Figure 12.1 A sandhopper, Talitrus saltator, on a sandy beach in Italy. The animal is just under 1 cm in length. (Photograph provided by Riccardo Innocenti)
Visual Orientation and Navigation
It is a sunny late afternoon on a beach on the west coast of Italy, not far from Pisa. With sunset approaching, the sandhoppers are on the move! Sandhoppers, smallish semiterrestrial amphipods that look like nothing more than animated brown beans (figure 12.1), hop up or down the beach as they commute between the damp sands where they burrow for safety or hide for the day and the patches of sand at the waters edge or the zones of debris where they forage. Look carefully—you can see that they are making their way up or down the beach eastward and westward, perpendicular to the north/south axis of the water’s edge. As the sun dips below the horizon, clouds cover the sky. Many sandhoppers have buried themselves in the sand, but the movements of the ones still visible in the gloom are haphazard and seemingly disoriented. A few hours later the sky has cleared once more, and the full moon is riding well above the horizon. It illuminates a scene where we see that the active little creatures have reemerged to the surface of the sand. They hop along, just as well oriented as they were during the day. On another beach, not far away, the shoreline runs east and west, and sandhoppers here are cheerfully making their commutes, but they hop mainly north and south when the sun or moon is out.
The sandhoppers, Talitrus saltator, are demonstrating their ability to use the sun or moon as a compass. Like most animals, these amphipods have a hierarchy of mechanisms that assist them as they move about on the beach, but one of the most reliable is the position of the sun or moon in the sky. Orienting like this is by no means simple—as Juliet herself pointed out to Romeo, the moon is inconstant. Although she was referring to the phases of the moon, the moon and sun both travel continuously, crossing the full width of the sky in 12 hours. Any animal that relies on their position must take this into account. Nevertheless, the sun has directed the movements of animals almost from the beginnings of vision itself, so we will return to these sandhoppers in a few pages to consider how sun compass orientation works.
Orientation refers to an animal’s ability to move or posture itself in a desired direction relative to its environment. The ability to orient is virtually a universal feature of animal life. Many animals go a step further and navigate through the environment, finding their way from their current location to a specific destination that might be meters or kilometers away. Orientation mechanisms, and even more those that underlie navigation, are often complex and multimodal, involving not only visual cues but also sensory information about gravity, magnetic fields, chemical stimuli, mechanical and auditory cues, and even internal stimuli. Earlier chapters have mentioned feats of orientation, such as how dung beetles roll their balls of treasure on straight paths, oriented by celestial cues such as the polarization of the sky or the axis of the Milky Way. The field of research on how animals orient and navigate is enormous, even just considering visual aspects of orientation and navigation, so we here offer what we hope is an appetizing sampler. As for so many other aspects of visual ecology, many of the critical observations have involved invertebrate animals, but work on vertebrates is very active as well. The main advantage of invertebrates is that they are motivated to do simple behaviors predictably. Vertebrates tend to mix simple behavior with more complicated activities, making interpretation difficult. We begin with a look at some of the very simplest mechanisms of orientation involving visual cues. These fundamental behaviors go back to the origins of photoreception and are still common today—even in some quite complex animals.
Figure 12.1 A sandhopper, Talitrus saltator, on a sandy beach in Italy. The animal is just under 1 cm in length. (Photograph provided by Riccardo Innocenti)
Simple Means of Visual Orientation: Photokinesis and Phototaxis
A “kinesis” is a response to a stimulus that leads to a change in locomotion, either in speed or in rate of turning. Photokinesis, of course, means that the response is to a light stimulus. Although it is definitely a stretch to call a kinetic response an “orienting” response, such an extremely simple behavior can produce something that looks like orientation, even in single-celled bacteria. If a creature stops moving or slows down appreciably when it perceives light, it will tend to spend more time in lighted places than in dark ones. Similarly, creatures that move in straight lines in the dark but initiate a series of turns in the light will tend to become concentrated in patches of light in an otherwise dark world. So even in the absence of the simplest possible imaging device, such beings do show a sort of ability to end up in the right place.
Taxes (pronounced “tax-eez”), in contrast, are true orienting responses. Phototaxis just means a directional response to light. In general, positive phototaxis means moving toward a light source, and negative phototaxis produces a response in the opposite direction. Many animals demonstrate simple phototaxes—every diver who has switched on her dive light in a dark sea knows that within seconds the lamp becomes enveloped in a swarm of tiny creatures unable to resist the siren song of a bright light source. But clearly, there is more to the story of the lives of these plankton than this, because the same animals do not find themselves trapped at the surface of the ocean when the sun rises or the moon shines. Consequently, many behaviors that have been ranked as taxes by photobiologists do not reflect what animals do in their natural lives. Dive lights did not exist when phototactic marine animals evolved, and we will quickly leave them behind. But first we should take a short look at a couple of animals that are themselves biological dive lights—fish that inhabit the dark, mesopelagic zone of the ocean.
Figure 12.2 shows two deep-sea fish (and one shallow-water one) that use their own light—bioluminescence—to attract prey (full disclosure: these animals have never been observed doing this, for the obvious reason that seeing rare behavior in rare fish deep in a dark sea, depending only on bioluminescence, is virtually impossible). The light organ of the grim-faced angler fish, an undescribed species, extends like a brilliant torch over its mouth, bringing planktonic prey or other small swimming creatures to the predator through their own strongly phototactic behavior. This does risk bringing in a predator instead of prey, of course, so the angler must be judicious in using the lamp. The dragonfish Bathophilus nigerrimus uses its light lure more cautiously, suspending it on a long, dangling appendage below the jaw, reducing the chance that an encounter with a predator will be lethal. Other deep-sea fish, and even some shallow-water ones like the flashlight fish Photoblepharon, also have well-placed lights that surely serve as lures for unsuspecting prey (Morin et al., 1975). Photoblepharon can turn its bacterial light source on or off at will with a retractable shutter (figure 12.2C,D).
A good question is why so many planktonic creatures are devotedly phototactic, given the risks involved. It appears that the response is an unavoidable byproduct of a desirable behavior, the ability to regulate depth or to engage in other simple photobehaviors that evolved in the context of natural light fields. Planktonic larvae of crabs have jewel-like little compound eyes (figure 12.3) and show strongly positive phototaxis to bright lights. The light response is useful for determining their spectral sensitivity and thus for modeling their behavior in the field (figure 12.3). The consistently strong phototaxis suggests that they should congregate at the water’s surface during the day, but in fact they migrate downward at sunrise and only move to shallow waters again in the evening (Forward et al., 1984; reviewed in Forward, 2009). The paradox was resolved when larvae were tested for their light responses in downwelling light fields that have angular distributions like those actually occurring in natural waters (chapter 2). In a natural light field, larvae show responses that duplicate their behavior in the field (Forward, 2009). The behavior is simple but extremely flexible, in that environmental variables such as temperature, salinity, and hydrostatic pressure interact with phototaxis to generate vertical movements that are adaptive for survival in nature. One particularly impressive phototactic behavior shown by crab larvae and many other zooplankton is the shadow response. When subjected to a sudden decrease in light intensity, these animals instantly sink, initiating a strong negative phototaxis (figure 12.3). The response is adaptive for escaping predators, and in fact the strength of the response increases in water that contains the chemical odors of common predators such as ctenophores or fish. So even the supposedly simplest orientation responses to light can generate quite sophisticated behavior. In the end, though, phototaxis has its faults, most notably when it leads victims straight to the mouth of a deep-sea anglerfish.
Figure 12.2 Top two photographs are of deep-sea fish with bioluminescent lures. (A) An undescribed species of anglerfish with a luminescent lure above its mouth. (B) Bathophilus nigerrimus displaying a lure that it places on the end of a long trailing appendage below the chin. It also has a bioluminescent patch under the eye. (Photographs by E. Widder, copyright 1983, 1999 Harbor Branch Oceanographic Institution) (C,D) The flashlight fish Photoblepharon, which has a bright luminescent patch directly under the eye; this animal feeds on many forms of zooplankton, all of which are positively phototactic. (The patch is closed with a shutter in frame D.) The other bright patches are reflections from the strobe flash used to photograph the fish; they are not self-luminescent. (Photographs by J. Morin)
Figure 12.3 (A) The third zoeal stage of the larva of the crab Rhithropanopeus harrisi. (B) Phototaxis to various wavelengths of light by early-stage zoea larvae, showing two spectral sensitivity peaks near 380 and 500 nm. (C) Descent by these larvae (a mixture of negative phototaxis and passive sinking) on a sudden decrease in light intensity in clean water and in water that had contained ctenophores, a predator on larvae. Note that the responses occurred in much smaller light decreases when ctenophore odor was present. (Graphs modified from Forward, 2009)
Compasses, Landmarks, and Panoramas
Orientation Based on Celestial Cues
Beaches are dangerous places—exposed to sun and wind, inundated by tides and waves, and infested with predatory crabs, birds, and small mammals. Sandhoppers cope with these threats by foraging carefully and by burying themselves where they minimize the physical and biological threats of beach life. Moving up and down the beach perpendicular to the tide line to reach the water, their feeding region, or the best place to bury themselves, they are guided by many visual orienting cues, a major one being sun compass orientation. This a basic form of celestial orientation, useful because the positions of astronomical objects are physically determined. Having obtained the solar angle, sandhoppers innately “know” the right direction to go. When animals from beaches with different orientations are tested in the lab in sunlight, they continue to orient as if they are on the beach where they were collected, and thus head off in a direction specific to their home beach (figure 12.4). The knowledge of direction clearly is under heavy selection, not surprisingly, given that a mistake can easily be lethal. Thus, even inexperienced animals generally choose the right orientation when raised in the lab and tested away from any cues other than the sun (figure 12.4; Pardi and Scapini, 1983). See Scapini (2006) for a review of the biology of sun compass orientation in sandhoppers.
Figure 12.4 Genetic inheritance of orientation in different populations of Talitrus saltator on the west coast of Italy. (B) Circles show the directions of hopping under a natural sky, with each dot showing the orientation angle of one individual and the arrow indicating the mean direction of travel of all animals. Triangle outside the circumference of the orientation circle shows the seaward direction at the home beach of each population, also indicated in the satellite view of the region (A). Both experienced adults and naive immature animals that had been raised in the laboratory oriented in the proper direction to the sun. (Modified from Pardi and Scapini, 1983) (Image data for A: Google, DigitalGlobe, European Space Imaging)
Simple sun compass orientation will point a sandhopper in the right direction but by itself cannot send the animal to a specific location. To do this, the animal would also need to know where it is at the start, what path angle is required to reach the goal, and how far it needs to go. The zonal orientation of sandhoppers seems to be all they need—it takes them to the water for feeding and hydration and to a damp location up the beach where they can bury themselves safely out of sight. Dung-ball rolling by beetles uses many cues to maintain a bearing, including the sun when it is visible (and the Milky Way at night; chapter 11), but successive runs by individual beetles vary considerably in their orientation to the sun (or other cues) (figure 12.5). Not surprisingly, if a beetle and its ball are picked up and then placed back on the ground, the beetle takes off in about the same direction as before. On the other hand, if the beetle constructs a new dung ball after the displacement, its next path’s bearing is quite random (figure 12.5; Baird et al., 2010).
Figure 12.5 Orientations of ball-rolling dung beetles, Scarabaeus nigroaeneus, in the field, South Africa. Each small black circle shows the direction an individual beetle selected. Left panels show the orientations of individual beetles in the morning and afternoon, oriented so that the sun is at the top (indicated by the arrow) in both cases. Note that each beetle selected an apparently random direction for rolling. On the right are examples of directions selected by individual beetles after being placed back in the dung pile on the same ball (top), or removed from the previous ball and obligated to construct a new one. In these panels, the arrow indicates the direction rolled with the first dung ball. Animals continued along the same axis if continuing to roll the same ball but selected a random rolling direction after making a second ball. (After Baird et al., 2010)
Other animals orient to the sun with much more subtlety and sophistication. Honey bees use a sun compass (or the polarized-light pattern in the sky—chapter 8—as a backup when the sun is hidden in clouds) to get the reference angle to fly to their foraging patches and back home again. Knowing the angle to the sun, together with an innate knowledge of the offset angle from the solar azimuth to the goal and the use of optic flow (chapters 6 and 10) to measure distance as they fly to it (addressed later on), honeybees can travel kilometers to get to a given destination. Similarly, the monarch butterflies inhabiting the eastern parts of the United States migrate all the way to central Mexico to overwinter, getting directional information in part using a sun compass.
Birds, some of the greatest migrators on Earth, are famous for their use of both solar and star-based orientation systems (Sauer and Sauer, 1960; Emlen, 1970). Their knowledge of the nighttime sky is not innate; instead, it depends on experience with seeing a rotating field of stars. Indigo buntings, for example, will orient to any polestar when trained in a planetarium. To these animals, “north star” is defined as “the star that rotates the least” (Emlen, 1970). Their knowledge of constellations is limited (if it exists at all), but they have an innate mechanism to learn the direction to fly based on any concentric, moving pattern of stars in the sky. Birds that have never encountered a rotating sky (even if only in a planetarium) are disoriented when tested with a normal night sky, one that presents no problems to birds that have viewed the sky in motion.
Sun compass (and star compass, and moon compass) orientation is useful because the positions of astronomical objects are physically determined and fully reliable. Of course, their reliability is compromised by their apparent movements through the sky caused by the Earth’s rotation. Animals such as sandhoppers, honeybees, and monarch butterflies must possess a mechanism that compensates for the changing positions of sun or moon caused by the Earth’s rotation. Because solar (or lunar) azimuth is mostly determined by time of day, the mechanism requires (1) internal knowledge of the required bearing to the goal, (2) an internal clock, and (3) knowledge of the angle from the sun to take to achieve the desired bearing at each time. It is not difficult to establish that sandhoppers “know” the right direction to go and thus meet requirement (1)—if they are artificially entrained to a light:dark cycle that is offset from the natural cycle, they confidently head off in the predictably wrong direction when they see the real sun (see similar results below for monarch butterflies). Not surprisingly, given that the sun and moon move with independent time courses, they maintain separate internal clocks for the sun and moon compasses (Ugolini et al., 1999; Scapini, 2006). Like the sun compass clocks of sandhoppers, those of monarch butterflies also produce incorrect bearings if the monarchs are entrained to an artificial light:dark cycle that is offset from the natural one. For example, if the artificial cycle is advanced by 6 hours relative to the natural one, the real sun at noon would have moved 90° past its expected position, which is internally near its position around sunrise, at 6:00 a.m. Therefore, if the butterflies want to fly south but perceive the sun to be in the east, they head about 90° to the right of the correct bearing (figure 12.6). Most insect clocks tick away in their brains, but an odd thing about monarchs is that their sun compensation clock resides in their antennae. Thus, antennaless butterflies completely lose their sense of time (figure 12.6; Martin et al., 2009). In fact, the antennae have their own sets of photoreceptors. If they are covered with black paint, the butterflies maintain a sense of time, but their internal clock drifts away from the natural cycle of the solar day. Sandhoppers, too, have special photoreceptor sets for measuring time, different from those used for sun compass orientation; but in these little crustaceans both receptor types are in the compound eyes (Forward et al., 2009).
Being able to orient may be all that an animal needs to survive in its niche, but many animals need more. They have to reach a destination that is specific and necessary for their survival. A sun compass is a valuable tool to have in such a task, as it can give a very accurate estimate of the vector the animal must maintain during its travel. The same is true of the various orienting mechanisms based on celestial polarization patterns discussed in chapter 8, which produce accuracies every bit as good as those of direct sun (or moon) orientation. Having clock compensation makes these compasses valuable at any time of day and over any duration of travel. The azimuth of the sun’s path across the sky, and the polarization patterns it produces, are not linear functions of time. They change most slowly near sunrise and sunset, and the rate of change varies with time of year and latitude. Many animals that are superb navigators correct very accurately for these irregularities, implementing a function called the “solar ephemeris” (see Wehner and Lanfranconi, 1981). As a result, they are able to determine which way to go—and maintain that course—at any time of day or year with outstanding accuracy.
Figure 12.6 The orientations during flight outdoors near noon of intact and antennaless monarch butterflies, Danaus plexippus, before and after being subjected to a 6-hour phase delay in the light:dark cycle. Animals on the left were in their normal light cycle, whereas those on the right had been entrained to the shifted cycle. The arrows show the mean vector for each group; the longer the arrow, the stronger the orientation. Intact animals showed time compensation, treating the southward sun as if it had been in the west. Butterflies that had their antennae removed were unable to entrain to the light:dark cycle and thus unable to orient. (After Martin et al., 2009)
But navigation is more than holding an accurate course. An animal must also know where it is when it starts, what absolute course is required to reach its goal, what route it should use to get there, how far it has gone as it moves along the way, and even how to recognize its goal when it gets there. We turn to the last question first—just how does an animal come to know that it is in the right place?
Visual Landmarks
Many animals commute between their homes, nests, or hives and places that they have learned to find food. Their behavior is not so different from our commutes between the house and the grocery store. They are very familiar with the endpoints and know their environs in great detail, but the terrain along the way is largely ignored except for a few crucial waypoints. Animals such as bees and wasps make their commute by air, and in many cases the actual travel is simply a matter of distance and direction. As mentioned above, direction generally comes from the celestial compass, and we will get to distance measurements later in the chapter. At the endpoints of the trip, orientation is handled by a thorough knowledge of local landmarks. These flying insects inspect the home environment visually and make a similar inspection at the destination as they prepare to land at the food supply. This inspection involves visual scans of the locales using flight patterns that are both stereotyped and highly varied in detail. Because these scans are made on departure from home and are particularly pronounced the first time a bee leaves a newly discovered food source, they have been called “turn back and look” behavior in which the departing bee stops, reverses its direction of view to face the reward (or nest opening), and enters a very characteristic swaying flight as she examines the surrounding features (Lehrer, 1993). Among other things that she learns while “turning back and looking” are the shapes, colors, and locations of landmarks near the point of interest. But what, exactly, is a landmark?
Humans have a pretty clear idea of what makes a good landmark, but our view is not necessarily shared by other animals. Furthermore, although we mostly choose landmarks by their visiblity, many animals use chemical or auditory landmarks in their travels. Here, as visual ecologists, we restrict our focus to visual landmarks. It seems obvious that a good landmark should be prominent and distinctive, but how these properties are evaluated by various species will certainly vary. Zeil et al. (2009) suggest that good landmarks have three properties: salience, permanence, and relevance. Salience refers to visual-system-specific cues such as shape, size, or color and to good discriminability from background. Permanence (or reliability) means that the landmark should be adequately long-lasting and fixed in place. Relevance refers to a clear connection between the place of interest or a waypoint on the way to it. Short-lived animals such as many insects can clearly judge the salience of a landmark on first encountering it, but it is not clear how they evaluate other properties of a landmark without experience.
We first consider landmark learning in flying insects. To be honest, these, together with their cousins the ants, are really the only animals in which the process has been investigated in detail, simply because it is not difficult to reconstruct both the geometry of their walks or flights and their lines of sight as they make their inspections. Figure 12.7 illustrates one process of visually learning a landmark, the turn-back-and-look flights of honeybees (Lehrer, 1993). On the first departure from a newly discovered food source (the canonical one, sugar water), the bee immediately turns around—so quickly that it has happened by the first video frame. It then makes a number of up-and-down, back-and-forth (and side-to-side, not visible in this projection) flights, mostly facing the opening to the food source but also looking around the area, eventually flying home at the upper right. The second departure, after the bee has returned once for food, is followed by a reduced version of the first one, and this time the bee moves a bit further away before turning around and before completing many fewer scans. The third departure is even more cursory, with a straight flight out, a few small scans, and a flight away. Once bees are fully familiar with the target’s surroundings and appearance, they just shoot straight out of the opening on their way home, like miniature darts (figure 12.7, lower right).
Visual learning flights by wasps are much like those of bees, not a surprise considering their kinship. Solitary, ground-dwelling wasps of the genus Cerceris were encouraged to memorize carefully the nest opening each time they left home, by partially concealing the nest and thus making it difficult to find when they returned (Zeil, 1993). On the next departure, they engaged in a very specific and characteristic nestorientation behavior, somewhat reminiscent of the turn-back-and-look behavior just described for bees. The wasp walked out from the nest entrance and turned around. Then she flew backward and side to side in a series of arcs, growing steadily in angular extent and also increasing in range from the nest entrance (figure 12.8). This behavior lasted for many seconds, often more than a quarter-minute. This initial series can lead to larger circles at a greater altitude around the nest, and often around the surrounding area, before she finally departs. During the nest-inspection period, the wasp does not fixate the nest on the frontal part of the eye, but rather to the side by around 45°, probably as a compromise between seeing the nest and watching where she’s flying. When a landmark is present, she consistently faces toward it across the nest entrance (figure 12.8). All this behavior is thought to provide a series of snapshots of the view of home for comparison with the view when the wasp returns from foraging (Zeil, 1993). The snapshots could include both geometric cues and image-motion cues derived from motion parallax (see chapter 10).
Figure 12.7 “Turn-back-and-look behavior” in foraging honeybees, Apis mellifera. Bees’ positions at video-frame intervals (40 ms) are indicated by a dot for the head and a line for the body axis. Number above each illustration indicates the number of flights the bee has made to the feeding station, the hole in the center of the disk (the first visit is number 1; n means “many visits,” after the turn-back-and-look behavior phase has been completed). (After Lehrer, 1993)
Having gathered information about the view of home or of the surrounds of a feeding location, what do bees learn? It turns out that this visual data gives extremely robust information about how to find the desired destination. If a bee learns the position of a food source relative to a single landmark, this is all that is required to search in the proper location. This is seen in figure 12.9, on the left (from Cartwright and Collett, 1982). The landmark was a black cylinder, 40 cm tall, and during training the food was placed some 50 cm away. On removal of the food, the bee initiated a search centered on the position of the food, so the earlier visits clearly had given her quite precise information about its location. Most bees were able to judge the proper distance from the landmark even if its size were changed, apparently through motion parallax (Lehrer and Collett, 1994). Giving a bee three landmarks (figure 12.9, right) permits extremely precise searches—note how much sharper the search peak is in this case. Significantly, moving the three landmarks away or even changing their sizes did not disrupt the search at all—the bee consistently searched at the point where the angles from the (missing) food source to the landmark set were the same as during training. This led Cartwright and Collett (1982) to propose the “snapshot model” of visual orientation (figure 12.10): the bees store a representation of the appearances of the positions of the landmarks in memory and attempt to match their current view to the stored snapshot. Modeling experiments using the snapshot model reproduce bee-havior very well.
Figure 12.8 Nest orientation in the solitary wasp Cerceri rybyensis on leaving its nest hole (marked by a cross). Position of the wasp’s head is indicated by a dot, and its body axis by a line. Positions are indicated at intervals of 40 ms and numbered at intervals of 1 s. The position of a landmark (a cylinder 2.2 cm wide × 6.3 cm tall) is indicated by the dark circle. (After Zeil, 1993)
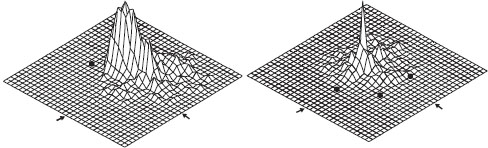
Figure 12.9 Landmark learning in honeybees. Vertical axis indicates the time spent in each square of the test area by a trained bee; black circles indicate positions of landmarks (cylinders 4 cm wide × 40 cm tall). Position where the bee has been trained to find food is at the center of the area, indicated by the arrows on two sides, but during testing no food was provided. (Based on Cartwright and Collett, 1982)
Figure 12.10 The “snapshot model” of landmark orientation in honeybees. The bee sees the indicated scene of landmarks (outer circle, 1) at position X1 and compares it to a “snapshot” in visual memory (inner circle). It then moves to position X2 in order to create a match between the viewed scene and the snapshot. (After Cartwright and Collett, 1982)
One other case of landmark orientation is particularly illuminating. The tropical bee Megalopta, which we met in chapter 11, lives in the Panamanian rainforest and does all its foraging at night. Its nests are tiny holes in twigs found among foliage under the rainforest canopy. Anyone who has attempted to walk without artificial lighting in a rainforest at night knows that this is an extraordinarily challenging situation—you literally cannot see your hand in front of your face, much less a twig with a hole in its end down in the bushes. Yet Megalopta routinely forages away from home in this level of darkness for many minutes, unfailingly returning to the nest and entering immediately. When leaving, the bee does an orientation flight similar to those described already, facing back toward the nest hole and orienting to landmarks around it (figure 12.11). Given an array of sticks, she chooses the one in the right position every time. This is a truly impressive visual feat for near-total darkness.
What makes a landmark salient? For bees, an important quality is its color. This is not so surprising in day-active honeybees (Cheng et al., 1986), which generalize learned colors when returning to a set of learned landmarks with subtly shifted hues. Now that you have heard about Megalopta, it should not be a shock to learn that a nocturnal bee, in this case an Indian carpenter bee, Xylocopa, also recognizes the colors of landmarks—in their case lit only by starlight (Somanathan et al., 2008a). This should come as no surprise; we saw in chapter 7 that nocturnal color vision, once thought to be rare, is reasonably widespread after all.
The other group of animals in which landmark orientation has been extensively studied is the ants. Taxonomic fellows of bees, adult ants have quite a different task to face when orienting. They have neither the ability to move in three dimensions nor an easy way to inspect a complex set of landmarks rapidly. Living on surfaces, and endowed with highly developed chemical senses, ants live in a world of scents and tastes. Their chemosensory abilities are less useful, however, in deserts and on hot, tropical soils because any scent cues that they might deposit are quickly degraded or vaporized. The ants that have attracted the attention of visual ecologists inhabit just such places—the flat, empty deserts of Tunisia and the brushy scrubland of the Australian outback. We now visit these arid environments to see how ants manage their travels.
Figure 12.11 Nocturnal learning and recognition of landmarks by the Panamanian bee, Megalopta genalis. (Left) A departure from the nest twig, videotaped from below, with an artificial landmark, a white square card, placed nearby. Bee’s position is indicated by a dot (head) and line (body axis) at intervals of 40 ms. (Right) Bees that have learned the position of their nest in an array of similar twigs return to the nest at the learned position, not the actual nest (marked with a star). (After Warrant et al., 2004)
Rüdiger Wehner, with many students and colleagues, has been studying vision, orientation, and navigation in the Tunisian desert ants of the genus Cataglyphis for nearly half a century. The work has revealed many unsuspected abilities in insects, making this elegant ant (figure 12.12) an invertebrate model organism second only to the honeybee for studies of its navigation biology (reviewed in Wehner, 2003). Cataglyphis is a trim, long-legged creature well adapted for keeping its body high above the hot sands of the daytime desert and traveling fast and far. In fact, it is a daytime forager, where its ability to tolerate the heat and direct sunlight lowers the risk of being picked off by a wandering predator. Nevertheless, the animal needs to make quick, wandering excursions in search of food. Once a portable morsel of food is encountered (or bitten off), the ant returns on a bee-line (ant-line?) home, even over distances exceeding 100 m, directly to the vicinity of the nest, which is only a small hole in the sand.
The task is accomplished by keeping accurate track of the vector home throughout the search by use of a compass and odometer. The compass is—not surprisingly—celestial, robustly based on the position of the sun and equally well on the skylight polarization pattern (using ultraviolet-sensitive dorsal rim polarization receptors). With it, the ant knows throughout each leg of its meandering outward journey which way it is heading. The odometer is not visual but is thought to be based on mechanical information generated while walking. Each vector during the meander is handled by a central path integrator (no small task for a 0.1-mg brain), and when the ant is ready to head home the length and solar (or polarizational) angle of the home vector are already known. The ant then runs down the vector, and if it does not find its nest at the end of the run it initiates an efficient search pattern to locate it.
Figure 12.12 Landmark orientation in the desert ant Cataglyphis bicolor, an individual of which is shown at the top left. Blue dots are paint marks used to identify individual ants. (Photo courtesy R. Wehner) Photograph at the right shows an array of artificial landmarks used to study orientation in this species in its actual habitat in Tunisia, arranged on the desert floor. (From Wehner, 2003) Graphs at the bottom show search behavior as paths (left) or time in a single grid square (right; darker indicates more time) by ants searching for food they have been trained to find midway between two landmarks. (From Möller, 2001)
The presence of any landmarks near a food item helps the ant to find it again precisely, and ants well remember the visual locations of landmarks and their sizes as seen from the position of the food (see figure 12.12, top right). Unlike bees and wasps, ants do not know the distances of landmarks from the target destination. If trained on two small cylinders, with the food source midway between them, they will search for it in the trained location (figure 12.12, bottom). However, if these small cylinders are moved further apart, the ants still recognize them as landmarks but do not know where to search for the food source. Double the distances of the landmark cylinders while simultaneously doubling their size produces a precise search back in the middle (Möller, 2001; original data from Wehner and Räber, 1979). The implication is that ants do not use motion parallax or other potential cues to measure distance, only perceived size. Perhaps this is because motion parallax generated by walking over irregular terrain is not as reliable as that available in flight. Cataglyphid ants use landmarks not only to locate the food but also as waypoints along the route to the food (Collett et al., 1992).
Mammals, of course, use landmarks (as you well know because this is probably how you find your way to the library from home). A special feature of the mammalian sense of space is that they (at least rodents, bats, and possibly primates) have a class of cells in the hippocampus of the brain called “place cells.” These fire when an animal is in a particular location in open space and could provide a way for the animal to know where it is, even in the absence of obvious external stimuli. Thus, they seem to act a little like internal landmarks. (A related cell type in the primate hippocampus is called a “spatial view cell” because these are active when an animal inspects an object in a particular absolute direction, independent of its own posture.) Place cells are at least in part fixed by visual cues. If a particular visual landmark, a “cue card,” is moved to a new location in an arena, place cells in a rat’s hippocampus reorient as if the arena rotated with it. They fire in the same relative location, with the cue card the reference for this location (figure 12.13). This is fairly strong evidence that the sense of place, at least in rodents, is established by vision.
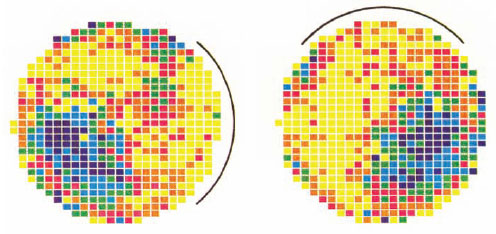
Figure 12.13 Place cells in the rat, Rattus norvegicus. Figure shows a small arena within which the rat could wander freely while the activity of a single cell in its hippocampus was monitored for spiking activity. Spiking rate is indicated by color: bluer and darker colors indicate the highest levels of activity. The arena had a white “cue card” placed along one edge (indicated by the arc), which was moved to a different location for the recording session on the right. Note how the favored locations of this place cell tracked the location of the visual cue. (After Muller and Kubie, 1987)
Panorama Orientation and Canopy Orientation
The sky is not the only source of potential compass information. The visual panorama itself is useful as long as the panorama is generated primarily by objects and terrain at a distance large enough to remain stable during locomotion, or at least during the travel between waypoints used along a route. Wood ants, Formica rufa, have a much richer visual environment than ants from deserts, and they use both local landmarks and views of the panorama to orient their trips to a food source (Graham et al., 2003). Ants were trained to find a feeder 3 m away from their starting point and were provided with a limited view of the laboratory as well as of a prominent black cylinder, which served as a landmark (figure 12.14A). Ants that found the feeder were allowed to continue training for several trials and were then tested in the same task, both with and without the landmark. With the landmark present, ants routinely headed in its direction and then diverted to the direction of the feeder (figure 12.14B). Somewhat surprisingly, even when there were no landmarks present, the ants continued to follow a path that initially directed them toward the [absent] landmark before turning back toward the feeder. Even when the landmark was placed on the “wrong” side, almost all ants continued to visit the location of the missing landmark rather than changing loyalties to the new one (figure 12.14C). The most likely explanation for the use of the dog-leg during travel is that the ants had learned to use the panorama of the laboratory for guidance, and because this was identical with and without the presence of the landmark, they could continue to orient properly throughout their familiar path (Graham et al., 2003).
Panorama use seems sensible for wood ants, but deserts like those of Tunisia often offer nothing to see in the panoramic view, especially from the height of an ant (see figure 12.12), although Cataglyphis does use local landmarks when available, as noted above. Other deserts do not offer such dire prospects. The scrub desert of central Australia is abundantly provided with visual landmarks such as gum trees, brush, and shrubs, and the Australian red honey ant Melophorus bagoti thrives there, foraging during the day in the southern summer. Like Cataglyphis, Melophorus is capable of using a celestial compass and odometer information to support a path-integration system, but with landmarks generally being convenient, it often prefers to use these for laying out regular travel routes to and from the nest (also a hole in the ground). Ken Cheng’s research group has used this tendency to inspect how ants might combine landmark information with view of the distant panorama to orient its foraging runs (Wystrach et al., 2011). After being trained to visit a feeder, ants would enthusiastically run home via a characteristic route through a string of landmarks of varying sizes and shapes provided between the feeder and the nest (figure 12.15). If the landmarks were shifted among their individual positions, the ants showed some disorientation on the homeward run, as if encountering the “wrong” landmark confused them, but still found their way home (figure 12.15). Ants even managed fairly well with no landmarks at all, suggesting that the presence of landmarks was useful to them, but views of the overall panorama were sufficient to get them headed in the right direction. Wystrach et al. (2011) concluded that the two sets of visual cues interact, and that the views of the landmarks and panorama together set up a series of visual objectives that move the ants to the nest.
Figure 12.14 Landmark learning and use in the ant Formica rufa. (A) The platform used to train the ants, showing the starting position, position of the black landmark, and location of the training feeder. (B) Tracks of a single ant trained with the landmark to the left, showing the tendency to head toward the landmark and then redirect toward the food. This behavior by trained ants continued during testing with no food and with the landmark removed (C, tracks of several trained ants). The ants continued the route they had learned when the landmark was present. (D) Even if the learned landmark was placed to the right, almost all trained ants continued to use the route they had learned initially. (After Graham et al., 2003)
In 1980, Bert Hölldobler (1980), working with the delightfully named African stink ant (Palthothyreus tarsatus), reported an entirely new form of panorama orientation that involved only the overhead features of the visual surround. These hymenopterans live in forests south of the Sahara and construct an enormous nest (up to some 25 square meters in area) with multiple entrances. Each worker ant uses only a single exit, which it must unfailingly find on return from a foraging excursion of several meters into the forest. The sky overhead is obscured by trees, and the sun (and even the polarization pattern of the sky) is seldom visible due to heavy cloud cover in these equatorial forests. Although chemical trails almost surely assist them, removing the soil from their favored routes does not disrupt their homeward orientation, even if they are randomly placed at any point along the newly scraped trail, and even if their view of all surroundings is obscured by a screen that is used to surround them as they travel. Instead, it became clear that they were fully oriented only when they could see the treescape above them. Rainforests have distinctive patterns of foliage seen against the sky (figure 12.16), and Hölldobler (1980) demonstrated that these were sufficient to produce excellent orientation in the laboratory. He termed this “canopy orientation,” and it is likely to be a common orientation mechanism for animals inhabiting forest floors.
Figure 12.15 Landmark use in the Australian desert ant Melophorus bagoti, an individual of which is shown carrying a reward of a cookie crumb in the photograph on the upper left. Ants were trained to return from a feeder to the nest (indicated by a star on the graphs) on a route (middle photograph) that involved a number of movable landmarks (“Training,” leftgraph). The trained ants rapidly threaded their way through the landmark sequence (“Trained”). When landmarks were transposed, as in the panel marked “Transposition test,” the ants were disoriented only slightly from their learned paths (far right panel). Melophorus apparently combines its views of landmarks and the visual panorama of distant objects (top right photographs, the blurred version approximates an ant’s view of the scene) to find its way through an unfamiliar sequence of landmarks. (Modified from Wystrach et al., 2011; ant photograph courtesy P. Schultheiss, others courtesy A. Wystrach)
Figure 12.16 Images of the Panamanian rainforest canopy taken at intervals along a trail through the forest. Note that each view is quite distinctive and that the sequence of views could serve for canopy orientation as a series of landmarks for a forest floor animal.
We end this section on panorama orientation with examples taken from opposite extremes of the animal kingdom. Box jellyfish, or cubozoa, have 24 eyes in four groups, of which eight (two per group) have superb (if defocused) lens optics (Nilsson et al., 2005). These simple animals have no central nervous system to which the eyes connect, only a rather diffuse nerve net serving a single nerve ring that circles the animal, with a ganglion found near each group of six eyes. Because they swim under the mangrove canopy in marine swamps, they are at risk of rapid deportation if they find themselves drifting into the main streams of mangrove channels. They use their eyes to orient effectively to the mangrove canopy, rapidly swimming toward cover whenever they are placed up to nearly 10 m from the canopy edge. This is not the finely oriented canopy orientation of stink ants, but considering the reduced nervous system present in jellyfish, it is an extraordinarily impressive orientation behavior for a simple organism.
The second example is from a vertebrate—specifically, hatchlings of the loggerhead sea turtle (Caretta caretta), when they emerge onto the sands of their natal beach. Although they hatch at night, these little creatures are at extreme risk of predation by stalking birds and roving mammals while on land. They are so motivated to get going they look more like tiny wind-up toys than living creatures, furiously racing to the water’s edge. Being oriented properly is a critical survival skill, and even though they have never seen a beach before (or even been out in the open), they normally have no problem heading in the right direction, even when the sea is not visible from their nest. The key to orientation is the elevated horizon found toward the landward direction, formed by dunes or beach vegetation. If hatchling turtles are released in an area with one side partially occluded by an elevated, dark horizon they head away in the opposite direction (figure 12.17; Salmon et al., 1992). The orientation remains true even if the sky above the artificial, raised horizon is brighter than the open and opposite one (a situation that would occur in nature if, for instance, the moon hung in the sky opposite the sea). A simple type of panorama orientation, it nevertheless gets a completely naive little turtle quickly into the relative safety of the ocean.
Figure 12.17 Seaward orientation by hatchling loggerhead turtles, Caretta caretta. The photographs at the top show hatchlings on their first emergence from the nest (left) and making their way down the beach to the ocean (right). (Photographs courtesy of J. Wyneken) When tested with a surround that was raised by 8° on one side, they oriented away from the occluded side, whether the light (indicated by a sun graphic) was toward the open side (left) or the occluded side (right). The dots show the orientations of individual turtle nestlings. The gray arrows show the average orientation in both cases. (After Salmon et al., 1992)
Visual Odometry
Above, we noted that ants are thought to use mechanical cues, most likely tied to their stepping movements, to gauge how far they have walked. If an animal uses landmarks, this is all the better because the “odometer” can be reset at each landmark, and a new compass heading and rezeroed odometer can handle the next phase of the trip. An animal like Cataglyphis does not often have the luxury of landmarks, and its path-integration system has to convert each compass bearing and odometric reading into a running calculation of the direction and distance to home—which it does with uncanny accuracy. But what about a flying animal? Surely it does not count wingbeats, running at hundreds of cycles a second? Or does it monitor its energy consumption, its “gas mileage”? The answer is more interesting, taking us back to vision and a familiar topic, optic flow.
We read in chapter 10 how honeybees and other flying animals use optic flow to monitor flight speed and to maneuver safely among obstacles. For the same reasons that make these useful insects amenable to studies of so many kinds, some already covered in this chapter, they have been the animals of choice for work on odometry in flight. The techniques follow some of the protocols that have been successful for other studies of their motion vision, flying the bees through tunnels (Srinivasan et al., 1997). The bees are first trained by placing a reward a certain distance down the tunnel, which is lined with vertical stripes, across the flight path (figure 12.18, bottom). If the reward is later removed, the bees are quite good at knowing where it was, and they will search in the vicinity of the trained distance (figure 12.18, top). That this is due to monitoring of optic flow is indicated by flying the bees in a tunnel with stripes of similar thickness and spacing but oriented parallel to the flight axis. In this situation, the bees lose almost completely their ability to estimate distance and fly back and forth throughout most of the length of the tunnel in their search (figure 12.18, top). When bees are flown in tunnels narrower than the one they were trained in, the apparent velocity of lateral optic flow is larger at the same flight speed (chapters 6, 10), and they underestimate the distance they have traveled; wider tunnels (with stripes flowing more slowly at the sides) produce overestimates (figure 12.18, middle).
Later experiments allowed the bees to fly through open air before reaching a feeder placed in a tunnel (Srinivasan et al., 2000). The bees “reported” to their nestmates how far they had flown when they performed their famous dances back at the hive. Bees that had flown to an outdoor feeder some 35 m from the hive, and with the food at the start of the tunnel (so they didn’t have to fly down it) reported that the food was far closer than when they had to fly down a tunnel for 6 m to reach food only 12 m from the hive. The bees had used the sum of all optic flow they encountered in flight to give them the distance to food. Outdoors, the optic flow is slow because it comes from far-away objects (like watching a distant mountain as you drive past it). The tunnel generates far more flow, and the bees treat this as a large distance (one wonders how fast they thought they were flying!). Srinivasan et al. (2000) could actually calculate how fast the odometer turns, based on the rate of optic flow. More recent work by Eckles et al. (2012) shows that bees that forage by flying into the forest canopy use optic flow to estimate how far up they have flown. Obviously, this is important information for a bee that has to ascend up to 40 m to find flowers.
Orientation, Navigation, and Multimodality
It is not easy to orient. Animals like sandhoppers only have to decide whether to go up or down the beach, and to do this they bring in a host of overlapping, redundant visual analyses: sky brightness, sky color, sun position, location of vegetation and trees, and even possibly polarization pattern. To this they add nonvisual cues such as beach slope, gradients of wetness, and chemicals present in the near-ocean air and in the sand. For an animal to navigate successfully is a challenge well above that of simple orientation; its only real hope of success is to bring in every possible sensory modality in a web of signal mixing and multimodal analysis. In this chapter so far, we have isolated a single sensory system, vision, in keeping with the subject of the book, but we end this chapter with an example of how vision itself is part of a multimodal sensory system, magnetoreception.
Figure 12.18 Visual odometry in honeybees trained to find food at a particular distance into a tunnel lined with vertical stripes. The food’s location is marked as “Reward” (bottom image), which was at the same distance from the entrance in all experiments. The bees were then tested in tunnels lacking food, with various diameters (middle graph). “Relative frequency” indicates the normalized proportion of bees in each test searching at the indicated distance. The top panel shows results from the control tunnel (at the bottom) and one with longitudinal stripes. In the presence of stripes parallel to the flight direction, and therefore not offering motion stimuli, the bees search throughout almost the entire length of the tunnel. (After Srinivasan et al., 1997)
Original formulations of how animals sense the Earth’s magnetic field were based on the use of internal magnets or some sort of sensory interaction involving ferromagnetic materials in the body. In some animals, this certainly could be the foundation of magnetoreception, but another hypothesis has gained ascendency in recent years, based on processes that require light to operate. The mechanism involves the interactions of excited states of molecules with earth-strength magnetic fields and is called the radical-pair hypothesis. For a discussion of how this could work, see Ritz et al. (2002). The relevance of the radical-pair mechanism to this chapter is that a reasonable way to create a radical pair involves photoexcitation. Paraphrasing Ritz et al. (2002), the system can work if it contains a fixed array of radical pairs that are functionally linked to light-sensitive pigments. Thinking of fixed arrays of photopigments leads one straight to the retina, and in the last decade or so, experimental support has been found for the notion that birds have a magnetic compass located in their retinas (Ritz et al., 2000, 2004; Mouritsen et al., 2004; Heyers et al., 2007). If a compass based on the radical-pair mechanism is in fact present in bird retinas, it appears that the photopigment involved is not opsin but is instead the nonvisual light-sensing pigment cryptochrome.
Cryptochrome is expressed in avian retinas in a class of retinal ganglion cells, and it has the required photodynamics to produce the radical pairs needed for magnetoreception. The retina is obviously itself an array, although how cryptochrome is oriented for the required directional sensitivity is not clear, and it is possible that bird head movements add to the sense of direction. Nevertheless, there is strong evidence that the cryptochrome-containing retinal ganglion cells send connections to visual centers in the brain (Heyers et al., 2007). It is quite possible that birds literally “see” their way to their migratory destinations.
With these observations, we have completed our all-too-brief tour of how vision is involved in keeping animals going in the right direction and taking them where they want to go. We now bravely proceed to an even higher-level view of visual function: the role of vision in advertising and hiding!