CHAPTER 11
Central Nervous System
Introduction
Anesthetic procedures almost always involve the central nervous system (CNS), whether inducing unconsciousness with general anesthesia, providing sedation, or impairing nerve transmission with regional blocks. An understanding of the fundamental anatomy and physiology of the CNS is important for the anesthesia technician. This chapter will introduce the anatomy of the brain and spinal cord, as well as the physiology of the neurons within.
Anatomy of the Brain
The CNS can be divided into two distinct anatomical structures: the brain and the spinal cord. The brain is a complex organ with many functions, including the processing of sensory input, the generation of movement, and higher functions such as thought, language, and emotions. The structures that collectively make up the brain are cerebral hemispheres, central subcortical structures, brainstem, and cerebellum (Fig. 11.1).
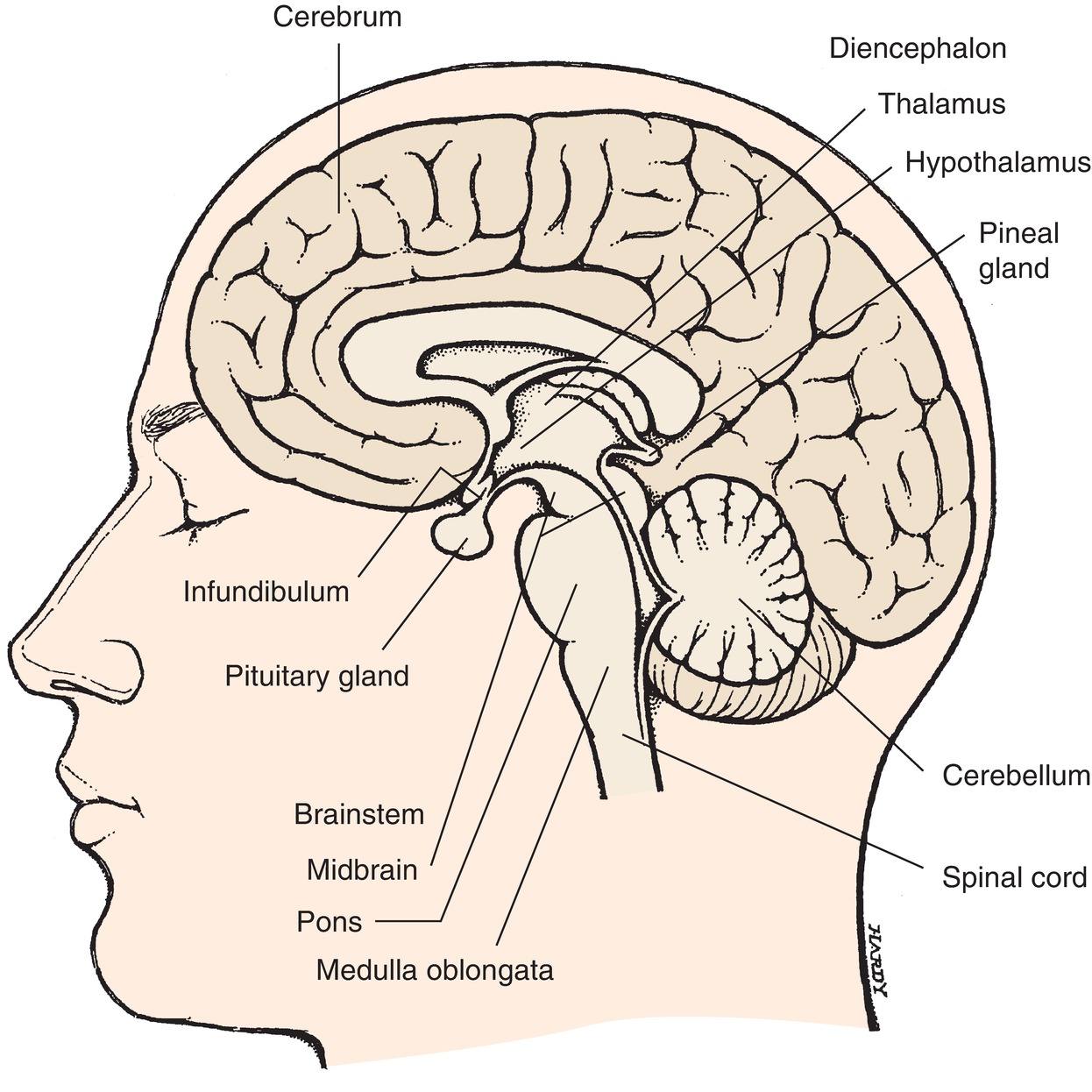
FIGURE 11.1. The brain (in situ; sagittal section). Showing the location of the four principal parts: cerebrum, diencephalon (which includes thalamus and hypothalamus), brainstem, and cerebellum. (From Stedman’s Medical Dictionary. 27th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000, with permission.)
The cerebral hemispheres are the most prominent of the brain’s structures and consist primarily of the cerebral cortex, called gray matter, and the white matter beneath, which contains the axons (“wiring”) that connects the regions of the brain. The cerebral hemispheres are divided into four lobes: occipital, temporal, parietal, and frontal (Fig. 11.2). Each lobe of the cortex contains specialized areas that are responsible for unique functions and responses. The occipital lobe is primarily associated with the processing of visual information. The temporal lobe is the primary site of auditory perception, performs language and speech processing, and also contains a structure called the hippocampus, which is responsible for storage and retrieval of memories. The parietal lobe integrates the senses and is also responsible for understanding language and spatial relationships. The parietal and temporal cortices together include the sensing (somatosensory) function and motor controls for the entire body, and this is the area of brain that helps us sense pain. The frontal lobe is responsible for executive functions, which include long-term planning, conscious motor movement, and behavior.
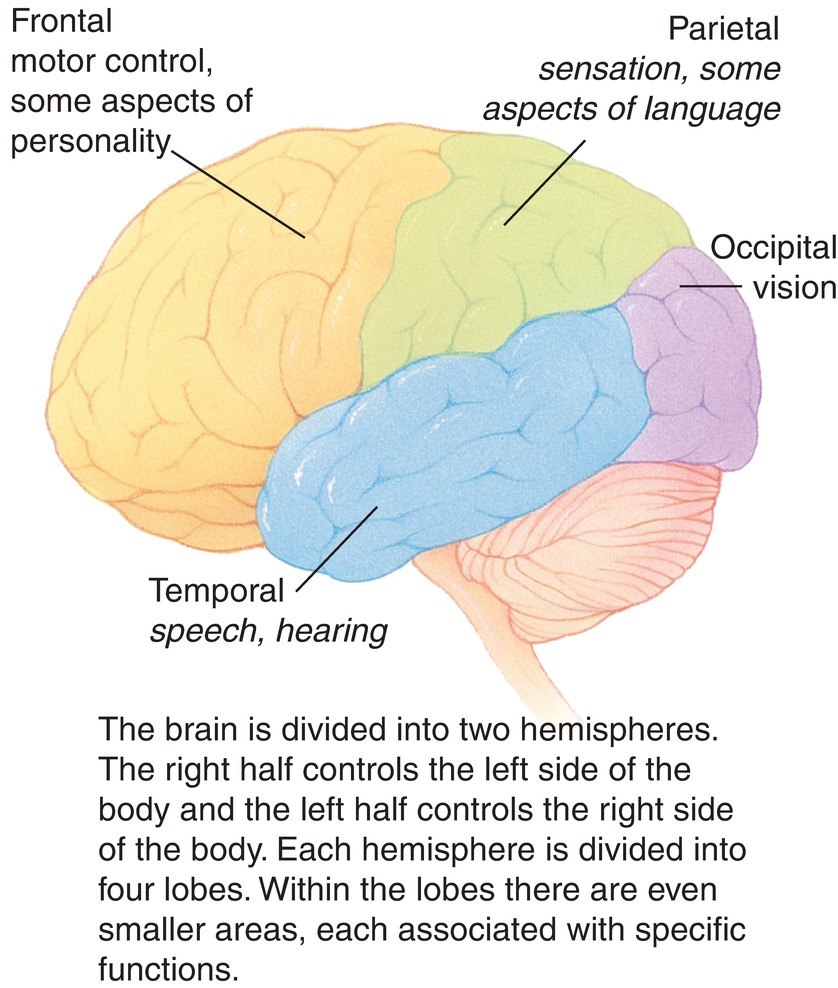
FIGURE 11.2. Segments of the brain and their role in the body labeled.
Deeper in the brain, several structures coordinate CNS functions. The basal ganglia, a set of nuclei below the cortex, are responsible for modulation of complex motor signals sent from the frontal cortex. The thalamus is a large central structure within the cerebral hemispheres, which acts as a sensory integration point and determines to which regions it should send various sensory inputs. Just below the thalamus, the hypothalamus links the brain and the endocrine system, regulating when hormones should be released with its direct input to the pituitary gland just below. The pituitary gland then secretes hormones that travel through the body to regulate growth, metabolism, blood pressure, and sexual development.
The cerebellum is in the back of the skull, below the occipital lobe of the cortex, and coordinates sensory and motor information to maintain balance and posture and perform movements smoothly.
The brainstem connects the brain to the spinal cord. The sensory and motor pathways between the brain and spinal cord pass through the brainstem to make their functional connections. The brainstem controls vital and unconscious functions, including breathing, heart rate, blood vessel tone, and vomiting. The brainstem also contains the nuclei for the cranial nerves, which communicate with the head, face, and neck.
Within the skull are the meninges and vasculature, which offer support to brain tissues. The meninges are a set of three layers of protective tissue that surround the structures of the CNS (Fig. 11.3). The outermost of the meninges, the dura mater, is a thick protective layer that directly contacts the skull. The next layer is the arachnoid mater, which surrounds the subarachnoid space, a cavity filled with a fluid called cerebrospinal fluid (CSF); the CSF provides a cushion for the brain against traumatic injury and provides a secondary (in addition to the venous blood flow) mechanism for removal of waste products. The pia mater is a thin layer that runs along the surface of the brain. The blood supply to the brain comes from the vertebral arteries and the internal carotid arteries, which flow together into the circle of Willis at the base of the skull before branching throughout the brain. Blood leaving the brain enters a network of venous sinuses within the dura mater before draining into the internal jugular vein.
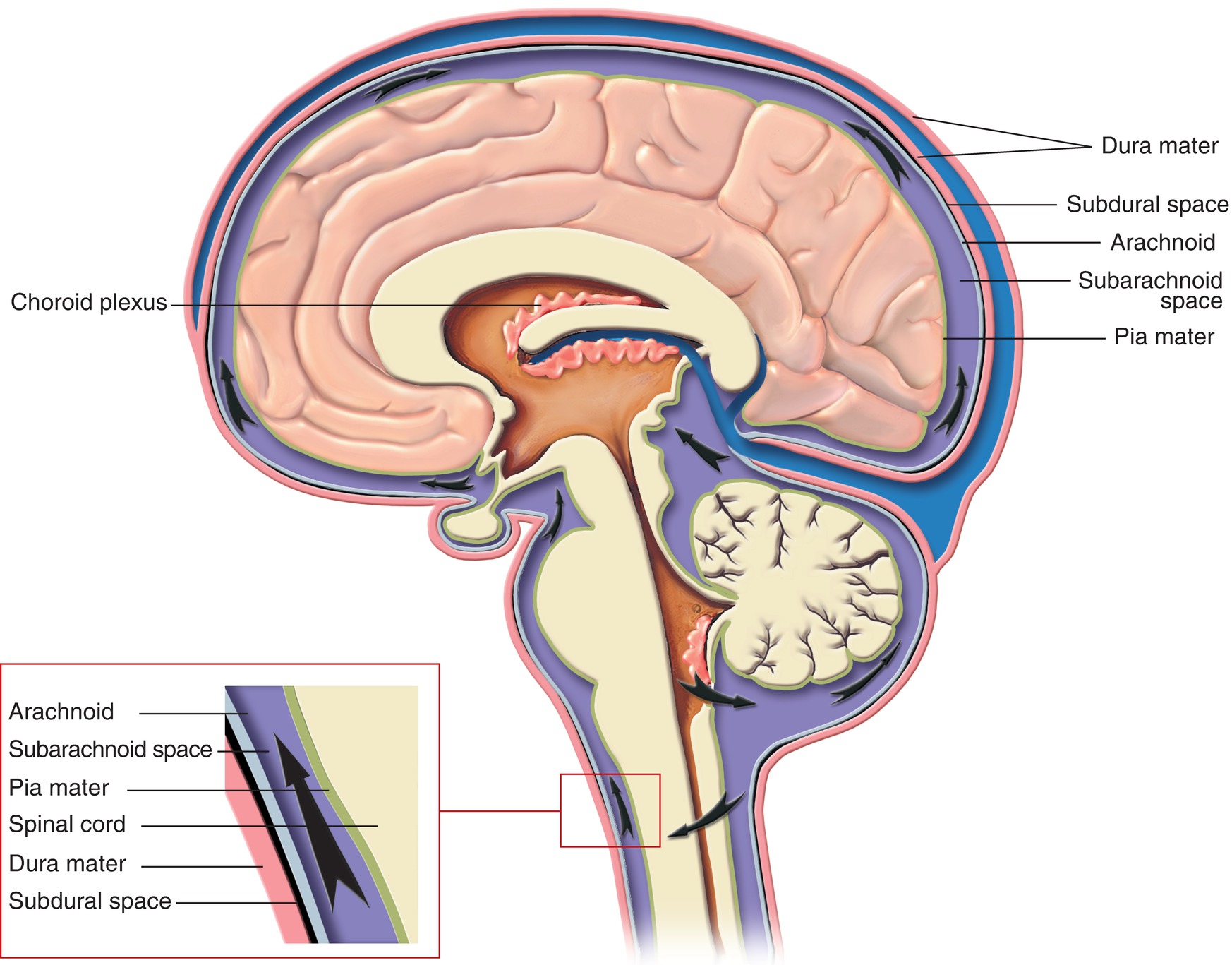
FIGURE 11.3. Meninges and cerebrospinal fluid flow.
Anesthetic Implications of Brain Anatomy
Due to the rigid structure of the skull and meninges, any increase in the volume of any component within the intracranial space (e.g., brain, CSF, blood, or foreign bodies) can result in an increase in intracranial pressure (ICP) and secondary brain injury. Blood volume can increase due to ruptured blood vessels (e.g., rupture of a cerebral aneurysm, which normally occur in large arteries originating from the circle of Willis). All of the inhaled anesthetics cause dilation of the cerebral blood vessels and can increase ICP: thus a typical neuroanesthetic will often include an intravenous anesthetic, such as propofol or opioids, so that the amount of inhaled anesthetics required for adequate anesthesia is reduced.
The brain tissue itself may increase in volume because of abnormal growth from a tumor or swelling from trauma or surgery, while the CSF component can increase from overproduction or poor reabsorption. Although each of these problems results in increased ICP, the definitive treatment is usually left to the discretion of the surgeon.
Pressure within the brain can be measured via a catheter placed in the central CSF-containing spaces (e.g., lateral ventricles), or directly into the brain tissue, or a pressure-transducing “bolt” can be placed through the skull (see Chapter 32, Neurologic Monitoring). Only the catheter in the lateral ventricle can be used therapeutically to lower ICP by withdrawing CSF.
Other means of decreasing ICP that are under the control of the anesthesiologist include intravenous administration of osmotic diuretics like mannitol and hypertonic saline, which reduce the amount of edema fluid in the brain. In this case, the anesthesia technician will observe an increase in osmolarity and chloride (with acidosis) in blood samples. The anesthesiologist may also use hyperventilation to lower PaCO2 and cause cerebral vasoconstriction to decrease blood volume in the brain. Conversely, increased PaCO2 causes vasodilation and is dangerous in patients with compromised ICP; even stable neurosurgical patients typically have tightly controlled ventilation. Due to the equilibrium shift between CO2 and H+, one will observe low PaCO2 and high pH values when the anesthesiologist uses hyperventilation to reduce ICP. Often these techniques (hyperventilation, removal of CSF and administration of hypertonic solutions) only cause a temporary reduction in ICP and are used so that the surgeon can take definitive action.
In the case of head trauma, definitive surgical care may include removal of a large portion of the cranial bone (i.e., hemicraniectomy) to give the brain room to swell, removal of blood clots in the cerebral spinal fluid space or brain, and/or removal of actual brain tissue (particularly from areas that are not responsible for critical body functions). If the increase in ICP is due to a ruptured blood vessel, the surgeon will clip (in the case of an aneurysm) or remove (in the case of an arterial-venous malformation) the blood vessel(s) and remove the blood clot from the brain.
Anatomy of the Spinal Cord
The spinal cord begins just below the base of the brainstem and continues down through the vertebral column, ending near the twelfth/last thoracic or first lumbar vertebral body. The vertebral column is divided into four segments based on anatomic location of the vertebrae (Fig. 11.4): seven cervical (neck) segments, twelve thoracic (chest) segments, and five lumbar (low back) and five sacral segments: the sacral bone is formed from five fused vertebral segments and forms the posterior element of the pelvis; the coccyx is a vestigial “tailbone” at the end of the sacrum. The spinal cord levels are divided into segments based on the level at which the paired sensory and motor spinal nerves exit the spinal column. Each set of nerves innervates a different set of muscles and a sensory area of the body in an organized, descending fashion (see Chapter 13, Peripheral Nervous System).
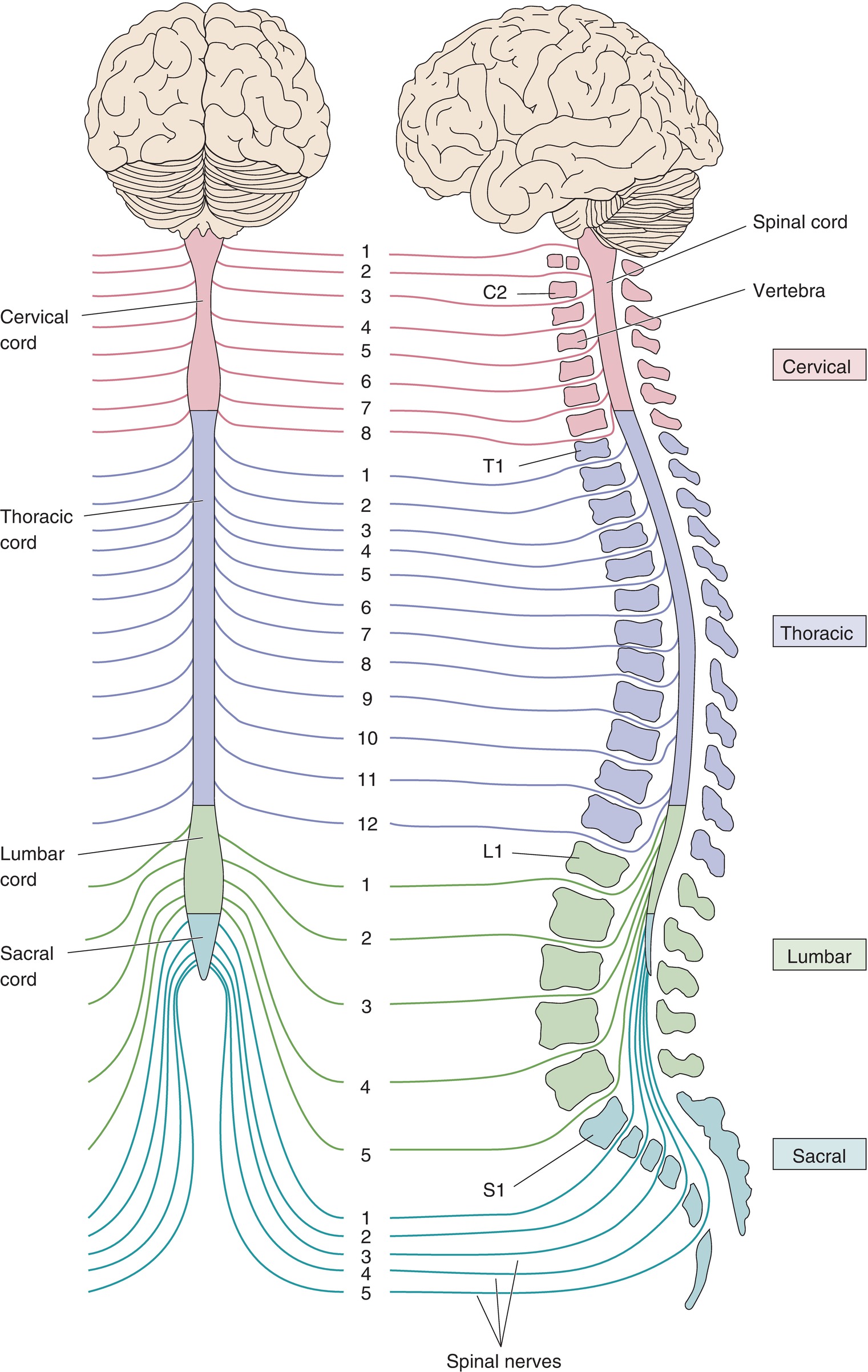
FIGURE 11.4. Segmental organization of the spinal cord. The spinal cord is divided into cervical, thoracic, lumbar, and sacral divisions (left). The right side shows the spinal cord within the vertebral column. Spinal nerves are named for the level of the spinal cord from which they exit. (From Bear MF, Connors BW, Paradiso MA. Neuroscience–Exploring the Brain. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001, with permission.)
Within the spinal cord, there is gray matter and white matter. The gray matter is composed primarily of neuronal cell bodies, and the white matter is composed of long bundles of nerve cell tissue for signal conduction. Just as in the brain, meningeal layers and CSF surround the spinal cord within the vertebral column.
Anesthetic Implications of Spinal Cord Anatomy
The meninges and ligaments of the bones of the spinal canal are used strategically by anesthesiologists for placement of spinal or epidural anesthesia. A needle can be placed between the vertebral bones into the bony canal of the spine. For spinal anesthesia, the needle is advanced into the subarachnoid space, and CSF flows back through the needle: local anesthetics are injected, resulting in regional anesthesia. For epidural anesthesia, the needle is advanced only until it has passed the ligamentum flavum, the innermost ligament of intervertebral space, without penetrating the dura. Once in this “epidural” (“on top of the dura”) potential space, a catheter can be placed, allowing medication to be injected for surgical anesthesia or for a smaller dose of pain relief (such as for labor). The medication diffuses into the nerves and spinal cord from outside the dural covering, making it less potent than spinal (inside the CSF) anesthesia and slower in onset, but permitting a longer duration.
The blood supply to the spinal cord comes from the vertebral arteries and a set of segmental branches off of the aorta, called medullary arteries. These arteries join at the surface of the spinal cord to form a network of arteries that supply the tissue of the spinal cord. Prominent in this network are the larger anterior and posterior spinal arteries, which run the length of the spinal cord, giving off smaller arterial branches at each level. Because these arteries run from the thoracic aorta to the spinal cord, patients who require surgery of the thoracic aorta (e.g., for aortic aneurysm) can be at risk for low blood flow during or after surgery, spinal cord injury, and paralysis.
Physiology
The CNS transmits information about the environment electrically. The major functional cell type in the nervous system is the neuron. In the peripheral nervous system, neurons interact directly with the environment, acting as sensors for pain, temperature, and tactile information and directly innervating skeletal muscle as well as other muscles and glands that regulate automatic body functions (see Chapter 12, Autonomic Nervous System, and Chapter 13, Peripheral Nervous System). In the CNS, neurons interact with one another and with peripheral nerves. A unique characteristic of neurons when compared with other cell types is the existence of a long cellular projection, called an axon, which terminates on other neuronal cells. The axon can be very long: spinal cord motor axons are up to a meter in length. An axon may also have as many as hundreds of thousands of terminal connections on other neurons to relay information in a complex network. CNS cells also have many dendrites, which act as receiving points for information. Each neuron also has a soma (cell body), which contains the nucleus and other important cell organelles and acts to integrate and process incoming signals from the dendrites.
Neurons function by propagating a signal in the form of an electrical impulse. The functional connection between two neurons is called a synapse (Fig. 11.5). To conduct a signal, the nerve cell membrane becomes permeable to sodium, which depolarizes the cell in a wavelike fashion and propagates the signal toward the end of the axon. When the nerve signal reaches the end of the presynaptic axon, it causes release of chemicals, called neurotransmitters, from the axon terminal into the synaptic cleft, a small space between the presynaptic axon and the postsynaptic cell. These neurotransmitters interact with receptors on the surface of the postsynaptic cell; these signal its depolarization, propagating the signal onward (Fig. 11.5).
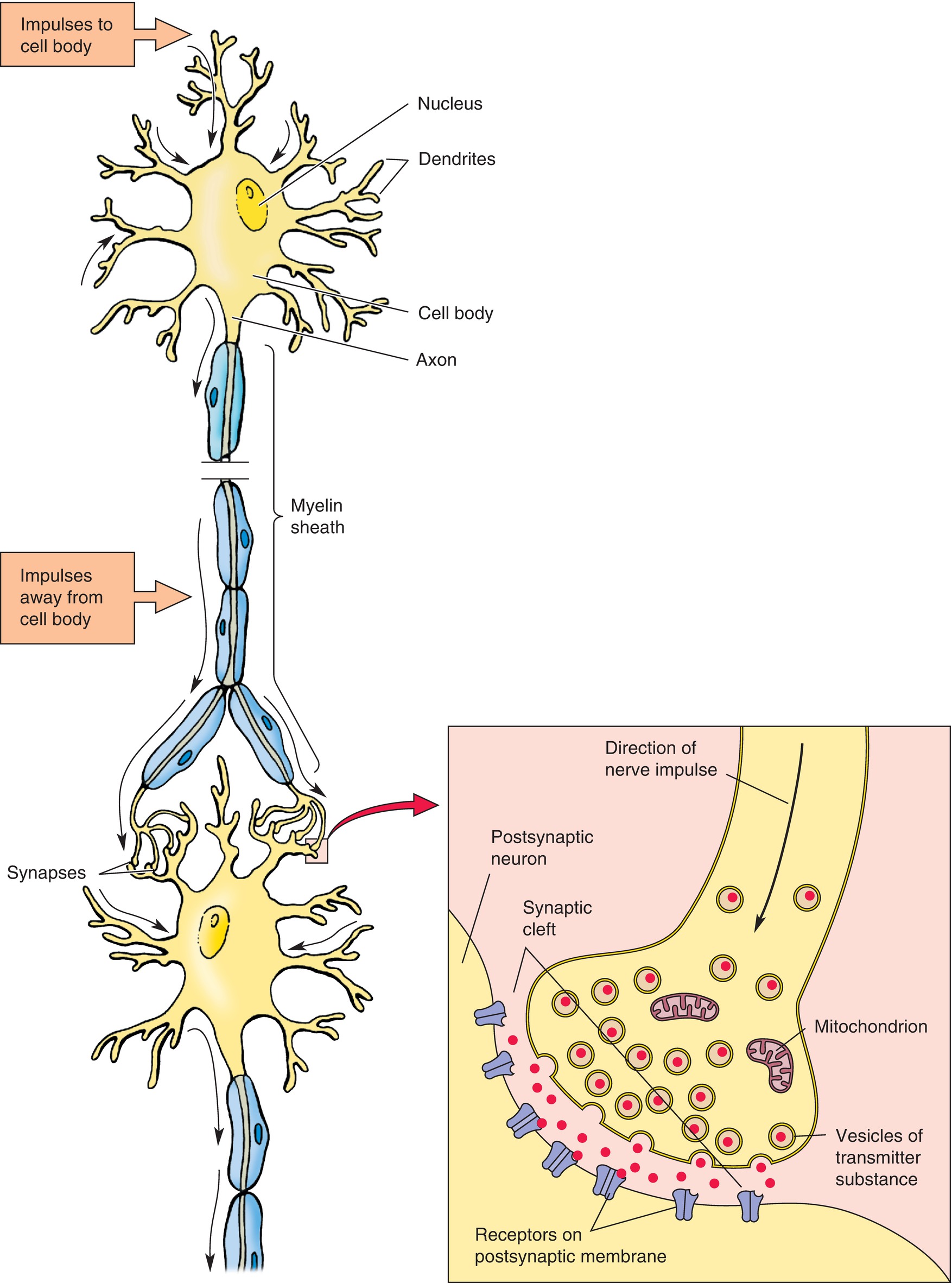
FIGURE 11.5. Structure of a motor neuron. A neuron or nerve cell is composed of a cell body having a nucleus and two types of processes—dendrites and axons. Impulses pass to the cell body through the dendrites (upper black arrows), and the axon carries impulses away from the cell body (lower black arrows). A neuron influences other neurons at junctional points or synapses. The detailed structure of an axodendritic synapse is illustrated (inset); neurotransmitter substances diffuse across the narrow space (synaptic cleft) between the two cells and become bound to receptors. (From Moore KL, Dalley AF II. Clinical Oriented Anatomy. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins; 1999, with permission.)
In addition to neurons, there are a variety of cells in the CNS that function as supportive structures; these cells are collectively referred to as glial cells. Their functions include support of neuronal growth and signaling, scavenging neurotransmitters and other debris, and providing immunologic protection within the CNS. One glial cell function is to produce myelin, which wraps neuronal axons and allows for greater speed and efficiency of nerve signal conduction. Myelin coating insulates the axon against sodium ion movement; thus, the ions can enter the axon only at gaps in the myelin sheath. The signal jumps from node to node, propagating the signal more quickly. Myelin is rich in lipids and gives white matter, which is primarily composed of axons, its color.
Functional anatomy of the CNS can be divided into sensory systems, motor systems, and complex functions such as language, emotions, and memory. All sensory systems feed into the CNS, where they are integrated into an image of the environment and the body’s position within it. Sensory systems include not only mechanosensory (touch), pain, proprioception (sensation of bodily orientation), temperature, and vibration, each sensed separately; they involve both the spinal cord and the brain. These pathways begin as part of the peripheral nervous system (see Chapter 13) and ascend to the brain via afferent neurons of the spinal cord. There are two separate pathways for how touch/vibration/proprioception (lemniscal pathway) and pain/temperature (spinothalamic pathway) reach the brain; they travel different routes in the spinal cord, brainstem, and thalamus before reaching the cortex. As an anesthesia technician, you may see anesthesia providers examining these sensations separately in patients under regional anesthesia or with neurologic diseases.
The control of voluntary motor function within the CNS begins in the primary motor cortex in the frontal lobe, with motor planning and guidance coming from other areas of the frontal lobe including the premotor and prefrontal cortex. The axonal fibers from the primary motor cortex descend through the brainstem, crossing to the contralateral (opposite) side at the level of the medulla and continuing to descend in the lateral column of the spinal cord. These axons terminate within the ventral portion of the spinal cord at the appropriate vertebral level and synapse with lower motor neurons that exit the spinal cord within the spinal nerve to innervate skeletal muscle. Modulation of the motor pathway comes from various locations within the CNS, including the cerebellum, which is mainly involved in smoothing and coordinating movements, and the basal ganglia, which helps generate complex motor patterns.
Reflexes are simple sensorimotor circuits that involve only the spinal cord and not the brain. In a reflex pathway, the sensory neuron synapses directly on a lower motor neuron in the spinal cord or on an interneuron that then inhibits a lower motor neuron (Fig. 11.6). Reflexes may be monosynaptic or polysynaptic. A monosynaptic reflex involves only one pair of sensory and motor neuron; for example, in the patellar tendon reflex, the tendon stretch sensory neuron directly activates the nerves that signal the leg to extend while acting to inhibit those that would flex the leg. A polysynaptic reflex includes behavior of withdrawing from a painful stimulus in the foot—one would move the foot away from the painful stimulus reflexively, but the sensory neuron would also activate the motor neurons to maintain balance in the opposite foot; thus, multiple motor neurons must be involved in the reflex. In general, reflexes become more prominent during brain injury, as the brain normally serves to inhibit reflexes in healthy patients.
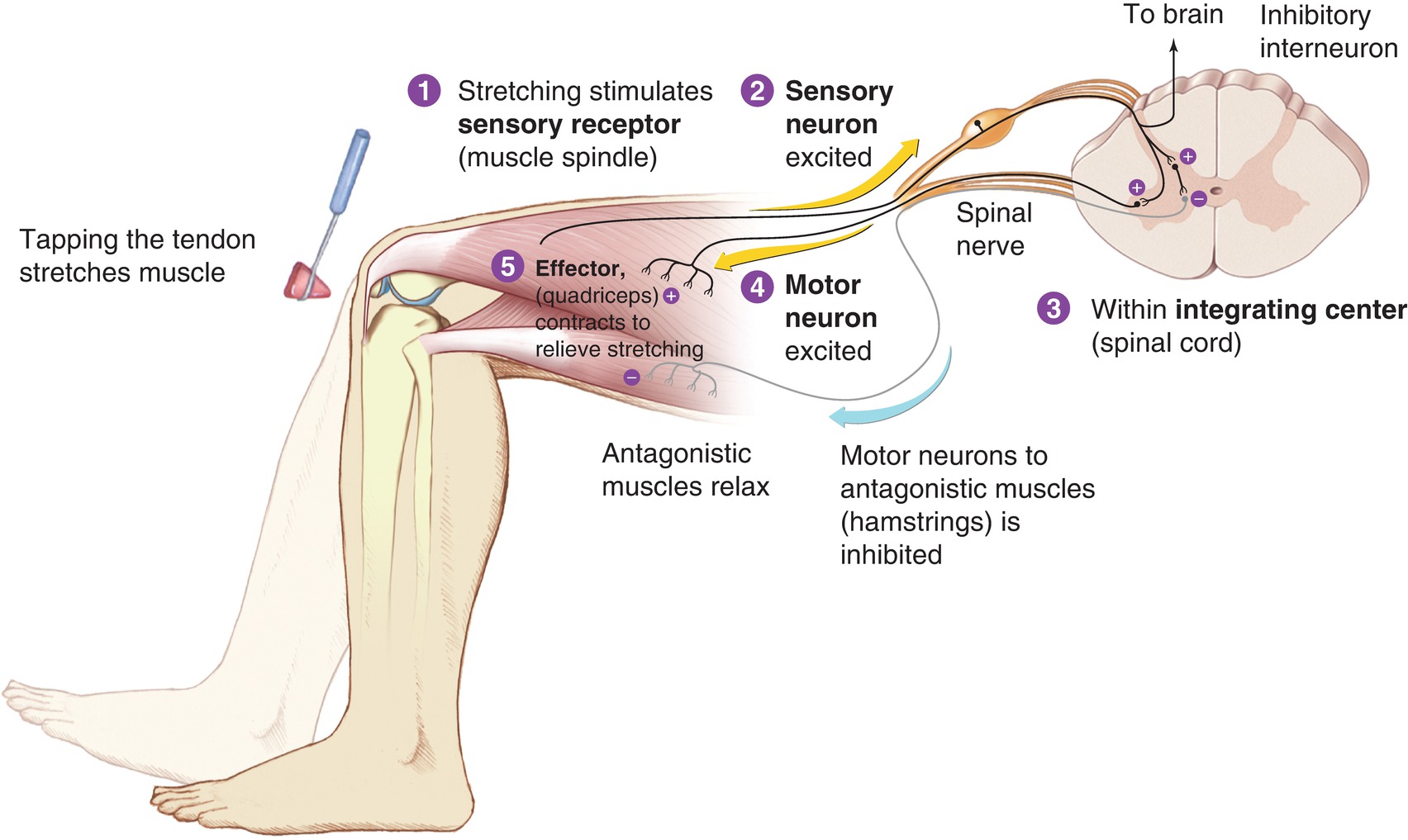
FIGURE 11.6. Reciprocal innervation. (From Premkumar K. The Massage Connection: Anatomy and Physiology. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2004, with permission.)
The CNS is also involved with the “special senses,” which includes vision, hearing, smell, and the vestibular (balance and body position) sense. Aside from olfaction (smell), the pathways of all the special senses involve nuclei in both the brainstem and the thalamus before arriving at the cerebral cortex. (Smell bypasses the brainstem and thalamus and projects directly from the olfactory bulb nucleus, just above the nose, to the cortex.)
The complex functions carried out by the CNS include memory, emotions, and language and mainly involve specialized areas of the cerebral cortex. Language is an important tool for communication among people, and it involves the production and comprehension of complex vocal and verbal patterns. Within the cerebral cortex, the areas involved with language fall within the temporal and frontal lobes, predominantly on the left side. Because of the importance of language in daily functioning, the left side of the cortex in most humans is considered the dominant hemisphere. The right hemisphere plays a role in language, but it is mainly involved with the emotional content rather than the semantic processing of language. There are two specific regions of the cortex that have been identified as major centers for language within the brain. The first, called Broca area, is typically in the left frontal lobe in the inferior frontal gyrus, anterior to the primary motor cortex, and it is involved with the efficient production of language and speech. Broca area is important for the motor planning involved in speech and for fluid expression of language. The second important area involved with language is Wernicke area, a region of the cortex in the left posterior temporal lobe in the superior temporal gyrus. Wernicke area is important in the understanding of language and is associated with the auditory cortex (Fig. 11.7). Damage to these areas of the cortex causes a specific language deficit, called aphasia, which can present as difficulty in language production (if Broca area is affected) or language comprehension (if Wernicke area is affected).
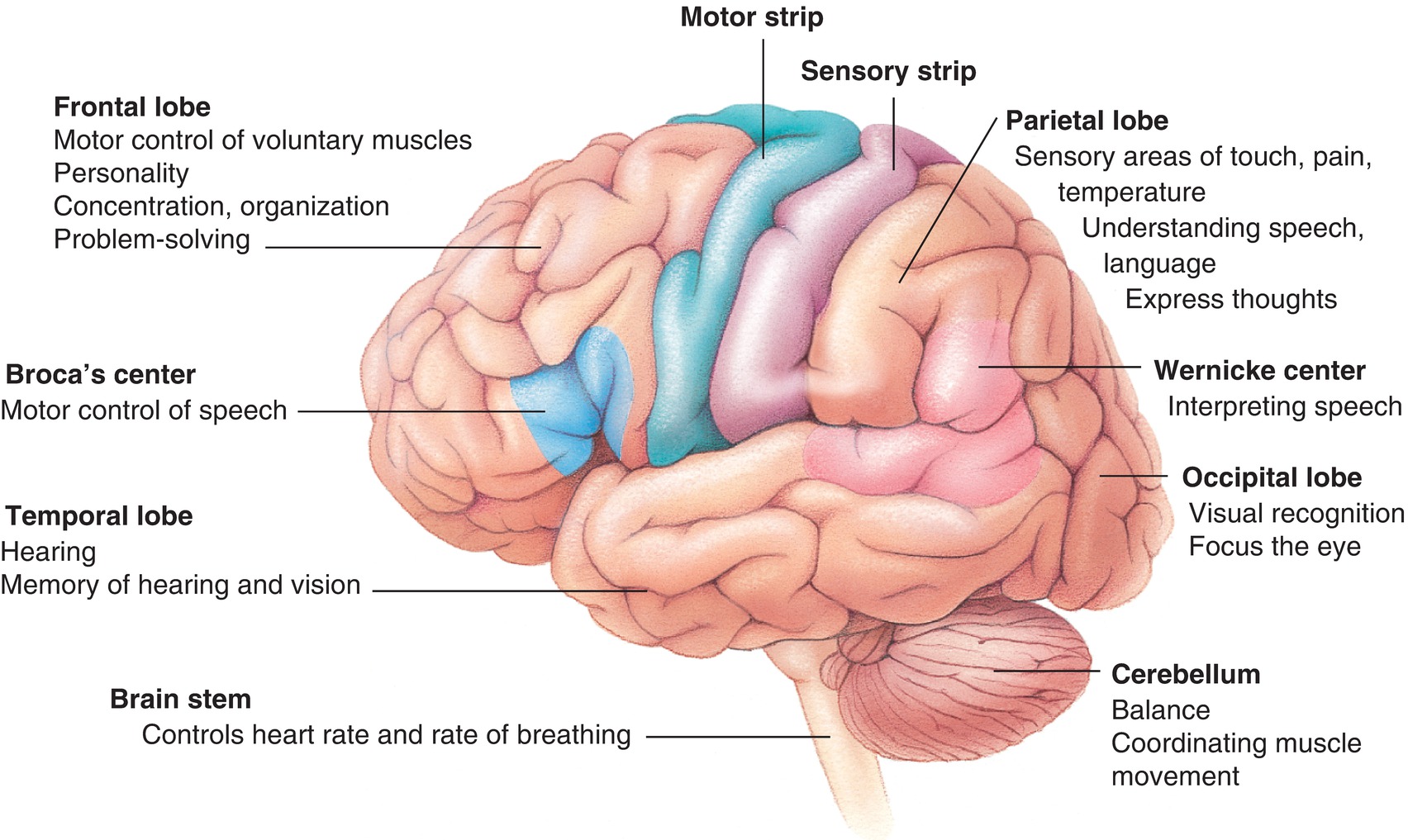
FIGURE 11.7. Topical anatomy of the brain.
Within the brain, emotional responses are dictated by a set of structures collectively referred to as the limbic system. The limbic system consists of deep areas of the cortex, like the cingulate gyrus and the hippocampus, as well as structures like the hypothalamus that affect the autonomic nervous system (see Chapter 12). When confronted with an emotionally charged stimulus, the limbic system activates and sends signals to various brain centers, including the prefrontal cortex, which is involved with decision-making; the pituitary, which controls the endocrine system, and the brain’s pleasure centers. Emotion and memory within the brain are intimately connected with many structures contributing to both processes.
An important brain structure involved with memory is the hippocampus, an area of the cortex found within the temporal lobe. The hippocampus is activated during the storage and retrieval of memory, and it is especially associated with memory for locations and events. The cerebellum is involved with procedural memory, also called muscle memory, which is important in learning motor-oriented skills like riding a bicycle or playing a musical instrument.
Strong emotional states encourage memory encoding, and so emotionally charged events are remembered more accurately and with more detail.
Pharmacology
There are many pharmacologic agents that have effects on the CNS. Psychomotor stimulants are drugs that increase the overall activity within the CNS by increasing neuronal firing rate or promoting neurotransmitter release. Examples include caffeine and d-amphetamine, a drug commonly used to treat attention deficit/hyperactivity disorder (ADHD). Anticonvulsants decrease neuronal firing frequency and enhance the effects of inhibitory neurotransmitters. The abnormally increased neuronal firing in the CNS associated with seizures can be treated with specific anticonvulsants like phenytoin (Dilantin) and levetiracetam (Keppra) or with sedatives like barbiturates (e.g., pentobarbital) and benzodiazepines (e.g., midazolam).
The CNS has an inbuilt array of molecules and receptors involved with analgesia (pain reduction). Specifically, three families of peptides, called endorphins, enkephalins, and dynorphins, are collectively referred to as the endogenous opioids and relieve pain via opioid receptors within the CNS. Morphine and the other opioid agonist drugs relieve pain by binding to these same receptors (see Chapter 19, Perioperative Medications).
General anesthetics are a class of drugs used during surgeries for sedation, analgesia, amnesia, and muscle relaxation (see Chapter 17, Overview of a General Anesthetic). These drugs are administered either through injection or inhalation. Dexmedetomidine is a unique short-acting injectable sedative and anesthetic that works through alpha-2 adrenergic receptors (see Chapter 12, Autonomic Nervous System). It is also very short-acting, so that it must be given by infusion, and its effects resolve quickly once the infusion is stopped.
In adult patients requiring surgery for intracranial pathology, general anesthesia is often induced with a quick-onset intravenous anesthetic (e.g., propofol) and a neuromuscular blocking agent (e.g., rocuronium or succinylcholine) to allow rapid control of ventilation, avoiding hypoventilation, elevated PaCO2, and elevations in ICP. Intravenous opioids are administered in order to blunt the hemodynamic response to noxious stimuli (e.g., intubation, placement of the patient’s head in pins, and surgical incision). Hemodynamic stability is important, as hypertension may result in brain swelling in areas where there is brain damage (e.g., in the region of a tumor or trauma) and hypotension may result in poor perfusion to important areas of the brain. During brain surgery, anesthesia is often maintained using a combination of intravenous agents (e.g., propofol infusion and/or an opioid) and inhaled agents (e.g., desflurane) in order to minimize the detrimental effects of the inhaled anesthetic agents administered in high concentrations on brain blood volume. As discussed above, an increase in brain blood volume may result in an increase in ICP and secondary brain injury. Patients also often receive neuromuscular blocking drugs so that they will not move in the pin headframe or while the surgeon is operating within the brain. However, a surgical request for monitoring of motor pathways (see Chapter 32, Neurologic Monitoring) may prevent the use of neuromuscular blocking agents and make it more likely that the patient may move at a critical point in the surgery.
Inherent Toxicity of Anesthetic Agents in the CNS
Previously, anesthetic agents were thought to “protect” the brain from injury by their ability to put the brain in a quiescent state. More recent investigations have demonstrated that all of the inhaled anesthetic gases and several of the intravenous anesthetic agents cause direct neurotoxicity in very young infants, but not in adults. The mechanism of this toxicity is not completely understood, but may involve generation of toxic oxygen radicals within the brain. Unfortunately, there is no current consensus on anesthetic agents that are free of neurotoxic effects on the neonatal brain. Ongoing research is trying to establish which, if any, of the anesthetic agents is truly safe and if there are clinically applicable strategies that can be applied for care of patients in the most vulnerable periods for anesthetic-induced brain injury. Proposed strategies may include administration of additional drugs to protect the brain (e.g., lithium oxygen radical scavengers) or development of new anesthetic agents (e.g., xenon). In the meantime, the most reasonable approach is to minimize exposure of patients to anesthetic agents during their most vulnerable age periods (i.e., neonates).
Anatomy, Physiology, and Pharmacology of Nausea and Vomiting
Emesis (vomiting) is a process in which the normal direction of digestive propulsion is reversed and the contents of the stomach are brought upward through the esophagus and out of the mouth. The process is often forceful and unpleasant and can be induced by a number of factors including gastrointestinal irritation, motion sickness, and drug reactions. Nausea (a subjective awareness of the urge to vomit) may precede emesis; persistent nausea can be extremely distressing to patients even in the absence of emesis.
Within the medulla, there is a nucleus called the chemoreceptor trigger zone (CTZ) that, when activated, triggers the emesis response. The CTZ has receptors for a number of neurotransmitters, including dopamine, acetylcholine, histamine, serotonin, and opioids. Drugs that modulate the effects of these chemicals and their receptors may alter the emetic response. Drugs with proemetic effects are often those that increase the activity of neurotransmitters to which the CTZ is sensitive. Opioid drugs can also activate the CTZ through its opioid receptors, but the proemetic effect of these drugs is not apparent in all patients. Other ingested substances, such as syrup of ipecac, trigger emesis by irritating the gastric mucosa.
There are a number of drug classes that antagonize emesis, mainly by inhibiting the neurotransmitters and receptors within the CTZ. Dopamine receptor blockers are used as antipsychotics and have antiemetic effects by blocking dopamine 2 (D2) receptors within the CTZ. Scopolamine is a drug that is used to combat motion sickness through its anticholinergic effects at the CTZ. Additionally, selective serotonin antagonists (e.g., ondansetron) are often given to inhibit the nausea that occurs as a side effect of anesthesia and chemotherapeutic treatments.
Summary
In summary, the CNS is a complicated organ system that requires an in-depth understanding by anesthesiology staff in order to establish the desired operating environment for the surgeon and outcomes for our patients. The anesthesia technician should understand the principles of how anesthetics affect the CNS in healthy patients as well as the goals of care for neurosurgical patients. This facilitates team communication, maximizing dose-dependent anesthetic beneficial effects while minimizing risks of CNS toxicity.
Acknowledgment
The authors would like to thank Dr. Josh Finkle for his contribution to the content of this chapter in the first edition.
Review Questions
1. Which of the following areas of the cortex is most likely to be involved with language production?
A) Left frontal lobe
B) Left temporal lobe
C) Right frontal lobe
D) Right temporal lobe
E) None of the above
Answer: A
Broca area is in the left frontal lobe, anterior to the primary motor cortex, and is involved with the efficient production of language and speech. Wernicke area, a region of cortex in the posterior temporal lobe, is important in the understanding of language and is associated with auditory cortex. The right hemisphere plays a role in language, but it is mainly involved with the emotional content rather than the semantic processing of language.
2. Blocking which of the following neurotransmitter receptors in the CTZ will have an antiemetic effect?
A) Serotonin
B) Acetylcholine
C) Dopamine
D) A and C only
E) A, B, and C
Answer: E
There are a number of drug classes that antagonize emesis, mainly by inhibiting the neurotransmitters and receptors within the CTZ. Dopamine receptor blockers are used as antipsychotics and have antiemetic effects by blocking D2 receptors within the CTZ. Scopolamine is a drug that is used to combat motion sickness through its anticholinergic effects at the CTZ. Additionally, selective serotonin antagonists (e.g., ondansetron) are often given to inhibit the nausea that occurs as a side effect of anesthesia and chemotherapeutic treatments.
3. Which of the following correctly describes the path of the electrochemical signal through a single neuron as it is received, then processed, then relayed down the length of the neuron?
A) Cell body → dendrite → axon
B) Dendrite → cell body → axon
C) Cell body → axon → dendrite
D) Axon → cell body → dendrite
Answer: B
Neurons receive most of their input signals through receptors on their dendrites. These signals cause changes in the cell’s electrical gradient, first within the dendrites themselves and then in the cell body. If the cell is depolarized past threshold, it will then send an action potential down the length of its axon.
4. Which of the following answer choices correctly matches the region of the spinal column with the number of vertebrae in that region?
A) Cervical, 12
B) Coccygeal, 5
C) Lumbar, 7
D) Thoracic, 12
E) Sacral, 7
Answer: D
The cervical region of the vertebral column spans the length of the neck and has seven segments. The thoracic region spans the upper part of the back and is divided into 12 segments. The lumbar region spans the lower back and is divided into five segments. The sacral region of the vertebral column extends into the pelvis and is composed of five bones fused to form a single structure. A single coccygeal bone (referred to as the tailbone) is found at the end of the spinal column. Found within the vertebral column is the spinal cord itself.
5. An endogenous peptide is discovered to act on receptors within the CNS and cause pain relief as well as euphoria and sedation. Which of the following CNS drugs acts on the same receptor as this molecule?
A) Isoflurane
B) Succinylcholine
C) Lithium
D) Morphine
E) Scopolamine
Answer: D
The endogenous opioids are peptides that act on opioid receptors within the CNS with analgesic effects. Opioid agonists, such as morphine, bind to and activate the brain’s opioid receptors and are used for analgesia. The inhaled anesthetics (such as isoflurane) directly interact with cell membranes within the CNS to cause analgesia and sedation. Succinylcholine is a neuromuscular blocking agent that interferes with cholinergic signaling between motor nerve terminals and muscles. Lithium can be used as a mood stabilizer to treat bipolar and other mood disorders. Scopolamine is a drug that is used to combat motion sickness through its anticholinergic effects at the CTZ.
6. In which part of the cerebral hemispheres are visual stimuli interpreted?
A) Frontal lobe
B) Parietal lobe
C) Occipital lobe
D) Hippocampus
E) Temporal lobe
Answer: C
The occipital lobe is primarily associated with the processing of visual information and forming an interpretation of the visual world. The temporal lobe is the primary site of auditory perception. The hippocampus is a structure inside the temporal lobe and is responsible for storage and retrieval of memories. The parietal lobe deals with language and spatial relationships. The frontal lobe carries out executive functions, including long-term planning, conscious motor movement, and behavioral control.
7. Which sense does not require processing in the brainstem or thalamus?
A) Smell
B) Hearing
C) Vision
D) Vestibular (balance)
E) Taste
Answer: A
The sense of smell is processed via the central olfactory pathway. It is the simplest of all the sensory pathways as it does not involve processing within the brainstem or thalamus. Specialized chemical sensors on the surface of the nasal cavity respond to interactions with specific chemical compounds in the inhaled air. These sensory neurons project to the olfactory bulb, which has neurons with axon that project directly to the primary olfactory cortex within the temporal lobe. All other pathways are processed first by either the thalamus or the brainstem before moving to their relative locations in the cerebral hemispheres.
8. Which brain structure is responsible for procedural memory?
A) Basal ganglia
B) Thalamus
C) Hippocampus
D) Cerebellum
E) Hypothalamus
Answer: D
Procedural memory is the memory of how to do things, also sometimes called muscle memory. It is important in motor- or procedure-oriented skills like washing dishes, riding a bicycle, or playing a musical instrument and is primarily the task of the cerebellum. The hippocampus is responsible for episodic memory or storage and retrieval of locations, events, and past experiences. The basal ganglia are responsible for coordination of sophisticated motor movements, the thalamus for integration of sensory information, and the hypothalamus for endocrine and autonomic regulation.
SUGGESTED READINGS
Clarke RS. Nausea and vomiting. Br J Anaesth. 1984;56(1): 19-27.
Hanes DE. Neuroanatomy: An Atlas of Structures, Sections, and Systems. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6(3):121-143.
Huffman JC, Alpert JE. An approach to the psychopharmacologic care of patients: antidepressants, antipsychotics, anxiolytics, mood stabilizers, and natural remedies. Med Clin North Am. 2010;94(6):1141-1160.
Ishizawa Y. Mechanisms of anesthetic actions and the brain. J Anesth. 2007;21(2):187-199.
Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242(4886):1654-1664.
Mansour A, Khachaturian H, Lewis ME, et al. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11(7): 308-314.
Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504-514.
Purves D. Neuroscience. 4th ed. Sunderland, MA: Sinauer Associates, Inc.; 2008.