CHAPTER 5
Bonding
Intramolecular Bond versus Intermolecular Bonds
This chapter examines what it is that holds matter together. The “glues” that are responsible for holding atoms together with other atoms and molecules with other molecules are called bonds. Intramolecular bonds are the bonds that are found within molecules. In other words, intramolecular bonds hold atoms to other atoms. These bonds vary depending on the types of elements involved in the bonding process. Intermolecular bonds are the bonds between molecules. These bonds are what give substances their varying melting points, boiling points, and vapor pressures.
The rules that govern bonds between atoms and molecules can be quite tricky. It may be worthwhile to review the chapters involving atomic structure and the periodic table/trends before you tackle the material presented here.
Ionic Bonding
Ionic bonds are very strong bonds that are formed between a cation and an anion. The ionic bond is formed when a metal loses or transfers an electron (or electrons) to a nonmetal so that the metal and nonmetal form ions that have a full outermost principal energy level. The cations and anions thus formed then attract each other’s opposite charges. The attraction between oppositely charged particles is called an electrostatic force.
The reaction between Na and Cl to form NaCl gives a good picture of how this works. Sodium has an electron configuration of 1s22s22p63s1, whereas chlorine has a configuration of 1s22s22p63s23p5. Sodium has one valence electron that needs to be given away to achieve an octet; chlorine has seven valence electrons and needs just one more to complete its outermost principal energy level. The one valence electron in sodium is transferred to chlorine as shown in Figure 5.1.

Figure 5.1 Electron Transfer in Sodium Chloride
The sodium and chlorine ions attracted by the opposite charges form a lattice in which each sodium ion is surrounded by six chlorine ions and six chlorine ions are surrounded by six sodium ions. The lattice demonstrates why ionic compounds do not form molecules. Instead, there is a continuous pattern of chlorine and sodium ions packed together as shown in Figure 5.2.

Figure 5.2 Example of a Crystal Lattice
Sodium oxide illustrates a slightly different situation. Here sodium has one valence electron and oxygen has six valence electrons. In this case it will take two sodium atoms to give up their one valence electron each to oxygen. This completes the octets for all three atoms as shown in Figure 5.3.

Figure 5.3 Electron Transfer in Sodium Oxide
On the basis of the diagram in Figure 5.3, you can see that the formula for sodium oxide is Na2O. There is no need to worry about predicting chemical formulas at this point. For now you just need to know where the two valence electrons came from to give oxygen a full octet.
Problem:
Diagram the reaction that takes place between calcium and oxygen to form calcium oxide.
Solution: Recognizing that calcium is a metal and oxygen is a nonmetal signals that the reaction will transfer electrons and the compound formed will be ionic. Because calcium is a metal, it will lose electrons to the nonmetal, oxygen. Calcium has two valence electrons and oxygen has six. Calcium will lose both electrons to the oxygen as shown in Figure 5.4.

Figure 5.4 Electron Transfer in Calcium Oxide
The electron configurations for both atoms also help clarify the reaction that takes place (see Figure 5.5).
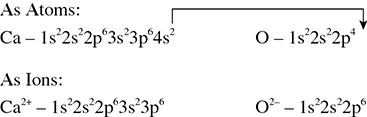
Figure 5.5 Electron Transfer and Electron Configurations for Calcium Oxide
Because it takes just one calcium atom and one oxygen atom to satisfy the octets for both atoms, the chemical formula for calcium oxide is CaO.
Covalent Bonding
Covalent bonds are formed when two nonmetal atoms share electrons in order to satisfy their need to have a full outermost principal energy level. Covalent bonds are not as strong as the bonds formed between ions. For example, it would take a high flame and a temperature of almost 800 degrees Celsius to break the bonds between the sodium and chlorine in sodium chloride. The covalent bonds found in methane can be broken instantly with the introduction of a lit match.
It is not enough to simply say that a compound has covalent bonds because there are different types of covalent bonds. One type of covalent bond is called the nonpolar covalent bond. In this case the sharing of electrons is equal between the atoms. This occurs because the electronegativities of the atoms involved are (almost) the same. For example, hydrogen gas has an equal sharing of electrons between its two atoms:
H . + . H → H:H
This diagram showing how the valence electrons interact is called a Lewis structure. In this case both hydrogen atoms have satisfied their need to have a full outermost principal energy level. Because both hydrogen atoms have the same electronegativity, the atoms will share the electrons equally. This will be the case with any diatomic molecule, such as chlorine gas (see Figure 5.6).

Figure 5.6 Electrons Shared in a Covalent Compound
Notice how each chlorine atom in Figure 5.6 has eight electrons around it. Also, notice the shared pair of electrons between the two atoms.
Because the hydrogen and chlorine atoms share a common pair of electrons, the two “dots” can be replaced with a “dash” to represent that a bond has been made. The valence dot diagrams can be rewritten as: H—H and Cl—Cl. The bond that is represented by the “dash” is called a single bond because there is one pair of electrons being shared between the two atoms.
The structure of diatomic nitrogen tells a different story. When you put two atoms of nitrogen next to each other, you see that each atom has three single electrons that want to pair up as shown in Figure 5.7.
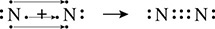
Figure 5.7 Electrons Shared to Make a Triple Bond
The two nitrogen atoms share six electrons or three pairs of electrons. This means that there are three bonds between the two nitrogen atoms, N ≡ N. This is called a triple bond.
The other case to be examined is one that involves double bonds. Carbon dioxide has two double bonds that form as shown in Figure 5.8.
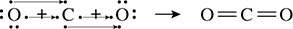
Figure 5.8 Electrons Shared to Make a Double Bond
The bonds found between carbon and oxygen raise a new issue regarding bonding. Because the electronegativities for carbon and oxygen are different (they differ by 0.5 to 1.7), the bond is called a polar covalent bond. The polar covalent bond is characterized by the atoms having an unequal sharing of electrons. Because the negatively charged electrons spend more time with the more-electronegative element, the more-electronegative element will experience a negative charge, hence the reason it is called electronegativity. Hydrogen chloride has a polar covalent bond between the hydrogen and chlorine atoms. The buildup of negative charge on the more-electronegative chlorine can be shown with the use of a dipole arrow as in Figure 5.9.
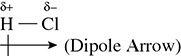
Figure 5.9 The Polar Bond of HCl
Because the hydrogen atom sees its electrons being attracted to the chlorine atom, the hydrogen atom experiences a positive charge as its one negative charge spends more time with chlorine.
Problem:
Draw the Lewis structure for formaldehyde, CH2O. Which bonds are going to be polar covalent? Nonpolar covalent?
Solution: Because carbon has the greatest number of single valence electrons it will be the atom that is placed in the middle of the molecule. The two hydrogen atoms will make single bonds with the carbon atom as shown in Figure 5.10.
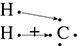
Figure 5.10 Hydrogen Bonds to Carbon
The remaining two unpaired electrons on the carbon atom will bond with the two unpaired electrons found around the oxygen atom as shown in Figure 5.11.
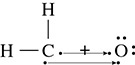
Figure 5.11 Carbon and Oxygen Form a Double Bond
The final structure is shown in Figure 5.12. Because there is little difference between the electronegativities between hydrogen and carbon, the bond between the two is nonpolar covalent. Because the difference in electronegativities between oxygen and carbon is greater, the bond between the two atoms will be polar covalent. In general, a bond is nonpolar covalent if the electronegativity difference between the atoms is 0 to 0.4. If the difference is 0.5 to 1.7, then the bond is polar covalent.
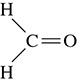
Figure 5.12 Formaldehyde
Sigma and Pi Bonds
The formation of single, double, and triple bonds in a molecule depends upon the types of hybridized orbitals that are sharing electrons. For example, when two hydrogen atoms bond to form H2(g), there is an overlap of s orbitals as shown in Figure 5.13.
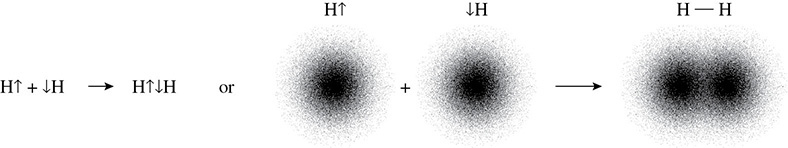
Figure 5.13 s Orbital Overlap
This overlap is what allows the hydrogen atoms to form a single bond. The first bond that forms between two atoms is called a sigma bond (σ). The sigma bond arises from the overlap of two s orbitals or from the overlap of one s and one p orbital, or from the overlap of two p orbitals. The bonds in a molecule of methane (Figure 5.14) are an example of a situation in which hybridized p orbitals overlap with an s orbital.
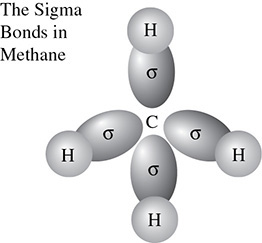
Figure 5.14 The Formation of Sigma Bonds in Methane
A pi bond is the second bond that is formed when two sp2 hybridized atoms have orbitals that overlap. The first bond that is made is from the joining of two sp2 hybridized orbitals. The second bond that is formed, the pi bond, is the result of the p orbitals in the y axis overlapping as the atoms get close enough to do so. Because the p orbitals that lie in the y axis need to be close enough to bond, a double bond is shorter than a single bond. However, the double bond is stronger than a single bond. The overlap is shown in Figure 5.15.
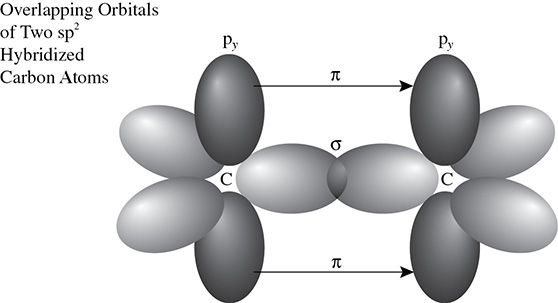
Figure 5.15 The Formation of a Pi Bond
Finally, there is the case for sp3 hybridization and the formation of a second pi bond. The second pi bond is the result of the overlap of the p orbitals in the z axis. Because a sigma bond is formed in the x axis and two pi bonds are formed in the y and z axes, a triple bond is formed. The triple bond will be shorter than the double bond and the triple bond will be stronger than the double bond as well. (See Figure 5.16.)
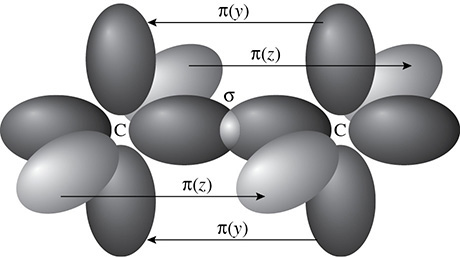
Figure 5.16 Two Pi Bonds Form to Make a Triple Bond
Network, Coordinate Covalent, and Metallic Bonds
There is another important type of covalent bonding besides the nonpolar and polar covalent bonds just discussed. An example is shown in Figure 5.17.
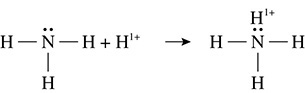
Figure 5.17 The Formation of a Coordinate Covalent Bond
In this example the free pair of electrons that is located on the nitrogen atom donates two electrons toward the bond that is formed with the hydrogen ion. Normally when a covalent bond is formed, one electron comes from each of the atoms that are bonding. In this case, the hydrogen ion did not donate any electrons toward this bond. When one atom donates both electrons in the covalent bond the bond is called a coordinate covalent bond.
Water provides another example of a coordinate covalent bond as shown in Figure 5.18.
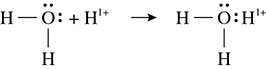
Figure 5.18 The Formation of a Coordinate Covalent Bond
In every example seen so far the covalent bonds have held atoms together in order to make molecules. However, there exist substances such as diamond and graphite where the carbon atoms are covalently bonded but do not bond to form molecules. Such cases are called network solids; the atoms bond to each other in a continuous network. The large network gives these solids a very high melting point. Also note that because both diamond and graphite are made up of the same element and are different substances, they are labeled allotropes of each other.
Finally, there is the metallic bond that occurs between metals. The atoms of metals hold onto their electrons very loosely, which is why metals conduct electricity so well. The loosely bound electrons are often referred to as the “sea of electrons.” The darker circles in Figure 5.19 represent the electron clouds of the metal atom.

Figure 5.19 The Electron Clouds of a Metal
The chart below summarizes the intramolecular bonds just studied.
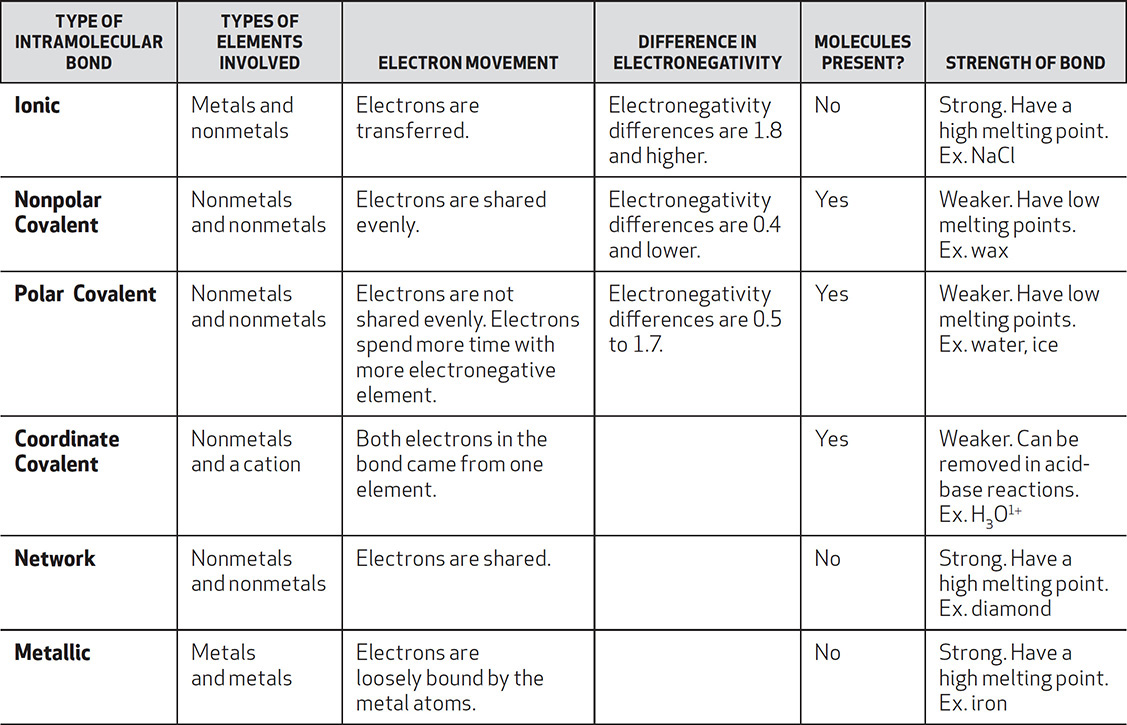
Problem:
Name the type of bonding found between the atoms in KCl, H3O1+, CCl4, SiO2, Fe(s), F2, and HBr.
Solution: KCl has a metal and a nonmetal ion attracted to one another, and it will be ionic. H3O1+ has polar covalent bonds and one coordinate covalent bond. The bond between C and Cl will be polar covalent because of the difference in electronegativities. SiO2 is sand and is a network solid. A sample of iron will have metallic bonds because only metal atoms are present. Fluorine is diatomic and will have nonpolar covalent bonds. HBr will have a polar covalent bond because of the great difference in electronegativity between these two nonmetals.
Dipole Forces and Polarities of Molecules
Because bonds can be polar and molecules can have certain shapes, electrons can “build up” on one side of a molecule and make one end carry a slight negative charge. When a molecule has this type of “buildup” of negative charge on one side and a positive charge on another side, the molecule is said to be a dipole. This is the case with HCl as shown in Figure 5.20.
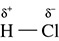
Figure 5.20 The Polar Bonds of HCl
In a molecule of HCl, not only are the bonds polar covalent, but because the electrons spend more time with chlorine than hydrogen, the chlorine end of the molecule has a negative charge on it. HCl is a dipole, or polar, molecule because the differences in electronegativity have created the “two poles.” Referring to the dipole arrow, there is no counterbalance of charges in this molecule and it is classified as polar.
A similar situation exists with water. In a water molecule the bonds are polar covalent because of the big difference in electronegativity between hydrogen and oxygen. Because of the bent molecular geometry of a water molecule, the dipole arrows cannot counterbalance. There is an overall dipole moment in the molecule as shown in Figure 5.21.

Figure 5.21 The Dipole Moment of Water
Reexamining the shape and polar bonds found in ammonia shows a situation similar to that of water. Because there is no symmetry in the molecule of ammonia, there is no counterbalance of forces and there is an overall dipole moment in a molecule of ammonia as shown in Figure 5.22.
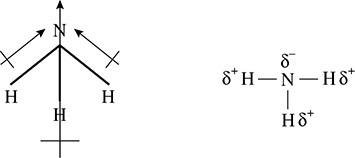
Figure 5.22 The Dipole Moment of Ammonia
Carbon dioxide and carbon tetrachloride tell a different story about polar bonds and overall dipole moment. Both carbon dioxide and carbon tetrachloride have polar bonds, as diagrammed by the dipole arrows shown in Figure 5.23.
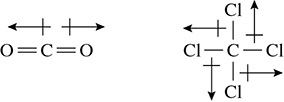
Figure 5.23 Nonpolar Molecules
In these two cases the dipole arrows cancel each other out because of the shape of the molecules. The linear shape of the molecule of carbon dioxide puts the dipole arrows in opposite directions to counterbalance each other. The same holds true for the tetrahedral molecular geometry found in carbon tetrachloride. Despite having polar bonds, these two molecules are nonpolar. There is no overall dipole moment in these molecules because the dipole arrows are of the same magnitude but lie in opposite directions in the molecule. This counterbalance causes the molecule to be nonpolar.
Resonance Structures
Resonance structures form when a substance has delocalized electrons that can shift within the substance. This allows for multiple Lewis Structures to be drawn. Some examples are the nitrate ion, ozone, and the carbonate ion as shown below.
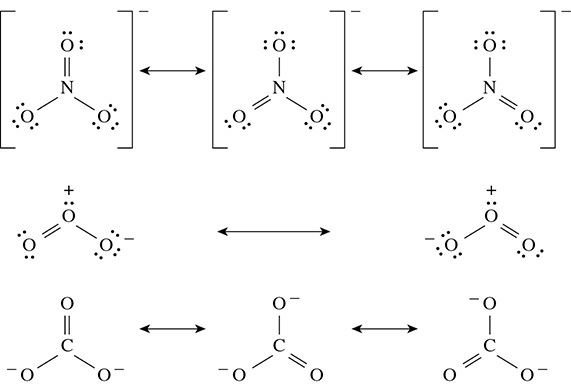
VSEPR Theory
VSEPR Theory can explain where the electrons are around the central atom of a molecule and the shape that the molecule takes on when it has a certain number of atoms bonded to the central atom. This can be summarized with the following chart:

Hydrogen Bonding
Hydrogen bonding is a weak force that comes about when hydrogen is bonded to fluorine, oxygen, or nitrogen. A good mnemonic device to use is “We heard about hydrogen bonding on the FON (phone).” When hydrogen is bonded to fluorine, oxygen, or nitrogen, the hydrogen will form a weak hydrogen bond with a neighboring fluorine, oxygen, or nitrogen atom. The dashed line in Figure 5.24 shows the hydrogen bonds being formed between the hydrogen and oxygen atoms in water.
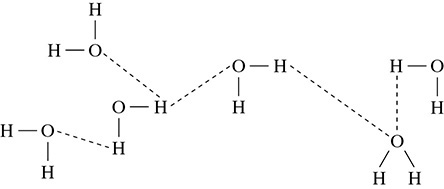
Figure 5.24 Hydrogen Bonding in Water
One important application of hydrogen bonding lies in our genetic code—DNA. In a molecule of DNA, two strands are held side by side at the nitrogen bases. It is hydrogen bonding that holds the nitrogen base of one strand to a nitrogen base of the second strand. Hydrogen bonds are strong enough in their greater numbers to hold the strands side by side and help DNA create its double helix structure. However, when DNA replicates, the hydrogen bonds are weak enough to be broken so that each strand can be replicated individually. (See Figure 5.25.)
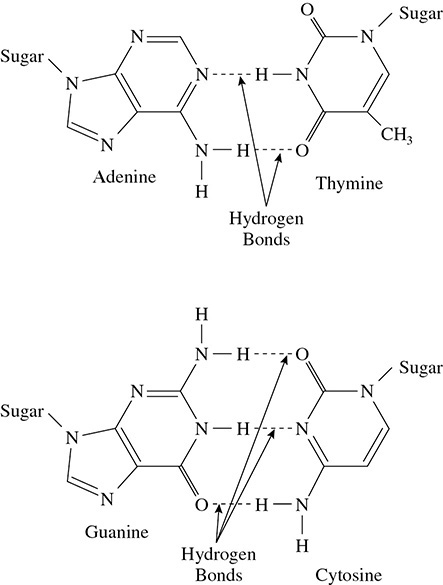
Figure 5.25 Hydrogen Bonding in DNA
Hydrogen bonds are also what give water an unusually high boiling point. Heavier molecules like H2S, which do not exhibit hydrogen bonds, should have a higher boiling point than the much lighter water molecule. But despite being lighter, water has the higher boiling point because it exhibits hydrogen bonding and hydrogen sulfide does not. (See Figure 5.26.)
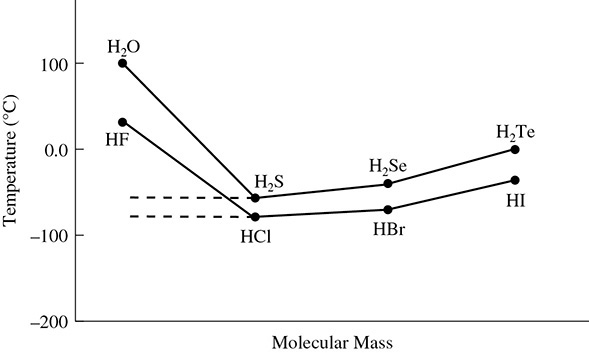
Figure 5.26 Difference in Boiling Points of H2O and H2S
THINK ABOUT THIS 
Van der Waals (London Dispersion) Forces
Even though nonpolar molecules are not thought of as having any attraction between them, there exists one very weak, temporary attraction called the Van der Waals force. You will often hear this force called the dispersion force as well. This force is a temporary force that comes about from the possibility of the electrons moving randomly and creating an uneven charge around the atom. The most important thing to know about the Van der Waals force is that, besides existing between nonpolar molecules, the force becomes stronger as the atomic masses of the nonpolar molecules become greater. This explains why the nonpolar diatomic molecular halogens exist in three different phases.

As the atomic mass increases, so do the Van der Waals forces between the molecules. This causes the molecules to be held together more tightly as the atomic masses increase. Iodine, the heaviest of the halogens listed, has the greatest mass and the greatest Van der Waals forces and exists as a solid.
Problem:
Of these three nonpolar substances, C8H18, C20H42, and CH4, one of them is a solid, one is a liquid, and one is a gas at STP. Which is which?
Solution: C20H42 with the greatest mass of the three is expected to be the solid. CH4 is the lightest in mass of the three and is expected to be a gas. C8H18 by process of elimination is the liquid. Just to let the cat out of the bag, C8H18 is called octane and it is the liquid that goes into the gas tanks of cars. C20H42 is called wax and exists as a solid. CH4 is methane gas.
Molecule-Ion Attraction
When salts are placed in water, they dissolve (some more than others) and form ions. After a soluble salt has been dissolved in water there is going to be an attraction between the charged ions and the water molecules that are polar. This is called the molecule-ion attraction. Because of these forces of attraction, the hydrated ions in solution cause the water molecules to orient themselves in a particular fashion. The oxygen portion of the water molecule has a negative charge because of its high electronegativity. This negative part of the water molecule will be attracted to the cations in solution. Because the hydrogen atoms in water have a slight positive charge, they will orient themselves toward the anions in solution. Such is the case when NaCl is dissolved in water as shown in Figure 5.27.
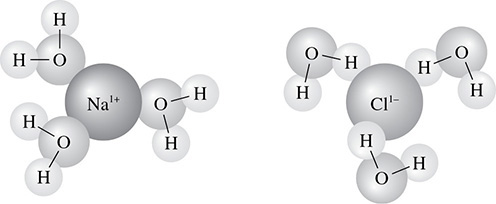
Figure 5.27 Molecule-Ion Attraction
Problem:
What type of bonding is found between the following molecules and atoms: F2(g), Ne(g), KBr(aq), H2O(l), NH3(l), and CH4(g)?
Solution: Fluorine gas is a nonpolar gas and will experience Van der Waals forces. Ne is a noble gas and will experience Van der Waals forces as well. KBr(aq) is an ionic compound that has been dissolved in water. The forces of attraction between the ions and water molecules are molecule-ion forces. Water and ammonia molecules will experience both dipole forces and hydrogen bonding. Finally, because methane gas is a nonpolar gas it will experience Van der Waals forces between the molecules.
This chart provides a handy summary of intermolecular bonding.

Naming Compounds
The ability to name compounds and determine the chemical formula for a compound comes from the ability to distinguish between ionic and covalent compounds. The name of a compound depends heavily on the type of bond present between the atoms. Besides being able to identify certain types of bonds, when learning to name compounds it is best to remember the rules that apply to the type of bond in question. The rules for naming four common kinds of compounds are outlined below.

Problem:
Name the following: S2−, N3−, CaF2, K2S, NaOH, Na2SO4, SO3, and CCl4.
Solution: The first two ions are single atom anions and are called sulfide and nitride. The next two are binary ionic compounds, calcium fluoride and potassium sulfide. The polyatomic ions hydroxide and sulfate are present in sodium hydroxide and sodium sulfate. Finally, the last two compounds are covalently bonded and are called sulfur trioxide and carbon tetrachloride.
Determining Chemical Formulas
This chapter has examined different ways for atoms to bond together. For certain problems it was noted that more than one atom of an element was needed to help complete the octet of another. Determining the chemical formula of a compound by putting together a Lewis structure is a tedious, laborious method. The crisscross method is a faster method to use provided that you understand how to find the charge of an ion. With the crisscross method, the number in the charge of one element becomes the number in the subscript in the other element. Here’s how this works in determining the chemical formula for magnesium chloride. First, determine the charges of the ions present in Mg2+ and Cl1−. Second, exchange the numbers in the ionic charge so that they become subscripts as in Figure 5.28.

Figure 5.28 Using the Crisscross Method
Finally, use the lowest ratio of subscripts if the compound is ionic, MgCl2.
The crisscross method also works for compounds containing polyatomic ions. One note of caution, however: when there are multiple units of a polyatomic ion present, you must use parentheses to indicate this fact. Using this note of caution you can determine the chemical formula for calcium phosphate. Calcium, a group 2 metal, will take on a charge of 2+, and using the reference tables you see that phosphate has a 3+ charge. So you have Ca2+ and PO43−. Next, crisscross the numbers in the charges as in Figure 5.29.
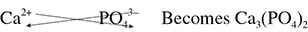
Figure 5.29 Using the Crisscross Method
Notice how the charges are no longer written in the formula. Also take note of the parentheses used around the polyatomic phosphate ion.
Problem:
Give the chemical formulas for ammonium sulfate and potassium dichromate.
Solution: Ammonium ion NH41+ and sulfate ion SO42− can crisscross to become (NH4)2SO4. Again, emphasis is placed on the use of parentheses. Potassium forms a 1+ ion and dichromate is Cr2O72−. After using the crisscross method the formula becomes K2Cr2O7.
Stock Method
The stock method for naming compounds helps clarify the names of compounds that contain the transition elements. For example, there are two types of iron chlorides, FeCl2 and FeCl3. How can you distinguish one from the other? In FeCl2 the iron has an ionic charge of 2+, whereas in FeCl3 the iron has an ionic charge of 3+. A Roman numeral in parentheses indicates the charge present on the cation. Therefore, FeCl2 is called iron(II) chloride and FeCl3 is called iron(III) chloride. After seeing the charge of the cation you can then apply the rules for the crisscross method.
Problem:
What are the chemical formulas for tin(IV) fluoride and lead(IV) oxide?
Solution: Using the crisscross method, tin(IV) fluoride will be SnF4. When using the crisscross method for lead(IV) oxide, initially the formula will look like Pb2O4 but remembering that all ionic compounds are written in the lowest ratios, the real formula is PbO2.
REVIEW QUESTIONS
1. Which substance has a polar covalent bond between its atoms?
(A) K3N
(B) Ca3N2
(C) NaCl
(D) F2
(E) NH3
2. Which kinds of bonding can be found in a sample of H2O(l)?
(A) Hydrogen bonds only
(B) Nonpolar covalent bonds only
(C) Ionic and nonpolar hydrogen bonds
(D) Both polar covalent and hydrogen bonds
(E) Metallic and ionic bonds
3. When an ionic compound is dissolved in water, the ions in solution can best be described as
(A) hydrated molecules only
(B) dehydrated ions and molecules
(C) both hydrated molecules and hydrated ions
(D) neither hydrated ions nor hydrated molecules
(E) hydrated ions only
4. Which substance represents a molecule that can combine with a proton (H1+)?
(A) NH3
(B) Na1+
(C) HCl
(D) H3O1+
(E) H
5. Which compound contains no ionic character?
(A) NH4Cl
(B) CaO
(C) K2O
(D) Li2O
(E) CO
6. The forces of attraction that exist between nonpolar molecules are called
(A) Van der Waals/dispersion forces
(B) ionic bonds
(C) covalent bonds
(D) electrovalent bonds
(E) metallic bonds
7. Which substance is a network solid?
(A) Li2O
(B) SiO2
(C) H2O
(D) CO2
(E) NaCl
8. Which molecule is a polar molecule?
(A) N2
(B) H2O
(C) CH4
(D) CO2
(E) KCl
9. Which is the chemical formula for iron(III) sulfate?
(A) Fe2SO4
(B) Fe3SO4
(C) Fe(SO4)3
(D) Fe2(SO4)3
(E) Fe2S3
10. In which of the following compounds are hydrogen bonds between molecules the strongest?
(A) HF
(B) HCl
(C) HBr
(D) HI
(E) HAt
11. When a salt dissolves in water, the water molecules are attracted by ions in solution. This attraction is called
(A) atom-atom
(B) molecule-molecule
(C) molecule-ion
(D) ion-ion
(E) atom-ion
12. Which element is expected to have a “sea” of electrons?
(A) hydrogen
(B) nitrogen
(C) cobalt
(D) chlorine
(E) oceanium
13. In which of the following liquids are the Van der Waals forces of attraction between the molecules weakest?
(A) Xe
(B) Kr
(C) Ar
(D) Ne
(E) He
14. Which molecule has both nonpolar intramolecular and nonpolar intermolecular bonds?
(A) CCl4
(B) CO
(C) HF
(D) HCl
(E) F2
15. The name of the compound MgBr2 is
(A) manganese bromite
(B) manganese bromide
(C) magnesium bromite
(D) magnesium bromide
(E) magnesium dibromide
16. The anion S2− is called
(A) sulfide
(B) sulfite
(C) sulphorus
(D) sulfuron
(E) sulfate
17. The compound PF5 is called
(A) monophorofluoride
(B) phosphorus pentafluoride
(C) pentaphosphoro fluoride
(D) phosphorus tetrafluoride
(E) potassium pentafluoride
18. Element X forms the compounds XCl3 and X2O3. Element X would most likely belong to the group called
(A) alkali metals
(B) alkaline earth metals
(C) group 13
(D) halogens
(E) noble gases
19. A nonmetal (X) reacts with a metal (M) to give the formula of M2X. Which pairing below is most like elements represented by M and X?
(A) M = Ca and X = N
(B) M = Li and X = S
(C) M = Si and X = O
(D) M = Rb and X = F
(E) M = Mg and X = Cl
20. How many sigma and pi bonds are found in the following molecule?
H—C≡C—CH2—CH2—CH═CH2
(A) There are 3 pi bonds and 13 sigma bonds.
(B) There are 12 sigma bonds and 5 pi bonds.
(C) There are 12 sigma bonds and 2 pi bonds.
(D) There are 2 pi bonds and 4 sigma bonds.
(E) There are 8 sigma bonds and 2 pi bonds.
ANSWERS AND EXPLANATIONS
1. (E) The first three compounds are ionic. The bonds in ammonia are polar because of the big difference in electronegativity between the nitrogen and the hydrogen atoms.
2. (D) Within a water molecule the bonds are polar because of the big difference in electronegativity between the oxygen and hydrogen. Between water molecules you can expect hydrogen bonding. Also, water molecules are polar and will attract other polar molecules.
3. (E) When an ionic compound dissolves in water, the ions will be surrounded by water molecules, making them hydrated ions.
4. (A) While choice D shows a compound that already has a coordinate covalent bond, the hydronium ion cannot combine any further with a hydrogen ion. However, ammonia has a free pair of electrons on the nitrogen that can combine with a hydrogen ion to form a coordinate covalent bond.
5. (E) Both choices A and E have substances that are made of all nonmetals. This makes them look as if both are covalent substances. However, because choice A contains an ammonium polyatomic ion bonded to a chloride ion, choice E is the only covalent compound—a compound with no ionic character.
6. (A) Van der Waals/dispersion forces explains the weak attraction between nonpolar molecules. As these nonpolar molecules get heavier, the dispersion forces become stronger.
7. (B) Silicon dioxide is a network solid whereas the other substances are either ionic or covalent in nature.
8. (B) Water is a polar molecule that contains polar covalent bonds.
9. (D) The iron has a plus three charge as indicated by the roman numeral, III. Sulfate ions have a negative two charge. When the crisscross method is applied, it takes two iron ions to combine with three sulfate ions to balance the charges of 6+ and 6-.
10. (A) Hydrogen bonding is strongest when the hydrogen is bonded to F, O, or N.
11. (C) The attraction between an ion and the water molecules it is dissolved in is called a molecule-ion attraction.
12. (C) The electrons are mobile in a sample of a metal. Cobalt is the only metal present in the choices.
13. (E) Helium, like the other choices, will have Van der Waals forces. However, they will be weak as helium is the lightest molecule present.
14. (E) Fluorine, like any diatomic molecule, will be completely nonpolar because there is no difference in electronegativity in the atoms that are bonded.
15. (D) Mg is magnesium, not manganese. The bromine ion is called “bromide.” Because this is an ionic compound, we do not use any prefixes.
16. (A) A single atom ion that is an anion must end in “ide.” Sulfide is the correct choice.
17. (B) This is a covalent compound and we need to use prefixes. The compound is phosphorus pentafluoride.
18. (C) Knowing that both compounds used the crisscross method to determine the formula, the cation must have a charge of plus three. This indicates that the element was in Group 13.
19. (B) It takes the charge of two M’s to balance the charge of one X. This will work when a Group 1 metal (1+) reacts with a Group 16 element (2–). Lithium and sulfur are elements in these groups.
20. (A) Keep in mind that there are some bonds drawn out and some that are not. In this molecule there are three pi bonds as the first bond between the atoms is sigma and the second and third bonds are classified as pi. All of the C-H bonds are sigma and the first C-C bonds are sigma as well. It is the second and third bonds between the carbon atoms that makes them pi in nature.