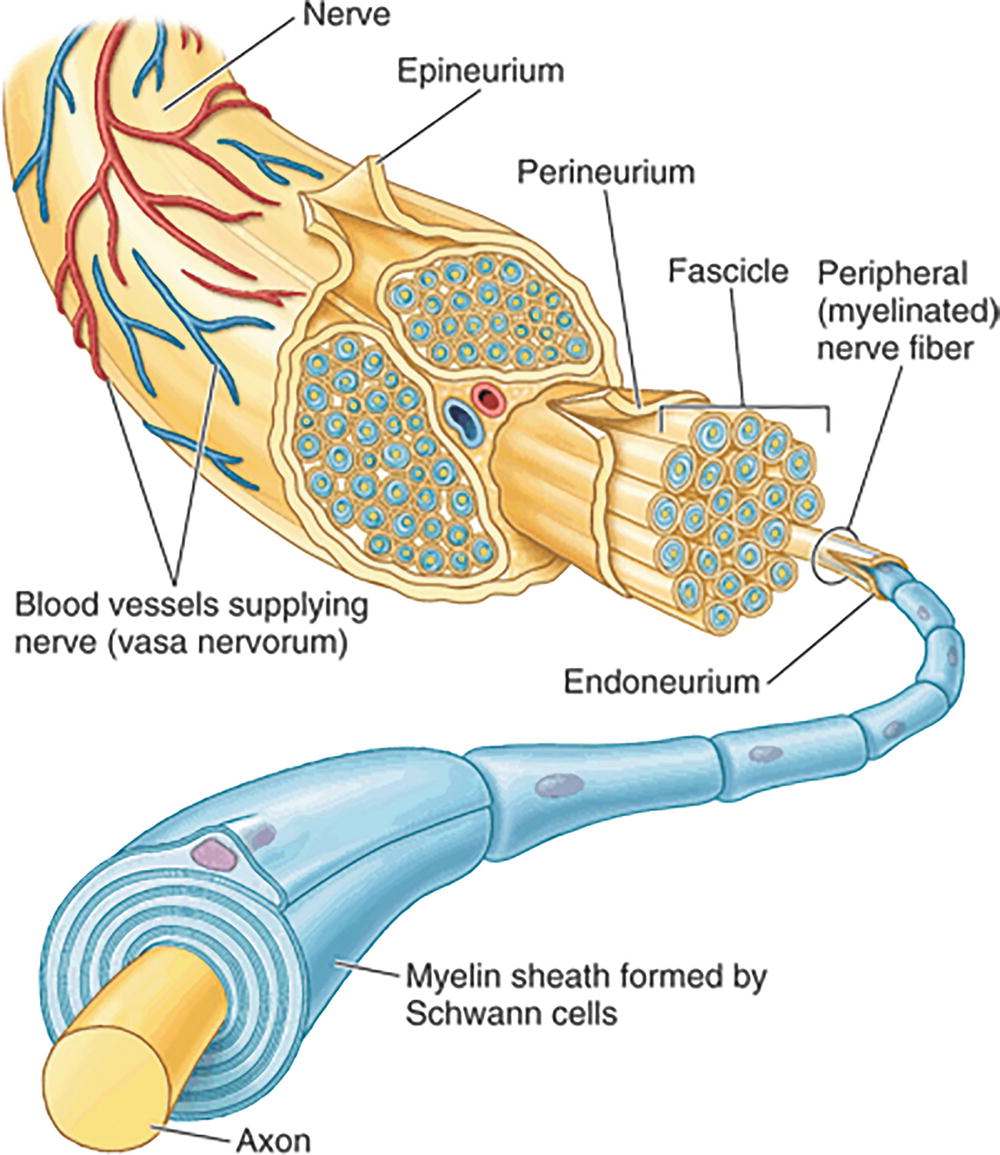
FIGURE 12.4. The nerve. Arrangement and ensheathment of myelinated nerve fibers. Nerves consist of the bundles of nerve fibers, the layers of connective tissue binding them together, and the blood vessels (vasa nervorum) that serve them. All but the smallest nerves are arranged in bundles called fascicles. (From Moore KL, Agur AM, Dalley AF. Clinically Oriented Anatomy. 7th ed. Philadelphia, PA: Wolters Kluwer; 2012, with permission.)
Neurons: The Nerve Cell
Both in the central and PNSs, neurons are the basic structural and functional cell (Fig. 12.5). The nervous system is also filled with many different types of glial cells, which support neurons but do not conduct information. Individual nerve cells come in many shapes and sizes depending on their locations throughout the body, but throughout the nervous system, neurons have four basic structures in common: dendrites, the soma (or cell body), an axon, and axon terminals.
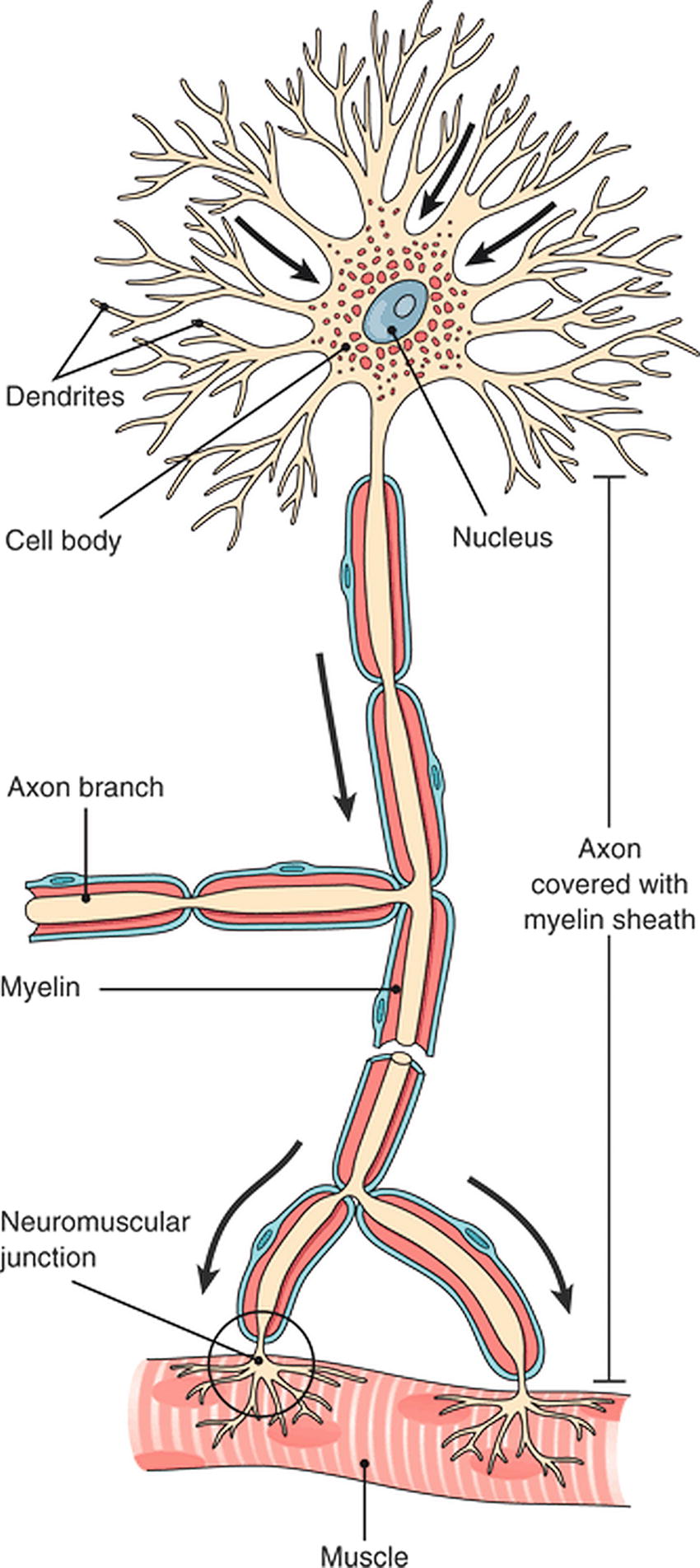
FIGURE 12.5. The neuron: the cell body containing the nucleus and other organelles, dendrites to receive information, an axon to transmit information over a distance, and axon terminals to transmit a signal to an end organ. (From Cohen BJ. Medical Terminology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003, with permission.)
Dendrites are the antennae of the nerve cell. They are highly branched terminal processes that receive input signals from outside the cell and relay this information to the cell body of the neuron. The cell body houses the nucleus and other cellular organelles necessary for the nerve cell’s metabolic processes. The cell body is the integration center of the neuron; it is responsible for processing all incoming signals received by the dendrites.
Axons extend from the cell body of the neuron and transmit action potentials over distance. Just as in the CNS and the ANS, in the PNS, axons can be short or very long (e.g., from the spinal cord into the foot).
Peripheral Nerve Physiology
Nerve signals are conducted by the net movement of positively charged ions sodium (Na+) and potassium (K+) into and out of nerve cells (Fig. 12.6). When no signal is being transmitted, a nerve cell is at rest. At rest, a neuron contains more potassium ions inside the cell and more sodium ions outside the cell and has a net difference in electric charge between inside and outside. This phenomenon is known as an electrochemical gradient. Constantly maintaining this gradient requires energy-using pumps embedded within the cell membrane. Nerve tissue, both in the PNS and the CNS, is very metabolically active and consumes a great deal of oxygen and energy.
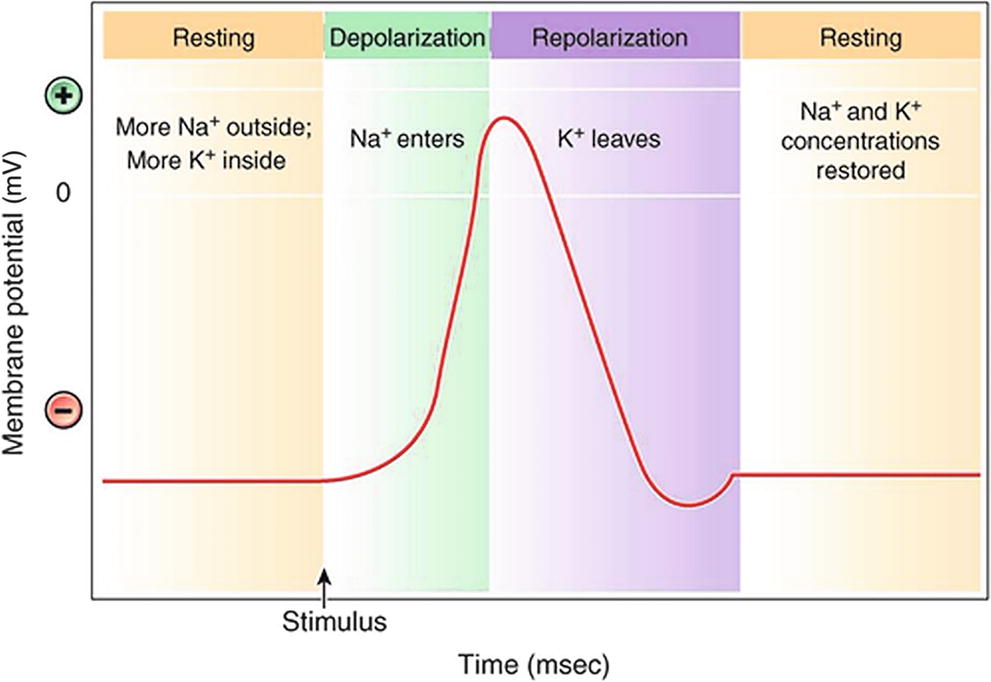
FIGURE 12.6. The action potential. A neuron that is not transmitting a signal is said to be at its resting potential. The inside of the neuron is negatively charged relative to the outside because of the large, negatively charged molecules in the cytoplasm. The sodium-potassium pump maintains concentration gradients for both sodium and potassium ions. (From Cohen BJ, Kerry KL. Memmler’s Structure and Function of the Human Body. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015, with permission.)
Depolarization
When a neuron is stimulated by neurotransmitter arrival at the dendrite, sodium ion (Na+) channels on the cell membrane open, allowing for rapid flow of Na+ ions into the cell. Rapid influx of Na+ changes the electrical gradient, and the inside of the cell becomes more positively charged compared to the outside of the cell. This is known as depolarization of the membrane. This impulse then moves rapidly along the axon, as the sodium channels are “voltage gated”; that is, they open in response to a change in voltage. This movement of an impulse from one localized area of membrane to the next is known as an action potential.
Once an axon begins to approach its target cell, it divides into smaller branches called axon terminals. The axon terminals make specialized contacts with target cells. This specialized cell-to-cell contact is called a synapse. If the target cell is a muscle fiber, the distal termination is referred to as a motor endplate and the contact is called a neuromuscular junction. The signal-sending neuron is the presynaptic neuron; the target cell is referred to as the postsynaptic cell.
There is a small gap between the end of the presynaptic neuron and the receiving cell. This gap is called a synaptic cleft. Electric impulses do not cross these gaps. Once an electric impulse transmitted along an axon reaches the axon terminals, it triggers the release of chemical messengers known as neurotransmitters. Axon terminals release neurotransmitters outside of the cell into the synaptic cleft. Neurotransmitters diffuse across the synaptic cleft, bind to target receptors, and activate the postsynaptic cell.
If the postsynaptic cell is another neuron, depending on the intrinsic properties of the postsynaptic neuron, it is either stimulated and initiates its own electric impulse or inhibited and the signal is terminated. If the postsynaptic cell is a gland, it secretes its hormone; if it is a muscle, it contracts.
Conduction Velocity
Many factors influence how fast impulses travel along a neuron. The most important factors include cell diameter and presence of myelin. The larger a neuron’s diameter is, the faster the conduction velocity of impulses will be. The greater cross-sectional surface area of larger neurons results in less electrical resistance and easier propagation of action potentials. Myelin is a fatty insulating substance made by glial cells, which coats some, but not all, peripheral nerves. Myelinated membrane cannot conduct an impulse. Paradoxically, this makes conduction faster in myelinated nerves. Voltage-gated ion channels are concentrated at the nodes between myelin wrappings. In a myelinated axon, action potentials occur only at these nodes; the myelin thus enables the impulse to jump quickly down the length of an axon (Fig. 12.7). This “jumping” from node to node is called saltatory conduction (Fig. 12.8).

FIGURE 12.7. Formation of a myelin sheath. Key point: The myelin sheath is formed by Schwann cells in the peripheral nervous system. A: Schwann cells wrap around the axon, creating a myelin coating. B: The outermost layer of the Schwann cell forms the neurilemma. Spaces between the cells are the nodes (of Ranvier). (From Cohen BJ. Memmler’s Structure and Function of the Human Body. 10th ed. Philadelphia, PA: Wolters Kluwer; 2012, with permission.)
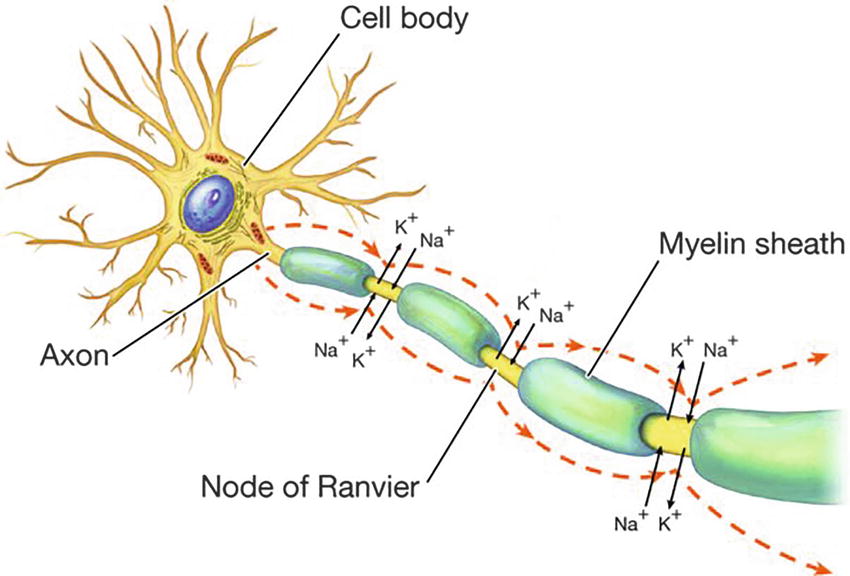
FIGURE 12.8. Saltatory conduction. An action potential is propagated along a myelinated fiber by jumping from one node of Ranvier to the next, which accelerates the speed of conduction. (From Wingerd B. Human Body. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2013, with permission.)
Repolarization
Shortly after depolarization, the change in polarity of the neuron’s membrane triggers the closure of voltage-gated sodium channels and opening of voltage-gated potassium channels. Sodium ions are no longer able to enter the neuron; instead, potassium ions now leave the cell, restoring the membrane’s relative negative charge back to the resting membrane potential. The change in electrical gradient and membrane potential back to that of the neuron’s resting state is known as repolarization. The original chemical gradients are reestablished via the active pumping of sodium out and potassium in again. This process again consumes energy. Nerve cells use a great deal of oxygen and energy compared with other cells in the body and are thus the most vulnerable cells in the body to ischemia.
Peripheral Nerve Pathophysiology
Nerve damage of peripheral nerves is known as peripheral neuropathy (Fig. 12.9). A number of diseases cause peripheral neuropathy including mechanical trauma, disruption in blood supply, metabolic diseases, genetically inherited disorders, toxins, inflammatory diseases, and vitamin deficiencies. Any of these causes can produce sensory dysfunction and/or pain.
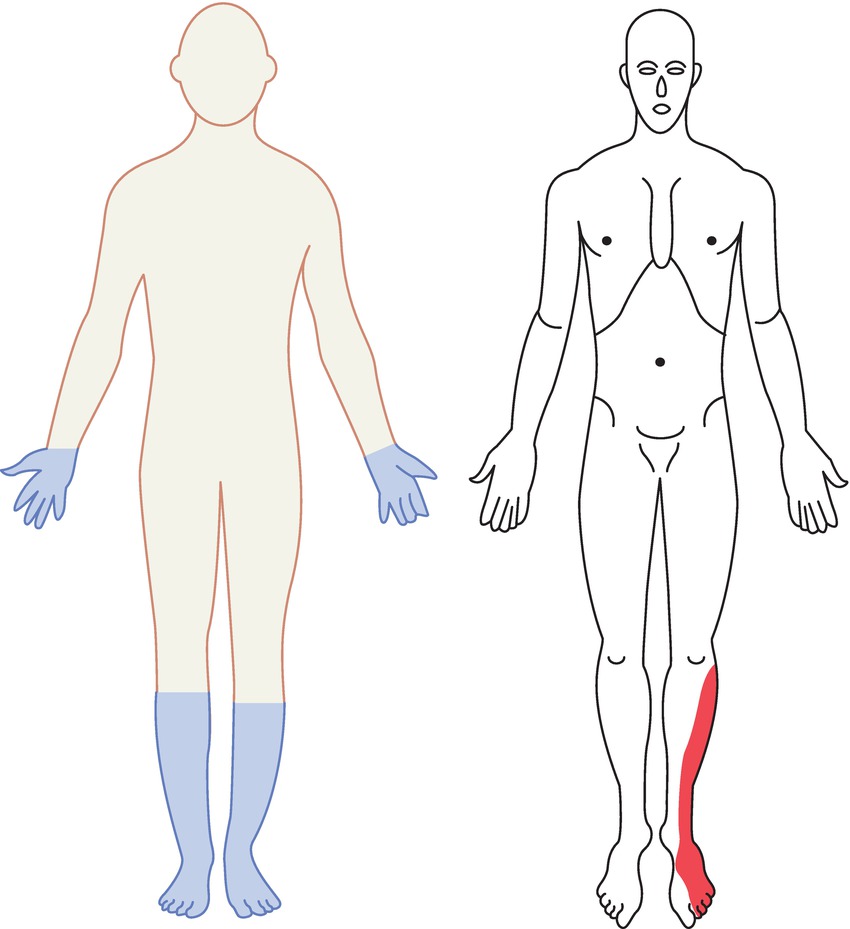
FIGURE 12.9. Distribution of sensory and lower motor neuron deficits in two types of nerve injury. On the left, a patient with peripheral polyneuropathy. Notice the “stocking-and-glove” pattern of sensory loss present in a systemic disease that causes problems for multiple peripheral nerves where they are smallest and most distal. Contrast this with the patient on the right, who has a pattern characteristic of injury to a single peripheral nerve, in this case the superficial peroneal nerve. This is a combined sensory and motor nerve; this patient is likely also to have a foot drop, as this nerve also innervates the muscles that dorsiflex the foot. This is a common positioning injury in the operating room (see Chapter 20, Patient Positioning).
Local Anesthetics and Peripheral Nerve Blockade
Local anesthetics inactivate voltage-gated sodium channels. This prevents propagation of action potentials in peripheral nerves. As discussed earlier, sodium conductance through voltage-gated sodium channels is necessary for depolarization of nerve cell membranes and propagation of impulses down nerve axons. Thus, nerves exposed to local anesthetics temporarily lose function, resulting in loss of sensation or weakness of muscles in areas supplied by the anesthetized nerve. Local anesthetic binding and blockade of nerve transmission depend both on intrinsic properties of the given local anesthetic and characteristics of the nerve such as nerve fiber size, baseline nerve activity, and whether the nerve is myelinated. See Chapter 16, Regional Anesthesia, for a more detailed discussion on how local anesthetics are used in regional anesthesia.
Basic Local Anesthetic Pharmacology
Chemistry
All local anesthetic compounds contain three structural components (Fig. 12.10):
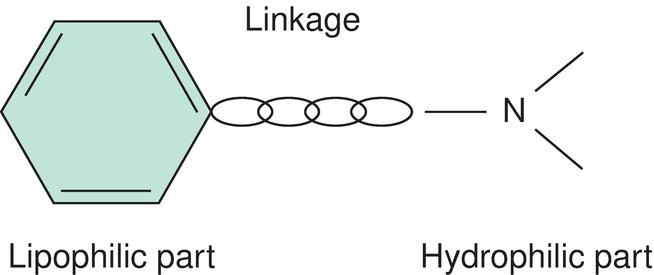
FIGURE 12.10. Structural backbone of a local anesthetic molecule.
1. Aromatic group—lipophilic portion (fat-loving) —this is usually a benzene ring.
2. Intermediate chain—the central hydrocarbon linkage (amide or ester bond)—determines the classification of the local anesthetic. Amides have an intermediate bond containing an amide bond (–HNC–), while esters have an intermediate bond containing an ester bond (–CO–) (Fig. 12.11).
3. Amine group—hydrophilic portion (water-loving)—this is the portion of the local anesthetic that acts as a hydrogen ion (H+) acceptor (more on this below).
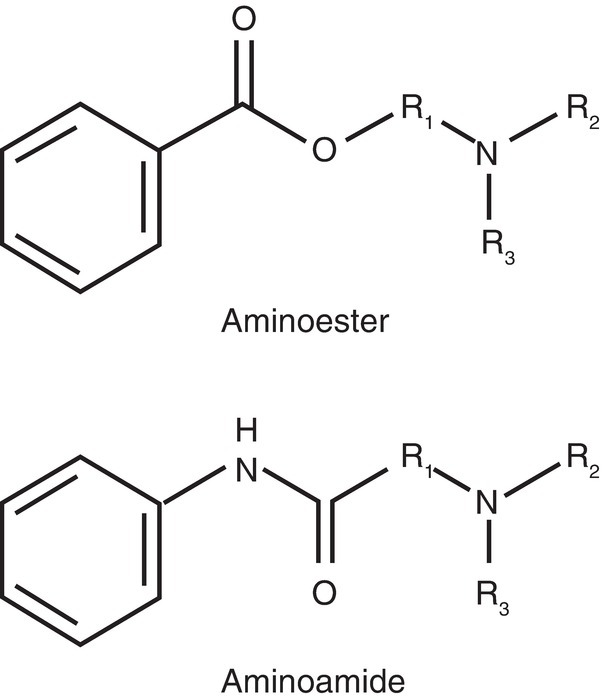
FIGURE 12.11. Ester vs amide linkage.
The two classes of local anesthetic are metabolized differently: amide local anesthetics are metabolized primarily by liver enzymes, while esters are primarily metabolized in the blood by pseudocholinesterase enzymes. Thus, any alteration in a patient’s liver metabolism can affect the elimination of amide anesthetics. Caution must be used in patients with liver or cardiac disease when using an amide local anesthetic as its elimination is likely to be slower. Likewise, caution must be used when using an ester anesthetic in a patient with pseudocholinesterase deficiency as esters will not be broken down as quickly. Ester anesthetics are more allergenic, and allergy to one ester suggests caution using another; these patients can usually receive amide local anesthetics safely. Allergic reactions to amide local anesthetics are extremely rare; often, patients who present with a listed “allergy” to an amide local anesthetic will have had another type of adverse reaction, and the allergy can be investigated and ruled out by the anesthesia provider.
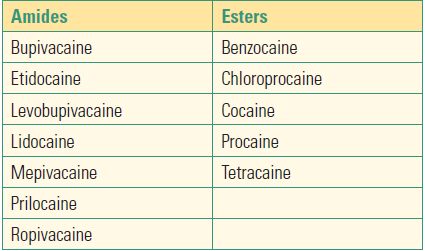
A trick on how to know if a local anesthetic is an amide or ester is to look at the generic name. If the spelling of the name contains two “i’s,” it is an amide (e.g., lidocaine). If there is only one “i” it is an ester (e.g., chloroprocaine). Table 12.1 lists common local anesthetics.
Table 12.1. Common Local Anesthetics
Structural Influences
The specific chemical structure of individual local anesthetics plays a role in determining the activity of the drug. pH and lipid solubility play a role in the onset, duration of action, and intrinsic potency of a local anesthetic.
Local anesthetics are weak bases, which means they have the ability to become positively charged, or ionized, by accepting a hydrogen ion. In solution, local anesthetics exist in equilibrium between nonionized molecules and positively ionized molecules. The pH at which these two forms (ionized and nonionized) are in exact balance is called the pKa and varies slightly between local anesthetics. Since nonionized molecules are the lipid-soluble form of a drug that can cross the cell membrane, the more nonionized drug present, the faster the onset is of the drug. You may sometimes see an anesthetist request bicarbonate as an additive for a local anesthetic in order to alter the pH, increase the amount of nonionized molecule, and further speed onset. In general, a local anesthetic with a pKa that is lower (closer to 7.4) will have a faster onset, since there is more nonionized drug available to enter the neuron. Lidocaine has a pKa of 7.8, whereas bupivacaine has a pKa of 8.1; lidocaine is in a state with more nonionized compound than is bupivacaine and has a faster onset. (Chloroprocaine is a notable exception with a pKa of 9.0 and yet a rapid onset of action.) Protein binding is yet another property that affects how a local anesthetic behaves. Local anesthetics exist in protein-bound and unbound forms. The unbound form is pharmacologically active.
Pharmacokinetics
Like all medications, local anesthetics can be toxic when absorbed systemically. The typical doses of local anesthetic used to produce peripheral nerve blockade or epidural anesthesia can be fatal if injected directly into the bloodstream by mistake (see Chapter 63, Local Anesthetic Toxicity).
Blood levels of a local anesthetic after regional block depend on absorption, distribution, and elimination. Rate of absorption of a local anesthetic depends on its site of injection, dose, and additional factors such as the addition of adjuvants like epinephrine. Well-vascularized tissues like the intrapleural space and paravertebral space result in more rapid absorption; absorption from the epidural space is very slow. When local anesthetics are injected into vascular regions, they are more avidly absorbed systemically than when they are injected into areas containing more fat. Higher total doses of local anesthetics also result in greater absorption and higher blood levels. The drug itself also matters: increased lipid solubility and increased protein binding both result in less systemic absorption. Once absorbed into the bloodstream, local anesthetics tend to rapidly distribute to highly vascular regions such as the heart and brain before distributing to less vascular regions such as muscle and fat. Distribution of the local anesthetic depends on organ blood flow, pKa, and the protein-binding affinity of the local anesthetic.
Toxic effects of local anesthetics are primarily focused on the nervous system and the heart.
Local anesthetics easily cross the blood-brain barrier and can result in CNS toxicity. CNS toxicity tends to be reversible; it has traditionally been viewed as the herald of the more serious cardiovascular toxicity; thus, anesthesia providers are alert to signs of CNS toxicity in any patient receiving high doses of local anesthetics. Classically, beginning signs of systemic toxicity are that of CNS excitement, which includes metallic taste, mouth or tongue numbness, tinnitus, and light-headedness. These can all be easily masked by low doses of sedation. At higher doses, signs of CNS depression ensue, such as generalized seizures and coma.
Local anesthetics block the sodium channels of the heart as well as those of the nervous system. Local anesthetics can affect the electric conduction system of the heart and thus cardiac function. By virtue of their sodium channel blockade, local anesthetics produce dose-dependent conduction blockade on the heart. Bupivacaine is commonly considered the most cardiotoxic of the local anesthetics.
The formulations of local anesthetics can also be harmful to nerves, depending on their routes of administration. In general, surgeons and nurses frequently perform infiltration anesthesia in skin or peripheral tissues, are not near major nerves, and can safely use local anesthetics packaged with preservatives. These may be less expensive and may be packaged as multiuse vials. Anesthesia providers performing blocks very close to large nerves, or in the epidural or spinal fluid space, however, must use preservative-free local anesthetic solutions, as some preservatives can be toxic to nerve tissues.
Summary
As an anesthesia technician, you will find an in-depth understanding of the PNS underlies your clinical practice in many anesthetizing locations. As you will see in the next chapter (Chapter 13, Autonomic Nervous System), anesthetics alter the nervous system reflexes of the cardiovascular and other organ systems in a way that anesthesia providers must treat every day. Regional anesthesia plays an essential perioperative role, whether as primary anesthetic or adjunct, and an anesthesia technician is essential in its efficient and safe implementation. Local anesthetics are used widely, in a varied fashion, and potential complications can result from their use; the anesthesia technician is part of a team involved in critical patient safety around their use, including drug stocking, safe administration settings, and safe patient monitoring settings. Peripheral nerves are very metabolically active and thus are vulnerable to ischemic injury when compressed by positioning or local injuries. You will frequently see patients with disorders of the PNS and will now have an understanding of some interactions with anesthesia planning that will help you better anticipate and assist in their care. As you go forward in your learning, an understanding of the basic science of the nervous system will continue to support your clinical practice.
Review Questions
1. There are __ pairs of spinal nerves.
A) 12
B) 13
C) 21
D) 31
E) 43
Answer: D
There are 31 pairs of spinal nerves.
2. True or false, all peripheral nerves are myelinated.
A) False
B) True
Answer: A
Some, but not all, peripheral nerves are myelinated. Myelinated peripheral nerves are wrapped in concentric layers of myelin around the epineurium.
3. How does an action potential travel along a myelinated axon?
A) The action potential jumps from one myelinated area of the axon to the next.
B) The action potential jumps from one nonmyelinated area of the axon to the next.
C) There are no gaps in a myelinated axon: the action potential jumps from the beginning of the axon to the terminal.
D) Myelin helps the nerve impulse cross the synapse from one myelinated nerve to the next.
Answer: B
Myelin is an insulator and cannot conduct nerve impulses. Therefore, in myelinated axons, the action potential jumps between nonmyelinated areas of the axon; these gaps, or nodes, between myelin segments have a concentration of voltage-gated sodium channels. The process of conduction jumping from one node to the next is known as saltatory conduction.
4. Which of the following statements are TRUE about nerve action potentials?
A) A nerve membrane is only polarized when conducting an action potential.
B) Depolarization cannot be conducted from one stretch of nerve membrane to another.
C) Phosphorus ions flowing into the cell cause depolarization.
D) Potassium ions flowing out of the cell cause repolarization.
E) None of the above.
Answer: D
Nerves transmit signals from one area of the body to another through action potentials. At rest, the nerve is polarized. During an action potential, sodium channels open and sodium first rushes into the nerve to depolarize it. Potassium channels then open and potassium rushes out of the cell to repolarize the nerve back to its resting state. An action potential occurs in one place in the nerve, and this then triggers depolarization and an action potential on the neighboring segment, propagating the signal along the nerve in a domino-like fashion.
5. Which of the following local anesthetics is associated with the highest risk of cardiotoxicity?
A) Lidocaine
B) Bupivacaine
C) Chloroprocaine
D) Ropivacaine
E) Mepivacaine
Answer: B
In general, the cardiotoxicity of local anesthetics is proportional to their potency, with bupivacaine being more potent and more cardiotoxic than lidocaine or chloroprocaine, ropivacaine, or mepivacaine. Chloroprocaine’s cardiotoxicity is also limited by its very short (seconds to minutes) plasma half-life. Recall that chloroprocaine is an ester; it is very rapidly degraded by plasma esterases.
6. Signs of local anesthetic toxicity include all of the following EXCEPT:
A) Tinnitus
B) Burning smell
C) Perioral numbness
D) Respiratory arrest
E) Metallic taste
Answer: B
Ear ringing (tinnitus), perioral numbness, metallic taste, seizures, cardiac arrhythmia, coma, and respiratory arrest are all classically described signs of local anesthetic toxicity. A burning smell is a sign of a focal (starting in an isolated area of the brain) temporal lobe seizure rather than a generalized (whole-brain) seizure as would be expected due to local anesthetic systemic toxicity.
SUGGESTED READINGS
Barash P, Cullen B, Stoelting R. Clinical Anesthesia. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90-102.
Brown DL. Atlas of Regional Anesthesia. 3rd ed. Philadelphia, PA: Elsevier; 2006.
Hadzic A. Textbook of Regional Anesthesia and Acute Pain Management. New York, NY: McGraw-Hill Companies, Inc.; 2007.
Henderson CA. A Comprehensive Introduction to the Peripheral Nervous System. Hattiesburg, MS; 2010.
Neal J, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35 (2):152-161.
O’Brien M. Aids to Examination of the Peripheral Nervous System. 5th ed. Philadelphia, PA; 2010.
Waxman S. Clinical Neuroanatomy. 28th ed. New York, NY; 2013.