CHAPTER 17
Overview of a General Anesthetic
Early History of Anesthesia Agents
An anesthetic involves the administration of various intravenous and inhalational agents to produce a state in which a patient may safely and comfortably undergo a procedure. While the medical specialty of anesthesiology may be regarded as modern and “cutting edge,” many of the foundational and current medications have existed for centuries. Opium was described around 3400 BC by the Sumerians in the poppy, which they called the “joy plant.” The Egyptians wrote about its analgesic, or pain-relieving, properties in 1300 BC. In 40 AD, the Greek physician Pedanius Dioscorides coined the term “anesthesia” to describe the effects of the plant Atropa mandragora (which contains a variety of anticholinergic alkaloids; see Chapter 11, Central Nervous System, and Chapter 13, Autonomic Nervous System), used in combination with alcohol prior to surgery. Friedrich Wilhelm Sertürner of Germany extracted the active ingredient from opium in 1803 and named it morphine, after Morpheus, the Greek god of dreams.
The first inhalational anesthetic was described in 1800 by Sir Humphry Davy, who personally experimented with nitrous oxide (“laughing gas”); he described it as producing a feeling of “well-being.” In 1818, Michael Faraday discovered that the volatile inhalational anesthetic ether resulted in similar effects. Ether was then used publicly in gatherings known as “ether frolics” and observed to result in both unconsciousness and insensitivity to pain. Dr. Crawford W. Long as early as 1842 employed it to produce labor analgesia and anesthesia for surgical procedures. He did not, however, make his work known outside his own practice in rural Georgia. A dentist, William T. G. Morton, is thus credited with the first successful use of the first inhalational anesthetic for surgery in a public demonstration of ether at the Massachusetts General Hospital in 1846. The use of anesthetics quickly spread and surgery flourished with the use of new agents that could alleviate the pain and suffering of a surgical procedure (Figs. 17.1 and 17.2).
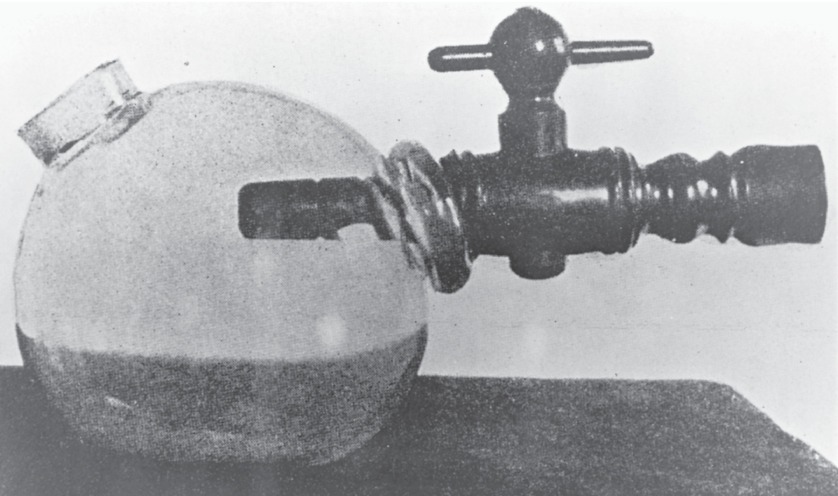
FIGURE 17.1. Morton’s ether inhaler (1846) consisted of a glass bulb, an ether-soaked sponge, and a spout to be placed in patient’s mouth. (From Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia. 8th ed. Philadelphia, PA: Wolters Kluwer; 2017, with permission.)
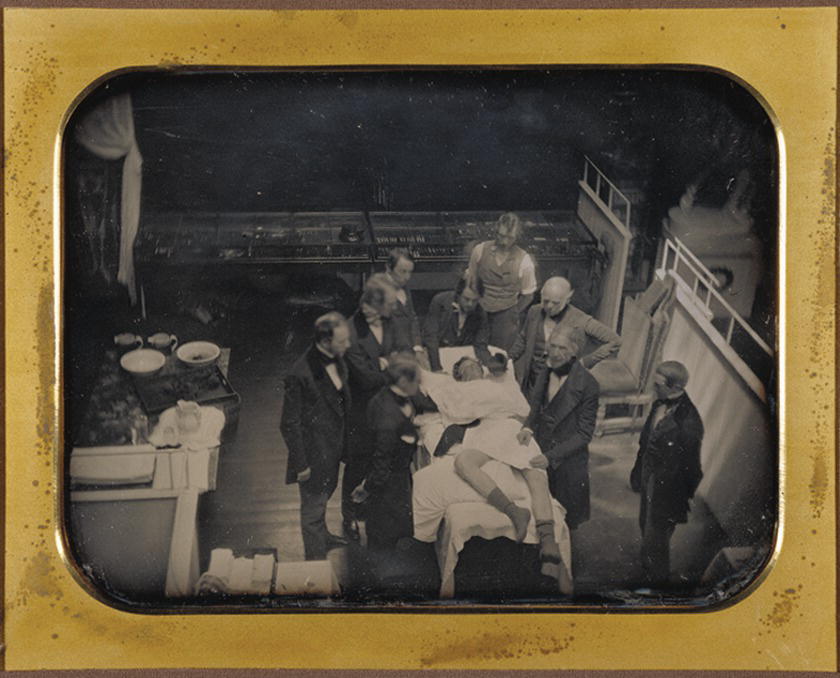
FIGURE 17.2. Southworth and Hawes (1846) “Early operation using ether anesthesia.” One of the first photographs of a patient receiving surgical anesthesia, with a sponge soaked in ether, at Massachusetts General Hospital in Boston. (Courtesy of The J. Paul Getty Museum.)
In Europe, chloroform was the preferred inhalational anesthetic. It was first described by Sir James Young Simpson, a Scottish obstetrician who administered it for labor pain. Dr. John Snow was recognized as the first professional anesthesiologist in England; he provided a chloroform anesthetic to Queen Victoria during the birth of her children in 1853 and 1857.
Intravenous anesthesia using sedative/hypnotic medications was not possible until the syringe and hypodermic needle were invented by Alexander Wood of Edinburgh in 1855. Barbituric acid was discovered in 1864 by Adolph von Baeyer, but it was another six decades before the first intravenous barbiturate was used to induce unconsciousness by the German obstetrician Rudolph Bumm in 1927. Barbiturates have been largely replaced by propofol, which was introduced in the 1980s. Benzodiazepines, which at low doses relieve anxiety but at higher doses may cause unconsciousness, have been available since 1960; the most commonly used benzodiazepine in anesthesia today is midazolam.
The local anesthetic cocaine was isolated in 1860 from the leaves of the coca plant, used by Peruvians for centuries as a stimulant. It was first used as a local anesthetic by Carl Koller in 1884 for eye surgery.
Curare was the first clinical neuromuscular blocker introduced in the 1940s. Like cocaine, curare was first found in South American plant preparations, described along the Orinoco River in 1800 by Alexander von Humboldt. Succinylcholine was introduced later by Bovet in 1949.
Inhalational gases, narcotics, local anesthetics, sedative hypnotics, and muscle relaxants remain the foundational medications of anesthesia.
Four Goals of an Anesthetic
The four goals of an anesthetic are to provide a lack of awareness or amnesia, pain relief, immobility, and patient safety. Ideally, medications should have a fast onset and offset, a predictable duration of action, and no unwanted or toxic effects. Because no single medication, to date, has all these qualities, an anesthesiologist often administers a “balanced anesthetic” with a small amount of a number of different drugs, thereby maximizing a drug’s positive effects while minimizing its side effects. Most anesthesia drugs have hazardous or potentially fatal effects even at therapeutic doses (e.g., most drugs that cause unconsciousness, also obstruct or stop breathing, which requires immediate treatment); this is one of the main reasons that anesthesiology requires skill and prolonged training.
As an anesthesia technician, you will find it useful to have a basic understanding of the desired and unwanted effects of the common anesthetic medications and the patterns in which they are used. Elsewhere in the text you will find a full discussion of principles of pharmacology, both pharmacodynamics and pharmacokinetics (Chapter 3, Pharmacology). As a reminder, pharmacodynamics is what a drug does to the body through interactions with receptors, cell membranes, enzymes and other proteins, and ion channels. A drug may either increase activity (agonist) or inhibit activity (antagonist). Pharmacokinetics is what the body does to the drug and includes its entry into the blood circulation (absorption), its spread through the fluids and tissues (distribution and, possibly, redistribution into reservoirs of inactive tissues like fat), its breakdown (metabolism), and its elimination from the body (excretion). You will also find a discussion of all the different classes of drugs used commonly during an anesthetic. This chapter will discuss inhalational and intravenous anesthetics.
Lack of Awareness
Lack of awareness is a continuum, ranging from fully awake to moderate sedation to deep sedation to unconsciousness/general anesthesia (see also Chapter 15, Sedation). A general anesthetic promises a total loss of awareness for the period from induction of anesthesia to emergence. This is assessed as unresponsiveness to surgical stimulation and as lack of recall (amnesia). The effects of various anesthetic agents on consciousness have been studied using the electroencephalogram (EEG), an instrument that measures electrical brain activity. The EEG can be processed into a single measure by commercial algorithms such as the bispectral index (BIS) monitor, occasionally used in the operating room to estimate the depth of anesthesia. Total unconsciousness is associated with a marked decrease in EEG activity.
By definition, a general anesthetic results in total unconsciousness, while other anesthetic techniques may result in only sedation or no reduction in consciousness at all. For example, a spinal anesthetic for a woman undergoing a cesarean section may avoid systemic drugs for mother and baby and may have minimal effects on consciousness. Sedation, even deep sedation, does not promise full loss of consciousness or full amnesia.
Inhalational Gases
Anesthesia Machine
Medications that can cause unconsciousness include both inhalational gases and injectable medications. Most general anesthetics are maintained with an inhalational gas. The anesthesia machine (Fig. 17.3; Chapter 24) is composed of four main parts: flow meters, the vaporizers, a breathing circuit, and a ventilator. Its purpose is to mix gases, including both oxygen and anesthetic gases, and deliver them safely to the patient. This central role of safe, precise gas delivery is unique to anesthesia and an essential part of your role as an anesthesia technician.

FIGURE 17.3. The anesthesia machine composed of the vaporizers, a flow meter, a breathing circuit, and a ventilator. (Reproduced with permission from LifeART image. Copyright © 2011 Lippincott Williams & Wilkins. All rights reserved.)
Nitrous Oxide
Nitrous oxide is a relatively weak inhalational anesthetic. The dose required to produce total unconsciousness would be more than 100% of the gas breathed by the patient: this would not provide oxygen for survival. Since the patient must also breathe oxygen, nitrous oxide cannot be delivered at a dose of more than about 70% of the gas breathed by the patient, with the remainder of the inhalational gas comprised of oxygen. This mixture is not enough to produce total unconsciousness; thus, nitrous oxide is not used alone in a general anesthetic but instead supplements other medications. It works through both ion channel and receptor activity and has a relatively benign side effect profile. Its primary advantage is a very rapid onset and offset and its cardiovascular stability compared with other inhalational anesthetics. Its primary disadvantage is that it has multiple contraindications; many patients or surgeries are inappropriate for nitrous oxide altogether. The scientific evidence on this old-fashioned drug (in continuous use since 1846) is controversial; many anesthetists prefer to avoid it, but some institutions still rely on it as a primary component of their practices. Nitrous oxide is not a volatile anesthetic (i.e., it is not a liquid that turns into a gas, supplied through a vaporizer), but a gas that is delivered under pressure, like oxygen or air, via a hospital supply from the wall or in a pressurized tank, and mixed with oxygen in the high-pressure system before the vaporizer in the anesthesia machine.
Sevoflurane, desflurane, and isoflurane are common volatile inhalational agents; all can be used as the sole agent for a general anesthetic. After decades of study and more than 150 years of use, the exact mechanisms by which inhalational general anesthetics work remain poorly understood. Inhalational anesthetics do not all resemble one another closely: nitrous oxide, ether, chloroform, isoflurane, and xenon all result in general anesthesia, but are not closely chemically related. Several theories have been proposed. On a molecular level, Meyer and Overton suggested that anesthetics work at the lipid portion of the nerve cell membrane. Later, Franks and Lieb determined that the site of action is at the protein layer of the cell membrane. The main receptor involved is the γ-aminobutyric acid (GABA) receptor. It is thought that certain anesthetic agents increase the action of this receptor, which inhibits nerve cell activity and consciousness.
Volatile inhalational anesthetics must be vaporized from the liquid state and are administered from agent-specific vaporizers (see Chapter 26, Vaporizers). A carrier gas passes through the vaporizer and takes up the inhalational agent, just as a breeze will carry steam. The agent will then be carried to the patient through the breathing circuit to enter the lungs. Once in the lungs, it diffuses into the bloodstream where it is carried to the brain and elsewhere throughout the body. The inhalational agent’s speed of onset is inversely proportional to its solubility, which is indicated by the blood/gas coefficient (Table 17.1). The minimum alveolar concentration (MAC) is the concentration of the inhalational agent in the lungs that prevents movement in 50% of patients undergoing a surgical stimulus (e.g., being cut by a scalpel). Inhaled anesthetics are mainly eliminated from the body by exhalation once the inhaled concentration is turned back to zero, although there is some metabolism in the liver and excretion in the urine and through the skin. Speed of onset and offset is also increased by increasing the rate of fresh gas flow. When fresh gas flow is high, the concentration of gas in the circuit is rapidly equilibrated with the fresh gas. If, for example, the patient breathes out anesthetic gas, that gas is immediately carried away to the scavenging system, and their next breath in is gas that has no anesthetic. When fresh gas flow is low, the gas in the circuit is largely recirculated from the patient, so that anesthetic gas concentration changes very little no matter what the concentration in the fresh gas flow is.
Table 17.1. Blood/Gas Coefficient, MAC, and Side Effects of Inhalational Agents
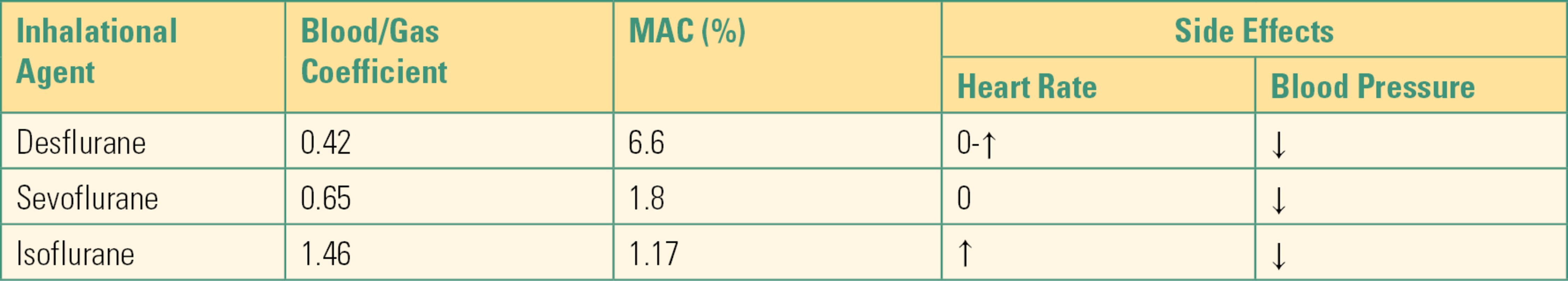
MAC, minimal alveolar concentration; ↓, decrease; ↑, increase; 0, no change.
As with all medications, gases have side effects. In the brain, volatile anesthetics increase cerebral blood flow and intracranial pressure while decreasing cerebral metabolic oxygen consumption. Their effect on the ventilatory system (airway and lungs) is to depress respiration slightly and bronchodilate. They also can decrease systemic vascular resistance and the heart’s contractility, thereby lowering blood pressure, possibly dangerously. Isoflurane and desflurane can cause increased heart rate, which can cause ischemia (inadequate oxygen supply) in a patient with coronary artery disease. Desflurane can cause airway irritation.
Injectable Medications
Common intravenous sedative hypnotic agents that produce unconsciousness include propofol, etomidate, benzodiazepines (e.g., midazolam), and barbiturates (e.g., sodium pentothal) (Table 17.2). These work by depressing the reticular activating system, an area in the brain responsible for regulating arousal/wakefulness, and by increasing GABA action, inducing a loss of consciousness. Ketamine is another intravenous sedative hypnotic used to produce unconsciousness, which induces a state known as “dissociative anesthesia” as well as profound analgesia via a different mechanism, as an N-methyl-d-aspartate (NMDA) glutamate antagonist. Their high lipid solubility in the brain results in a rapid onset of action. In many cases, their duration of action is primarily determined by redistribution (dilution) throughout the body rather than by metabolism or excretion. Thus, they accumulate when large doses are given and are more often used to start (induce) a general anesthetic than to maintain it. All of these agents are typically injected intravenously; alternate routes exist for a few agents, particularly useful for children or other uncooperative patients: intramuscular, oral, intranasal, and rectal.
Table 17.2. Commonly Used Intravenous Anesthetic Agents
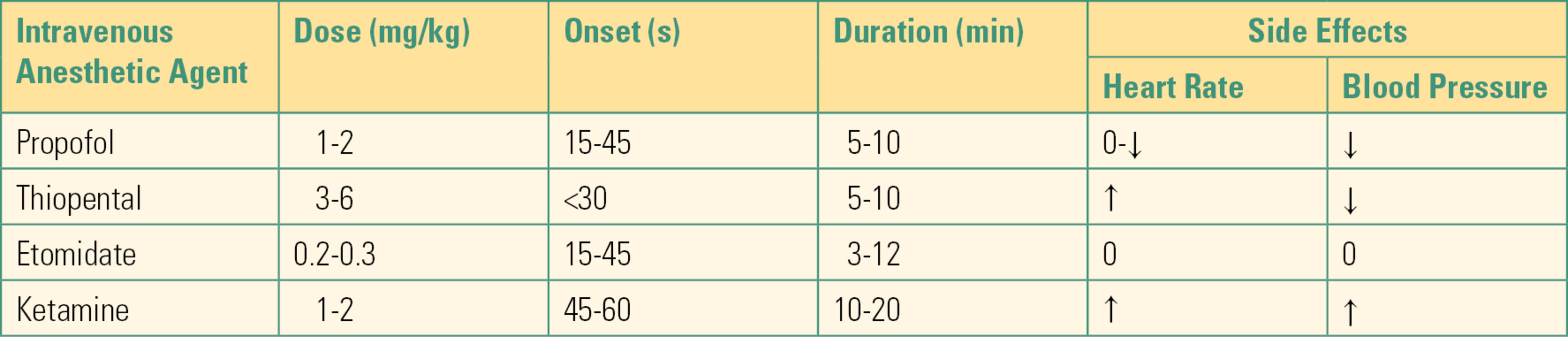
↓, decrease; ↑, increase; 0, no change.
Propofol often causes pain at the site of IV injection, so it is often preceded by or administered along with the local anesthetic lidocaine. It produces dose-dependent hypotension as a result of decreasing the heart’s contractility and lowering the systemic vascular resistance. At doses that induce anesthesia, it typically causes patients to stop breathing. Etomidate causes less hypotension than propofol, but it can suppress hormone (steroid) production and is relatively expensive and places patients at higher risk of postoperative nausea and vomiting; thus, it is typically reserved for patients who will not tolerate the hemodynamic effects of propofol. Midazolam is noted to have less adverse cardiovascular and ventilatory effects than any of the preceding drugs, but the doses required to cause unconsciousness require a long time to wear off. Ketamine also maintains hemodynamic and ventilatory stability and is often used in unstable patients. However, it does not block the hypertensive response to laryngoscopy and may cause dramatic increases in blood pressure and heart rate. Its postoperative psychological effects are a matter of current debate. As an anesthesia technician, you may see some of its unique characteristics. It can be given intramuscularly for induction to agitated or uncooperative patients who will accept neither an IV nor a mask. It may not cause apnea even in induction doses (patients may continue to breathe); because of its different CNS mechanism, some patients, though truly unconscious, amnestic, and unresponsive, may not spontaneously close their eyes and will not look “asleep.” In smaller doses, it is a potent analgesic and is given in many settings for pain relief.
Pain Relief (Analgesia)
Analgesia is defined as decreased pain sensation. Opioids are commonly used to produce analgesia and may be used alone or in conjunction with other analgesics such as local anesthetics, ketamine, dexmedetomidine, or clonidine. The administration of analgesic medications may reduce the reflex response to a painful stimulus that is often apparent as an increase in heart rate and blood pressure, each of which can endanger a patient if the increases are too great. Opioids act as agonists on receptors in the brain and the spinal cord and are therefore regarded as “centrally acting,” although there are also some effects throughout the body (“peripherally acting”) (Table 17.3). They have some sedativelike properties, can cause unresponsiveness to stimulation, and may reduce the dose requirement for general anesthetics, but they do not produce amnesia (loss of memory) and cannot, by themselves, produce general anesthesia. They are typically included as part of a balanced anesthetic. They are associated with hemodynamic stability but have the significant side effect of ventilatory depression, especially when used with other drugs that cause sedation. Other centrally acting analgesics include dexmedetomidine and clonidine, which are agonists for α-receptors, and ketamine, which acts as an antagonist on NMDA receptors.
Table 17.3. Commonly Used Parenteral Narcotics

Immobility
Neuromuscular blockers are used during many anesthetics, either to facilitate airway management, as part of a balanced anesthetic, or both. They are commonly administered after induction of anesthesia and before laryngoscopy and intubation and may be given in repeated doses throughout surgery. Nondepolarizing neuromuscular blockers often require reversal at the end of anesthesia and surgery (Table 17.4).
Table 17.4. Neuromuscular Blockers
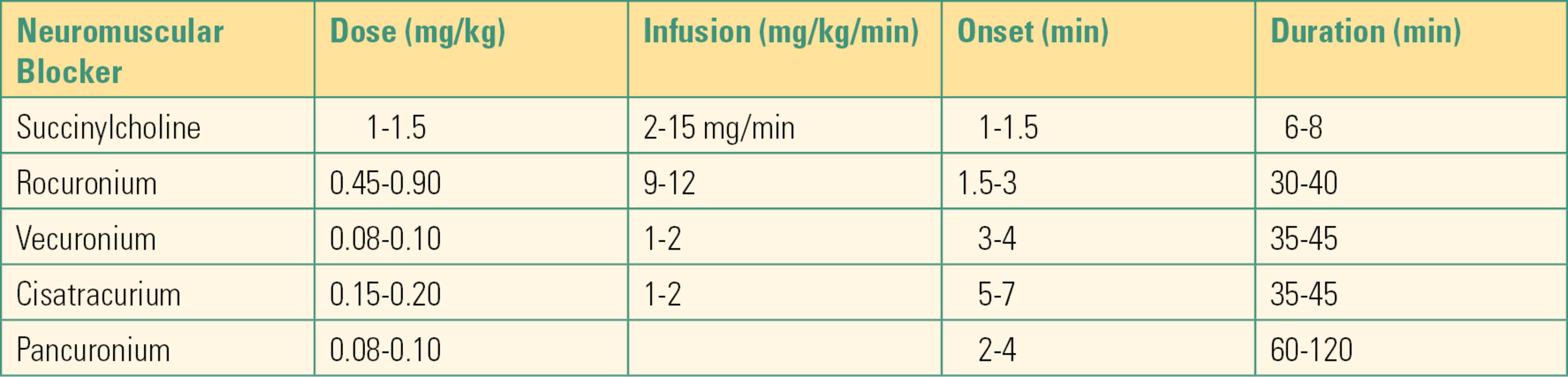
Laryngoscopy and intubation are easier and safer in the presence of neuromuscular blockers. A still, nonmoving patient is also essential for a successful operation. Volatile anesthetics depress blood pressure and heart rate in a predictable way: the higher the dose, the lower the blood pressure. More volatile anesthetic, sometimes an unsafe amount, is required to ensure immobility than is required to ensure total unconsciousness; when this is the case, a neuromuscular blocker can be helpful. Volatile anesthetics provide a modest amount of skeletal muscle relaxation, but many surgical procedures (such as abdominal and orthopedic surgeries) require more profound muscle relaxation. Some stimuli (such as bladder and vocal cord surgeries) cause spinal or autonomic reflexes that cannot be fully blocked with volatile anesthetics (see Chapter 14, Neuromuscular Anatomy and Physiology, for a full discussion of neuromuscular blockers).
Patient Safety
This goal is also referred to as homeostasis, which means keeping things as they are. The effects of anesthesia and surgery may interfere with the normal functioning of many vital organs, especially the heart and lungs, and the anesthesiologist must be able to quickly diagnose and treat any significant abnormality. Diagnosis is accomplished through continual and vigilant patient monitoring. The American Society of Anesthesiologists (ASA) has established standards for patient monitoring during anesthesia, and all anesthetics must incorporate these standard monitors at a minimum. They include monitoring the heart rate and rhythm with electrocardiography (ECG), blood pressure, and oxygen saturation with a pulse oximeter. Carbon dioxide (capnography) and temperature should be monitored when appropriate.
Stages of Anesthesia
The three types of anesthesia are general anesthesia, regional anesthesia, and monitored anesthesia care (MAC). This chapter focuses on general anesthesia. Arthur Guedel in 1937 described four stages of a general anesthetic for ether. These are still in use to describe today’s anesthetics in much the same form. Stage 1 is the time from administration of an anesthetic to the subsequent loss of consciousness. Stage 2 is sometimes referred to as the excitation phase, most commonly seen during emergence but occasionally during inhalation induction, during which patients may have irregular respiration and breath holding. They may also appear agitated, and their pupils are often dilated and their eyes divergent. This is a critical state as patients may have episodes of vomiting, laryngospasm (vocal cords closing), and uncontrolled movements. Stage 3 is the desired depth of anesthesia for surgery. The loss of the lash reflex (no response to stroking the eyelashes) is indicative of this stage. Respiration, if present, becomes regular, pupils constrict, and involuntary movements cease. Stage 4 is a state of anesthesia crisis and is considered to result from an overdose of anesthetic medications. Circulatory and respiratory arrest and death may occur.
Time Frames of a General Anesthetic
There are three periods in an anesthetic: the preoperative, intraoperative, and postoperative. The preoperative phase begins when the patient is admitted to the preoperative holding area, the intraoperative phase begins when the patient is in the operating room, and the postoperative phase starts when the patient leaves the operating room.
Preoperative Phase
During the preoperative phase, a thorough assessment of the patient is performed. This evaluation is an essential step in developing the anesthetic plan and includes a history of present illness, medical and surgical histories, and a review of pertinent laboratory, radiologic, and other tests. The patient’s current prescription and nonprescription medications are reviewed for potential drug interactions. Allergy and the type of reaction should be documented. Family history is reviewed, especially for a history of the rare inherited disorder known as malignant hyperthermia (see Chapter 60). Problems that may have been associated with a general anesthetic from a past surgical procedure, especially a history of difficult intubation, need further evaluation, including a review of any previous anesthetic records. A social history including a patient’s alcohol, tobacco, and illicit drug use should be documented.
Preoperative fasting is required to reduce the risk of aspiration (inhalation) of stomach contents during a general anesthetic, and the fasting interval should be assessed and documented. The recommendations include fasting for 2 hours for clear liquids; 4 hours for breast milk (neonates and infants); 6 hours for infant formula, nonhuman milk, and a light meal (toast and clear liquid); and 8 hours for protein or fatty foods.
A thorough physical examination should be performed. This includes an assessment of vital signs (blood pressure, heart rate, respiratory rate, pulse oximetry, temperature, and pain score), airway, heart, lung, and extremities. The airway exam is of utmost importance to the anesthesiologist. The administration of general anesthesia frequently involves placement of airway devices into the patient’s mouth and throat. In addition, anesthetics frequently depress respiration, and the anesthesiologist must ventilate the patient during periods where the patient is unable to breathe on his or her own. The airway exam is critical for assessing any potential difficulties that might be encountered with ventilation or the placement of airways due to the patient’s anatomy. The patient’s dentition, including loose, missing, or chipped teeth, caps, bridges, crowns, or dentures, should be documented. It is important to identify any loose teeth because dislodgement can lead to aspiration of the tooth into the lung. The mobility of the neck should be examined in terms of flexion and extension. The mouth opening and temporomandibular joint mobility should be examined. The Mallampati score is an assessment of the mouth opening that is used to suggest a difficult intubation. A higher score means it is more likely that a difficult airway will be encountered (Table 17.5; Fig. 17.4).
Table 17.5. Mallampati Score

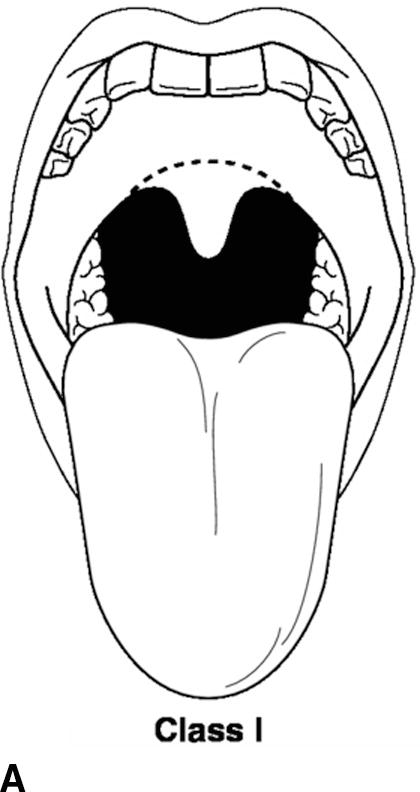
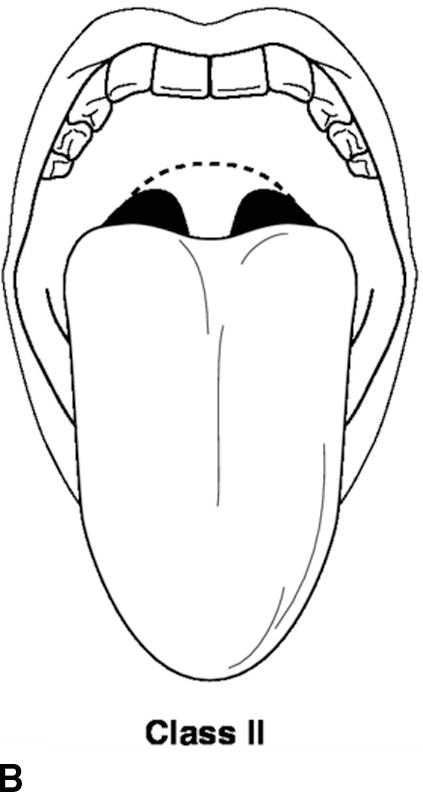
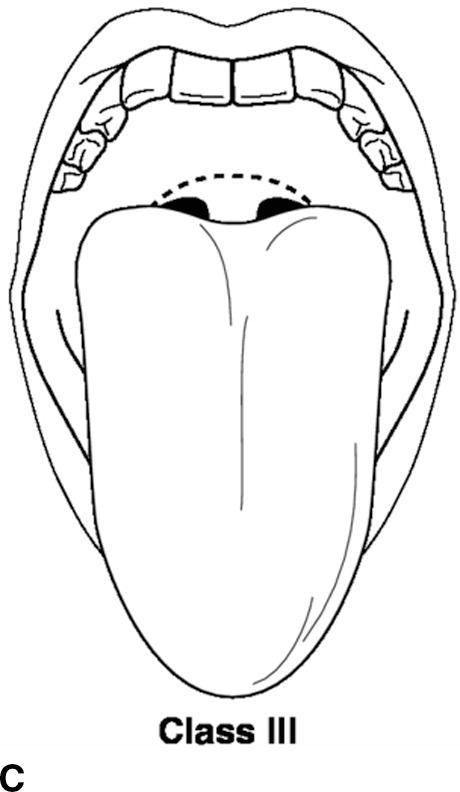
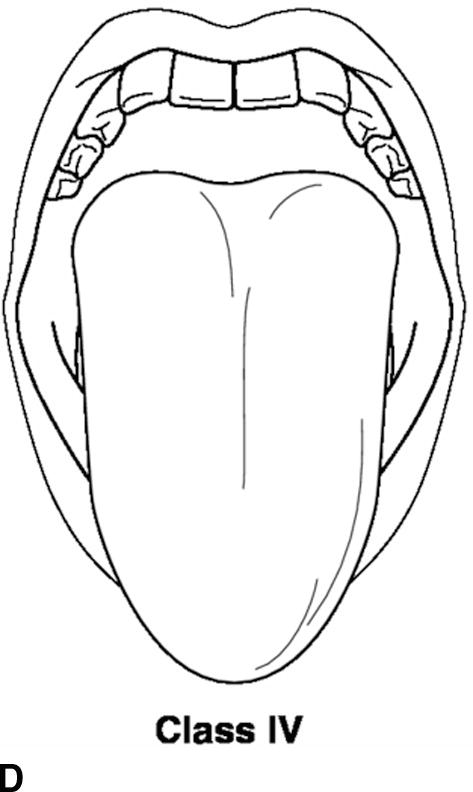
FIGURE 17.4. A-D: Mallampati score. (Reproduced from Blackbourne LH. Advanced Surgical Recall. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014, with permission.)
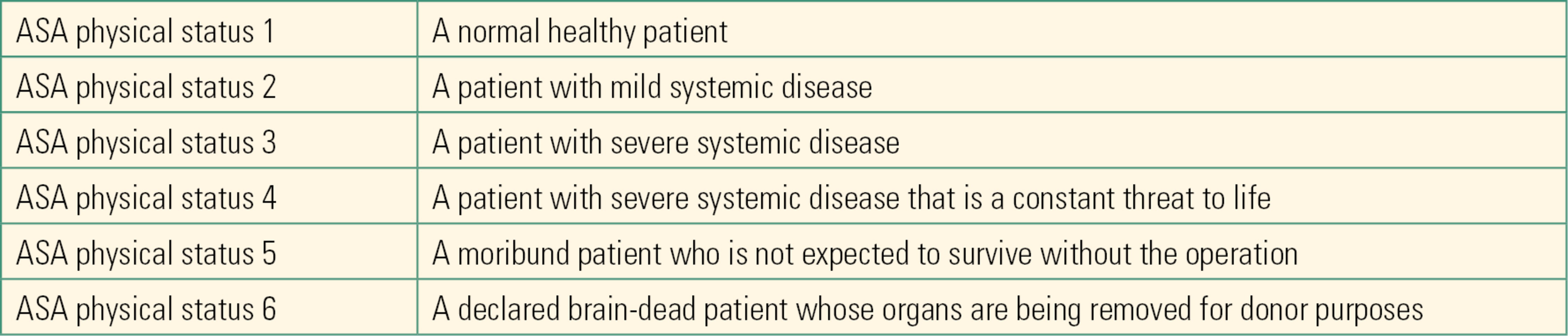
Once the anesthesiologist has gathered all of the pertinent information, the patient is assigned an ASA physical status number that indicates the patient’s overall health (Table 17.6). The ASA status is a global assessment that frequently varies between anesthesiologists for any given patient; despite this inherent “interrater variability,” it is a very strong predictor of patient outcomes. As an anesthesia technician, you should be familiar with ASA status and understand what each status means. You may find that your institution bases protocols for care on ASA status: for example, that ASA III patients have monitors for postoperative transport or that ASA IV patients are assigned to anesthesia providers for moderate sedation.
Table 17.6. ASA Physical Status Classification System
Reprinted with permission of the American Society of Anesthesiologists, 1061 American Lane, Schaumburg, Illinois 60173-4973.
After the preanesthesia assessment is performed, the anesthesiologist uses the information to decide what anesthetic technique, medications, and monitors should be used. The risks, benefits, and alternatives to the anesthetic technique are explained and discussed with the patient. An intravenous line is typically placed. The anesthesiologist may also choose to further assess the patient (e.g., obtain a urine pregnancy test, potassium level, ECG, or cardiology consult) or to administer various medications (e.g., midazolam to relieve anxiety) prior to starting the intraoperative phase. In some circumstances, the anesthesiologist may perform other procedures in the preoperative area to prepare the patient for surgery including arterial lines, central venous access lines, a peripheral nerve block, or an epidural.
Intraoperative Phase
The intraoperative portion of a general anesthetic can be divided into three phases: induction, maintenance, and emergence.
Induction
Steps in the induction phase of anesthesia include appropriately positioning the patient, placing the standard monitors, inducing general anesthesia, and managing the airway.
Monitors
Prior to providing a general anesthetic, patients are usually positioned supine (on the back, face up) with the head in the so-called “sniffing” position (head elevated off the bed and tilted back). Additional time may be taken to place obese patients in positions that are proven to improve their safe ventilation and intubation, lifting the chin away from the sternum. These may be an additional lift under the shoulders, in backup, reverse Trendelenburg (bed tilted foot down) or with blankets or a wedge under the back (a bariatric ramp). Standard monitors (ECG, pulse oximeter, automated blood pressure cuff, capnogram) are applied. The use of monitors, as well as ongoing visual assessment of the patient and surgery, provides critical information to detect the disruptions in homeostasis that occur with both anesthesia and surgery. Invasive monitors, such as a radial arterial catheter for continuous blood pressure monitoring and blood gas sampling, or an internal jugular vein catheter for measurement of central venous pressure, may be indicated for patients undergoing certain surgeries or with certain diseases. Transesophageal echocardiography (TEE), an ultrasound device placed in the esophagus next to the heart, is often used for heart surgery and can be placed after intubation. The various monitors used in anesthetized patients are extensively covered in this text, in Chapters 5 (Cardiovascular Monitoring), 8 (Respiratory Monitoring), 30 (Gas Analyzers), 31 (ASA Standard Monitors), 32 (Neurologic Monitoring), 37 (Intravascular Monitoring Equipment), and 42 (Transesophageal Echo).
Induction of General Anesthesia
Induction refers to the administration of sufficient anesthesia medications to cause unconsciousness. It is typically accomplished by intravenously injecting a sedative hypnotic (intravenous induction), by having the patient breathe a volatile anesthetic (inhalational induction), or a combination of the two. Induction of anesthesia can be accomplished by intramuscular injection if needed. The choice and amount of medication are largely determined by the patient’s health status and the surgical requirements. Care is taken to avoid extreme changes in a patient’s heart rate and blood pressure. Also, since an unconscious patient may lose the ability to maintain an open airway and/or lose the urge to breathe (ventilatory drive), patients are “preoxygenated” by having them breathe 100% oxygen via a sealed face mask on their own prior to administering induction medications.
Anesthetic Agents for Induction
Intravenous Induction
Intravenous agents are more frequently used for induction of anesthesia in adults because of their rapid onset and relatively short duration of action.
Inhalational Induction
Induction of anesthesia with volatile anesthetics is more common in children. Often, the odorless mixture of nitrous oxide and oxygen (70/30) is initially administered to the awake spontaneously ventilating child via a face mask, with the gradual addition of sevoflurane (the least pungent and noxious of the potent volatile agents). After anesthesia is induced, an intravenous line is typically established.
Airway Management
By definition (Fig. 17.5), any general anesthetic may involve impairment of the patient’s ventilation or obstruction of the airway. Thus, after the induction of general anesthesia, it is critical for the anesthetist to establish a safe airway and ventilation for the patient. If anesthesia gases are not being used (TIVA), a patient may breathe spontaneously without airway support; supplemental oxygen is usually given and capnography is mandatory. The least invasive method to ventilate an apneic patient is with a hand-held positive pressure mask, with the anesthetist relieving upper airway obstruction, or the possible use of an oral or nasal airway. Another option would be a supraglottic airway, like an LMATM. The usual definitive method to “secure” the airway is laryngoscopy to place an endotracheal tube. Some patients will have an invasive surgical airway. The duration, type of surgery, and patient health issues help the anesthesiologist plan which airway choice will be best. Each involves a different anesthetic and pharmacologic technique for placement. Details of airway management are covered in Chapter 18, Principles of Airway Management.
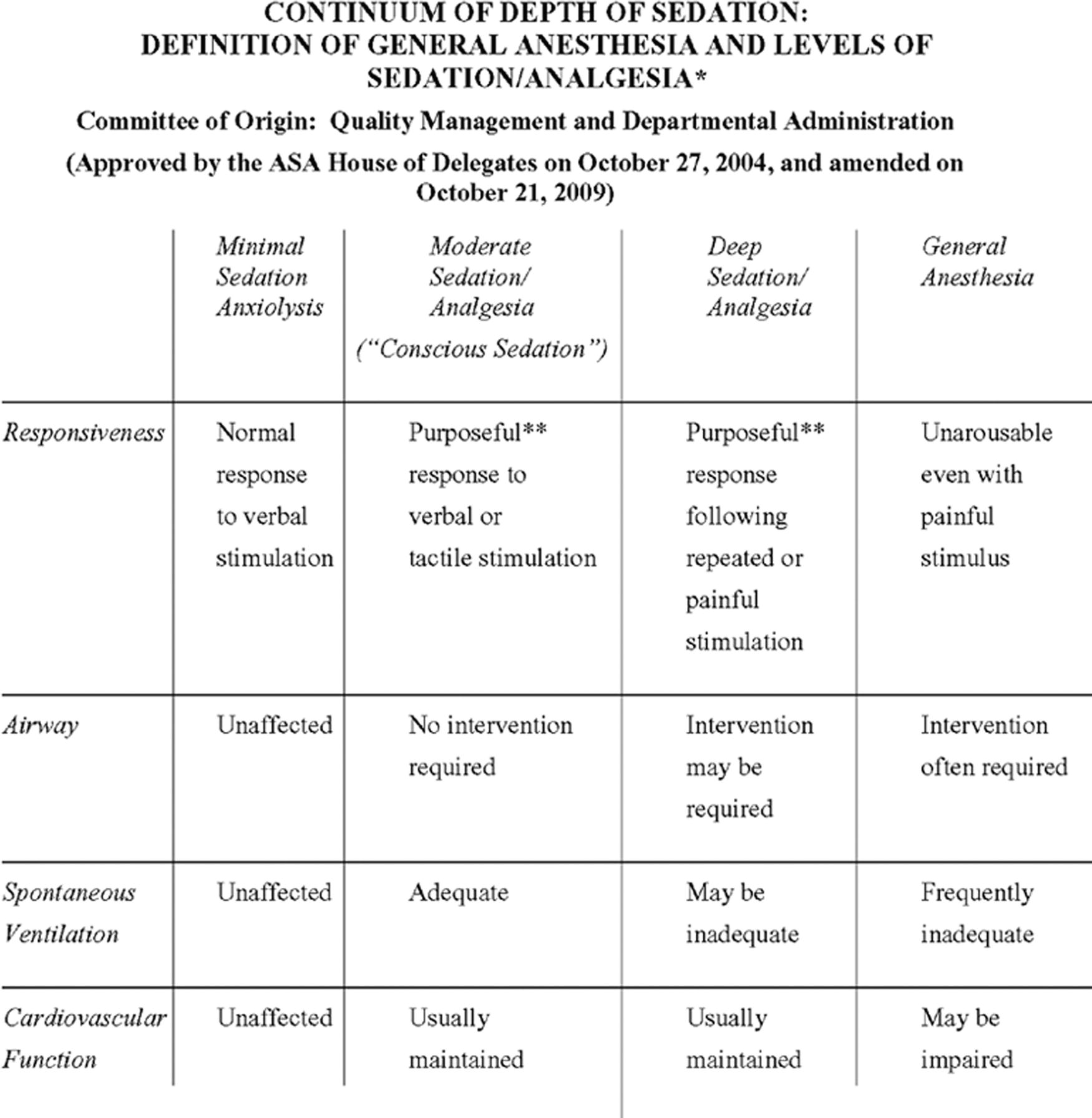
FIGURE 17.5. Excerpted from Continuum of Depth of Sedation: Definition of General Anesthesia and Levels of Sedation/Analgesia of the American Society of Anesthesiologists. Approved by the ASA House of Delegates on October 27, 2004, and amended on October 21, 2009. A copy of the full text can be obtained from ASA, 1061 American Lane, Schaumburg, Illinois 60173-4973 or online at www.asahq.org.
Maintenance
Because of the rapid elimination of intravenous induction agents by redistribution and the relatively brief duration of action of volatile agents, recovery from an induction dose of medication is quick. To ensure an ongoing state of anesthesia, the anesthesiologist immediately begins maintenance anesthetic agents after induction, possibly even during airway management. GA can be accomplished with a volatile anesthetic, an intravenous anesthetic such as propofol, or a combination of both. Frequently, other medications are used for additional muscle relaxation and analgesia.
Total Intravenous Infusion Anesthesia
Total intravenous anesthesia (TIVA) is another method for maintenance of anesthesia. A continuous infusion of an intravenous agent is delivered typically by a pump. The infusion rate is titrated (adjusted) until the appropriate depth of anesthesia is achieved. The additional use of opioid analgesics and muscle relaxants may be indicated depending on the type of surgery being performed. TIVA is utilized in patients with a history of malignant hyperthermia because all volatile anesthetic agents are a known triggering agent. TIVA is also frequently used in open airway surgery, in out of OR anesthesia if an anesthesia machine is not easily accessible, and in neuroanesthesia to avoid unfavorable alterations in cerebral blood flow and neuromonitoring capacity. TIVA may require additional support from you as the anesthesia technician, as it may require multiple infusion pumps. End-tidal anesthesia gas monitoring is one of many pieces of information available to the experienced anesthetist to assess a patient’s depth of anesthesia; when using TIVA, this anesthesia agent monitoring is unavailable, so some anesthesia providers may ask for EEG monitoring such as BIS when using TIVA.
Emergence
Emergence from anesthesia involves the gradual awakening of the patient and the return to baseline physiologic and psychological function. It is as critical a time as the induction of anesthesia, since hemodynamic variability, airway obstruction, and emergence delirium (postanesthesia excitement) can occur. Emergence from inhalational agents occurs with the diminishing concentration of the agents in the lungs as the patient breathes them out. Recovery from intravenous agents is dependent on their redistribution, metabolism, and excretion. Recovery from neuromuscular blockers may be spontaneous or may require the administration of a reversal agent.
Delayed emergence (failure to regain consciousness) can be multifactorial. Some factors are residual inhalational or intravenous anesthetic agents, analgesics, or sedatives. Electrolyte and glucose disorders can contribute, and as an anesthesia technician, you may be asked to assess arterial blood gas samples in these patients, even those without arterial lines. Naloxone and flumazenil can be used to reverse the effects of opioids and benzodiazepines. Hypothermia (decrease in body temperature) may be a contributing factor. Emergence delirium, a state of excitement after recovery from general anesthesia, is more commonly seen in children than adults. It can present as confusion, disorientation, or restlessness. In extreme episodes, the patient may thrash and scream. Possible reasons for emergence delirium are rapid emergence, anxiety, and pain in the postoperative period.
Postoperative Phase
The immediate postoperative period can be divided into two phases. Phase 1, in the postanesthesia care unit (PACU), involves intensive nursing care. Phase 2 involves a less intense level of care. Most patients will be discharged from the PACU to a postsurgical ward or to phase 2 recovery based on a scoring system that assesses their level of consciousness, cardiac and respiratory stability, and ability to move. Since patients in phase 2 are typically going home and no longer receive medical or nursing care, discharge criteria additionally take into account postoperative pain, bleeding, and nausea or vomiting. The PACU is fully equipped with monitors for pulse oximetry, ECG, and blood pressure measurements. It has a defibrillator with transcutaneous pacing and an emergency cart with medications for advanced life support. Other supplies available include oxygen cannulae, masks, airways, laryngoscopes, ETTs, an Ambu bag (self-inflating bag for ventilation), and ventilators. Patients who are unstable after a general anesthetic and require hemodynamic and respiratory support are usually admitted to an intensive care unit (ICU). These patients are transported with portable monitors, an oxygen tank, an Ambu bag, emergency drugs, and additional airway equipment (see Chapter 51, Patient Transport).
Summary
An anesthesiologist uses medications, devices, and techniques in a general anesthetic to accomplish four goals of anesthesia: lack of awareness or amnesia, pain relief, immobility, and patient safety. While the foundation for anesthesia has been the same for hundreds of years, the specialty continually applies cutting-edge technology to provide better and safer anesthetics. A modern general anesthetic has predictable stages: preanesthesia assessment; anesthesia induction, maintenance, and emergence; and postanesthesia recovery. It involves the use of potent inhalational and intravenous agents, management of the airway, and often blockade of the neuromuscular junction. At all times, the patient’s physiology must be monitored and adjusted to ensure patient safety, comfort, and optimal operating conditions. As an anesthesia technician, an in-depth understanding of this basic rhythm will form the foundation of your practice.
Review Questions
1. You are present during induction of a trauma patient. You witness the following drugs given. Which of the following agents induces a state of general anesthesia?
A) Fentanyl
B) Ketamine
C) Succinylcholine
D) Vecuronium
Answer: B
Ketamine induces unconsciousness and is an intravenous general anesthetic, though it works by a different mechanism than the other intravenous general anesthetics: ketamine is an NMDA antagonist, whereas propofol, the barbiturates, and the benzodiazepines are GABA agonists. Fentanyl is an opioid. Opioids block pain and depress patients’ responsiveness but do not cause amnesia or loss of consciousness. Succinylcholine and vecuronium are neuromuscular blockers and have no effect on the central nervous system or the level of consciousness.
2. You are paged to the operating room to assist in a case. The anesthesiologist tells you that they are “putting monitors on.” What is likely to be the NEXT event?
A) The start of surgery
B) Administration of intravenous induction agents
C) Informed consent for anesthesia
D) Placement of the breathing circuit and mask for preoxygenation
E) Placement of vascular access
Answer: D
Informed consent for anesthesia is typically obtained before patients are transported to the operating room, as is (in adult or cooperative patients) vascular access. Monitors are placed once the patient is positioned on the operating room bed. After this, the patient is preoxygenated to create a reservoir of several liters of oxygen in the lungs prior to induction of anesthesia.
3. Place in order the sequence in which drugs are administered for a general inhalational anesthetic in an adult patient.
A) Neuromuscular blocker
B) Intravenous induction agent
C) Inhalational agent
D) Sedative/anxiolytic
E) Reversal agent
Answer: D, B, A, C, EFirst = D. Sedative/anxiolytic; Second = B. Intravenous induction agent; Third = A. Neuromuscular blocker; Fourth = C. Inhalational agent; Fifth = E. Reversal agent. A sedative/anxiolytic can be administered before the patient is brought to the operating room suite to help alleviate the stress response associated with surgery. After application of ASA standard monitors and preoxygenation, an intravenous induction agent is administered for loss of consciousness. After loss of consciousness occurs, a neuromuscular blocker may be administered. Inhalational agents are used for maintenance. Reversal agents are given if the presence of a neuromuscular blocker requires them.
4. Which of the following monitors is NOT an ASA standard monitor in all patients?
A) Pulse oximeter.
B) Blood pressure.
C) Capnogram.
D) Electrocardiogram.
E) All of the above are ASA standard monitors.
Answer: E
All these are ASA standard monitors.
5. All of the following occur in the preoperative period EXCEPT
A) Performing the preanesthesia assessment
B) Obtaining laboratory data
C) Preoxygenating the patient
D) Assessing the nil per os—nothing- by-mouth (NPO) status
E) Explaining the risks, benefits, and alternatives of anesthesia
Answer: C
During the preoperative period, a thorough assessment of the patient is performed. Preoxygenating the patient is a step in anesthesia induction and typically occurs in the intraoperative period.
6. Nitrous oxide
A) Was the first successful demonstrated anesthetic in 1846
B) Is used for maintenance of anesthesia but not induction
C) Is chemically related to the other inhalational anesthetics
D) Cannot be delivered during patient transport
E) All of the above
Answer: D
Ether was the first successful demonstrated anesthetic in 1846: nitrous oxide, though attempted earlier, was unsuccessful because it was not potent enough for surgery when used by itself. Nitrous oxide is often used as the initial anesthetic induction gas because it is odorless. The inhalational anesthetics, including nitrous oxide, are chemically very different from one another, and their molecular mechanisms are not well understood. Nitrous oxide must be mixed with oxygen for safe delivery: this mixing in the high-pressure system happens in an anesthesia machine and cannot be done in transport.
7. “Total intravenous anesthesia” refers to which of the following?
A) Administering an intravenous agent followed by an inhalational agent for maintenance of anesthesia
B) Administering a continuous infusion of an intravenous agent titrated to the appropriate depth of anesthesia
C) Administering smaller amounts of a number of different anesthetic drugs in order to reduce the unwanted side effects associated with each one
D) Performing induction of anesthesia using a volatile anesthetic or nitrous oxide via mask and then placing an intravenous line
E) None of the above
Answer: B
The correct answer is B. Option A describes a routine general anesthetic in an adult. Option C describes the concept of a balanced anesthetic. Option D describes an inhalational induction (commonly used in the pediatric population).
8. Match the stages of anesthesia on the left with the correct definition on the right:

Answer: C, A, D, BStage 1 = C (time from administration of an anesthetic to loss of consciousness); stage 2 = A (commonly observed in the pediatric population); stage 3 = D (depth of anesthesia required for surgery); stage 4 = B (anesthesia crisis/overdose).
9. The Mallampati score helps estimate the patient’s
A) Overall health status
B) Risk of aspiration
C) Risk of anesthesia
D) Risk of intubation difficulty
E) None of the above
Answer: D
The Mallampati score is an assessment of the airway that is used to detect the probability of a difficult intubation. Visualizing fewer structures (e.g., tongue, uvula, and tonsils) gives a higher score and is one of several indicators of the likelihood of a difficult intubation.
SUGGESTED READINGS
Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia. 8th ed. Philadelphia, PA: Wolters Kluwer; 2017.
Benumof JL. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84(3):686-699.
Hewer CL. The stages and signs of general anaesthesia. BMJ. 1937;2(3996):274-276.
Horine EF. Episodes in the history of anesthesia. J Hist Med Allied Sci. 1946;1(4):521-526.
Morgan M. Total intravenous anaesthesia. Anaesthesia. New York, NY: 1983;38:1-9.
The American Society of Anesthesia Task Force. Practice guidelines for preoperative fasting and the use of pharmacological agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376-393.