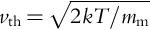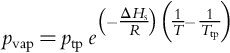Volatiles: Origin and Transport
Abstract
It may seem counterintuitive that volatiles are of critical importance to understanding the surfaces of airless bodies. By their nature, volatiles find the unprotected surfaces of such bodies to be unfriendly places. However, even relatively small quantities of these substances can have a major effect on surfaces. For some of these bodies, volatiles comprise a surprisingly high fraction of the material content. The delivery, transport, retention, and loss of these volatiles have connections to space weathering, surface roughness, material cohesion, and more. Our understanding of the volatile budgets of airless worlds has implications for our basic understanding of the solar system and its evolution, as well as the characteristics of extrasolar systems. The nature of future space exploration and resource management is directly tied to these issues.
Keywords
Volatiles; Sublimation; Ice; Permanently shadowed regions; Hollows
It may seem counterintuitive that volatiles are of critical importance to understanding the surfaces of airless bodies. By their nature, volatiles find the unprotected surfaces of such bodies to be unfriendly places. However, even relatively small quantities of these substances can have a major effect on surfaces. For some of these bodies, volatiles comprise a surprisingly high fraction of the material content. The delivery, transport, retention, and loss of these volatiles have connections to space weathering, surface roughness, material cohesion, and more. Our understanding of the volatile budgets of airless worlds has implications for our basic understanding of the solar system and its evolution, as well as the characteristics of extrasolar systems. The nature of future space exploration and resource management is directly tied to these issues.
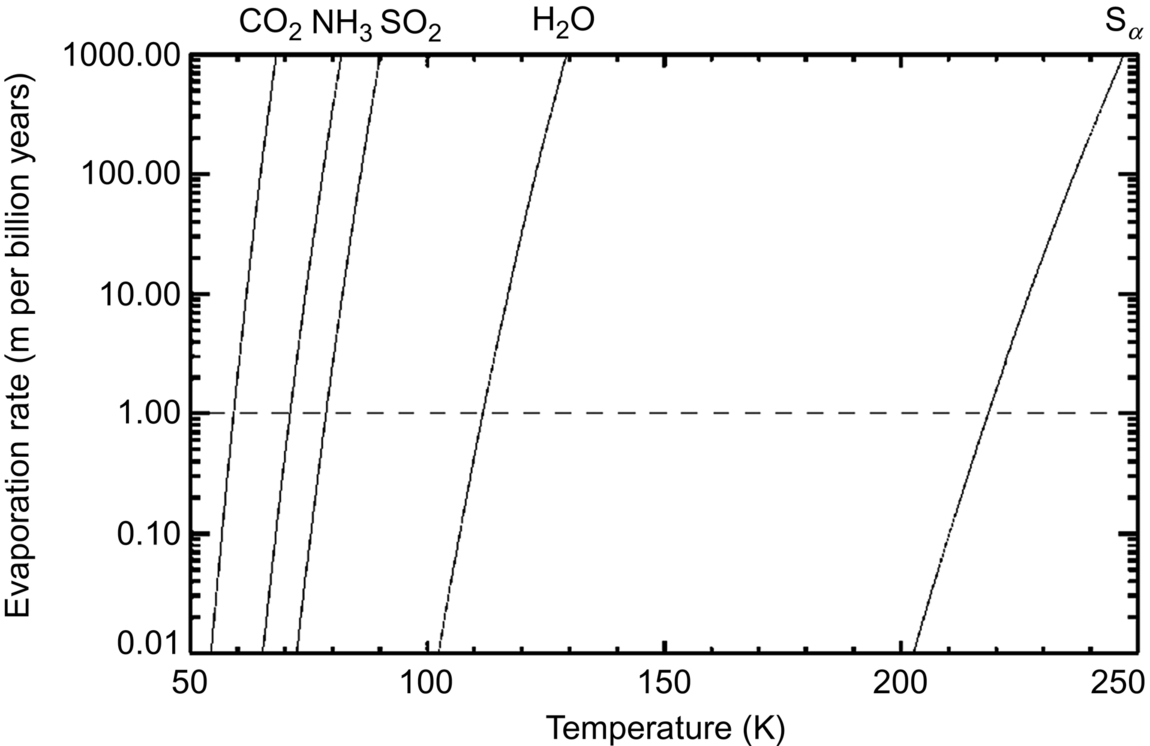
As a brief overview, we will note at the start that at temperatures above ~ 110–120 K, water ice is unstable in a vacuum. Survival of ices like carbon dioxide and nitrogen requires still colder temperatures (Fig. 10.1, from Vasavada et al., 1999). Water bound into minerals can survive at much higher temperatures in a vacuum and is by and large stable on airless body surfaces (though we discuss (3200) Phaethon and objects with very small perihelion distances in Chapter 11), and we know many asteroids with such minerals at their surfaces. The Moon, Mercury, and some asteroid classes have compositions that are naturally anhydrous, but may have volatiles delivered or experience reactions that create them. For those objects, volatile molecules that are at locations that are too hot to retain them will begin a series of ballistic hops—because these objects are airless, they are much, much more likely to encounter the surface than another molecule. Broadly speaking, they will continue to hop until they are moving fast enough to escape the object, are disassociated by solar UV, or reach a location where the temperature is sufficiently low for them to remain. These locations are termed “cold traps.” Cold traps can be temporary (for instance, a small area just past the dawn terminator that will soon heat up) or very long lasting—craters near the lunar and mercurian poles never see direct sunlight and are called “permanently shadowed regions” or PSRs. Given the nature of PSRs, we expect significant accumulation of volatiles in those areas.
Definitions
We define volatiles in this chapter as those chemical elements and compounds that are not stable in a vacuum at typical airless body temperatures in the inner solar system. “Typical temperature” is relative for a given situation, but for the most part these are substances that are found in the terrestrial environment as gasses and liquids (setting aside substances such as lead that are volatile in some situations). Examples of volatiles for our purposes are water, carbon dioxide, molecular nitrogen, molecular hydrogen, ammonia, sulfur dioxide, methane, and the noble gasses such as argon. Water is of specific interest given its potential effects on space weathering, exospheres, mineral hydration, etc., and it is an important resource for future space explorers.
Origin of Volatiles: Where Does an Airless Body Get Volatiles?
The sources of volatiles for airless bodies can be separated into two general categories—endogenic and exogenic. The endogenic sources are those that are native to the body itself and are potentially left over from its formation. These may be sequestered in fluid inclusions or tied to hydrated minerals. They also may be found underground, and can be liberated and outgassed by various heating events. Exogenic sources are those that deliver volatiles to the airless body well after its initial formation, these might include solar wind particles or cometary ice.
The Accretion and Evolution of Endogenic Volatiles
Volatiles were abundant in the nebula where our Solar System formed, if evidenced only by the gas and ice giants we have in our system today. But most of the volatile elements (and subsequent compounds) are also cosmically highly abundant, so their incorporation into the solar nebula is no surprise.
Table 10.1 lists the 10 most abundant elements in the solar system, with their abundances relative to silicon listed. The Big Bang produced the first elements, including a massive amount of H, lesser amount of He, and small amounts of B and Li. Subsequently, these materials were gravitationally gathered into stars, which produced all heavier elements through stellar fusion, supernova explosions, black hole interactions, etc. We see from the table that the elements and constituents of volatile molecules are highly abundant, and expected to dominate in protostellar nebulae. Of note are the high abundances of both hydrogen and oxygen, making the common presence of water in the solar system, in locations where ice is stable, no surprise. Carbon, nitrogen, and sulfur compounds would also be expected from these abundances, particularly in compounds with hydrogen and/or oxygen.
Table 10.1
| Element | Ab. Rel Si |
|---|---|
| Hydrogen | 28,000 |
| Helium | 2700 |
| Oxygen | 24 |
| Carbon | 10.0 |
| Nitrogen | 3.1 |
| Neon | 3.0 |
| Magnesium | 1.1 |
| Silicon | 1 |
| Iron | 0.9 |
| Sulfur | 0.52 |
The most common elements are not typically found in rocks, other than oxygen, one of the building blocks of silicate minerals. The most common elements are found in volatile ices like water ice, carbon monoxide and dioxide, ammonia, methane, etc.
We expect volatiles to be less abundant closer to the furnace that is our Sun, since these would be unstable or driven off by evaporation, vaporization, etc. However, we think even the inner planets, including the airless bodies of the inner solar system, had some amount of volatiles available when they formed.
The “snow line” (also “ice line”) is the distance from the Sun at which the conditions in the solar nebula would have allowed water ice to condense. The concept of a snow line can be applied to any of the substances we discuss here—the “carbon dioxide snow line” was always at a further distance than the water ice line, and the “nitrogen snow line” further still. We see abundant amounts of water and other volatiles beyond the ice line (at about the current distance of Jupiter) and we see much less inside that line (the rocky inner planets) (Fig. 10.2). However, once ice grains condensed beyond the snow line, they may have migrated inward toward the Sun through gas drag (Cyr et al., 1998). In addition, new dynamical models (such as the Nice Model and Grand Tack: O’Brien et al., 2014; Morbidelli et al., 2015) suggest a great deal more mixing throughout the solar system, on both small and large scales, than was previously considered, an idea supported by the range of materials returned by the Stardust comet coma sample return mission. As noted in Chapter 3, these models suggest that volatiles, especially ice, may have been delivered to the inner solar system by water-rich planetesimals early in solar system history. These models further suggest that the bulk of mass in today's asteroid belt may have formed in the giant planet region (Walsh et al., 2012).

The Earth, although inside of the snow line, stands as an example of a water-rich rocky world. Some bodies like the Moon suffered some form of major event or process (differentiation, giant impact, etc.) that drove many of the original volatiles out of the planet, leaving behind what were long considered to be utterly “dry” worlds. (See Chapter 3 for discussion of discovery of water on the Moon, and the following paragraphs for discussion of lunar volatiles.) Our paradigm has shifted. Now we know that even the “dry” airless bodies can have ample volatiles incorporated in their surfaces in places, and that such substances play a key role in the evolution of those surfaces.
Those volatiles original to the body that still remain can be liberated from deep within by impacts and by volcanic activity. Impacts are happening all the time on airless bodies (Chapter 7), so this process is always occurring and available to bring volatiles up from the depths, as well as to, conversely, degas surface volatiles. For many bodies, any meaningful volcanic activity happened billions of years in the past. But even on the Moon there is some suggestion that may still be enough volcanic activity to locally degas volatiles. Some features on the Moon have exceptionally low crater densities, and odd morphologies. Such areas might be the sites of very recent (~ 10 Ma age) volatile degassing. If this is the case, then it is possible that some of the volatiles currently detected on the Moon derive from recent events. Areas where this may be happening on the Moon, sites of “lunar transient events” are discussed in Chapter 11.
Finally, some asteroids accreted significant amounts of volatiles and maintain them to this day. For the most part, meteoritic hydrated minerals are confined to the C chondrites, though there are some exceptions mentioned here. The CM and CI groups of carbonaceous chondrite meteorites have abundant phyllosilicate minerals, which have OH as part of their structure, as well as other hydroxide minerals (Rubin, 1997). As a result, 10% or more of their mass can be water or hydroxyl. It is thought the CM and CI parent bodies formed as mixtures of ice and anhydrous silicates that were then heated beyond the melting point of ice, allowing aqueous alteration reactions to begin and hydrated minerals to form (Brearley, 2006). Many of these reactions are exothermic, so once they begin additional heat is generated to melt more ice and drive further reactions, creating a self-sustaining process until all the ice is melted. Depending on the specific situation, continued heating could occur, metamorphosing and eventually dehydrating and destroying the hydrated minerals. It is thought this happened to some carbonaceous chondrites (Nakamura, 2005). On the other hand, objects that never experienced sufficient heat to melt ice in the first place may retain their original ice in their subsurface and interiors. At least some of the so-called main belt comets or active asteroids appear to have activity driven by sublimation of near-surface ice, consistent with being such never-heated objects. Given that the CM and CI parent bodies can have ~ 10% of their mass in water, it is reasonable to expect any unreacted bodies to also have 10% of their mass in ice, if not more. Examples of all of these types of objects, from the parent bodies of hydrated and metamorphosed carbonaceous chondrites to potentially unreacted physical mixtures of ice and anhydrous minerals, may be present in the C-complex asteroid population, as further discussed here.
Models of the thermal evolution of Ceres by McCord and Sotin (2005) and Castillo-Rogez and McCord (2010) prior to Dawn's arrival predicted that body to be differentiated, with an icy mantle above a rocky core. With the benefit of data from Dawn, it is now thought that Ceres is only partially differentiated, with an ice-rich but not pure ice interior (Park et al., 2016). Whether this interior structure reflects a loss of ice early in Ceres’ history (Castillo-Rogez et al., 2016) or reflects a largely undisturbed state is a subject of ongoing research. While Ceres is the only object of its kind that we have visited with spacecraft, there is the prospect that other large asteroids have had similar histories and now have similar interior structures (Schmidt and Castillo-Rogez, 2012).
Added Volatiles, Exogenic
Sources for additional volatiles are varied, depending on the body. For the Moon, the primary sources are the Sun, the Earth's atmosphere, comets, and asteroids (Lucey, 2009). Many of these sources are pertinent for all airless worlds, although each contributes differently to the volatile budget depending on the unique situation of that world.
The Sun: Solar wind particles are constantly streaming away from the Sun. Without an atmosphere (or, in most cases, a magnetic field) to protect them, airless bodies are subject to the direct impingement of these particles on their surfaces, with potential consequences and effects ranging from space weathering (Chapter 6) to charging and potential movement of regolith (Chapter 8). As noted earlier, the solar wind implants hydrogen into the surface of the Moon. Some of this hydrogen chemically interacts with the target materials, and creates hydroxyl and, potentially, water. Spectroscopic evidence from the Chandrayaan-1 spacecraft is still being interpreted, but recent work suggests that this may be occurring across the lunar surface rather than at limited latitudes (Bandfield et al., 2018). Studies of (433) Eros by Peplowski et al. (2015) using NEAR Shoemaker GRS data and by Rivkin et al. (2018) using ground-based spectroscopy suggest that object has a few hundred ppm or more of hydroxyl, and Rivkin et al. found the same result for the asteroid (1036) Ganymed. Solar-wind-implanted hydrogen is a likely source for this asteroidal “water” as well as for the lunar case. A more detailed description of the connection between space weathering and volatile creation is found in Chapter 6.
Earth's Atmosphere: The Moon does not move directly through the Earth's atmosphere, of course. However, some particles from the Earth's atmosphere do find their way to the Moon (Ozima et al., 2005; Terada et al., 2017). As the Moon passes through the Earth's long magnetotail, it is irradiated by terrestrial atmospheric ions. The relative importance of terrestrial ion implantation in the lunar regolith is not yet clear.
Comets and Asteroids: We have noted that impacts are the dominant process shaping airless body surfaces, and the bodies doing the impacting are, of course, asteroids and comets. We note above that carbonaceous chondrite meteorites can have 10% water or more by mass, and there is evidence that carbonaceous chondrite-like objects dominate the main asteroid belt (Masiero et al., 2011). Comets impact planetary surfaces more rarely than asteroids but they are even more water rich, generally considered to be roughly 50% ice by mass. It is not clear how much in the way of volatiles are retained from these sources, but work (Ong et al., 2010) suggests that a substantial fraction of water from comets can indeed be delivered and then survive in polar sinks on the Moon and Mercury, further discussed here. Infall of hydrated dust and impactors is interpreted to be the source of hydrated minerals on Vesta (discussed in Chapter 11). Future work examining the D/H ratios of volatile sinks on airless bodies may lend insight into the prospective sources.
Detecting Volatiles on Airless, Rocky Bodies
The techniques for detecting volatiles are included among those discussed in Chapter 4. The most important remote-sensing techniques for volatiles on these bodies are reflectance spectroscopy and neutron spectroscopy, though others are also used. Volatiles like water, carbon dioxide, ammonia, and methane all give rise to absorptions in the 2.5–5 μm region. Working in this spectral region requires modeling and removal of thermal emission to study the reflectance spectrum, an undertaking that becomes more difficult as surfaces get hotter and as local/shape effects become more important. Main belt asteroids can be treated as point sources, and thermal flux removal from their spectra is straightforward, while measurements of Mercury in this wavelength region are dominated by their thermal component unless cooler areas are isolated, which in turn creates complications due to the high phase angles found at those cooler areas.
The 3-μm spectral region is the most important one for water and hydroxyl. OH has its strong fundamental absorption in the 2.7–3.1-μm region, with the exact position dependent upon mineral composition and other factors, plus H2O has another, very strong fundamental absorption near 6 μm, giving rise to a strong overtone band near 3 μm (Fig. 10.3). These lead to band depths upward of 20%–30% on CM chondrites and some asteroids. However, ground-based observations are strongly affected by the Earth's atmosphere, whose water vapor is also strongly absorbing over much of the same wavelength range. Nevertheless, ground-based measurements have established the presence of hydrated minerals on dozens of asteroids.

Another powerful technique is that of neutron spectroscopy, described in Chapter 4. Neutron spectrometers cannot be effectively operated on flybys or distant orbits, but have been used to detect hydrogen, and by extension water, on the Moon, Vesta, and Ceres. Relatedly, X-ray/gamma-ray measurements have been used to measure the hydrogen concentration on Eros.
Volatile Transport
As volatiles arrive or are created on an airless body, they either interact with the surface (chemical reaction, adsorption), begin a trek across the body via ballistic motion, and/or escape. This is also true for water vapor that has reached the surface after subliming from a subsurface reservoir, for instance. The speed of a volatile molecule is temperature dependent, and in calculations it is often treated as instantaneously adopting the temperature of the surface it is interacting with. There is a distribution of speeds associated with a given temperature with varying probabilities, along with slightly different definitions of thermal speed (and there is some disagreement about the most appropriate speed distribution to use in this case), but the most probable speed (vth) can be estimated as
where k is Boltzmann's constant and mm is the mass of the molecule in question. For a water molecule at 300 K, the most probable speed is 526 m/s. This is a significant fraction of the escape speed of the Moon, but a water molecule is likely to remain bound to the Moon and hop from one location to another as long as it exists or until it reaches a location where it can stably remain. The same is true for Mercury—the much higher temperatures that can be reached on its surface lead to higher thermal speeds, but the most likely value remains comfortably below the escape speed. On Vesta, however, the escape speed of 360 m/s is too low to hold onto a typical water molecule given the temperatures present over most of its surface. When considering molecular hydrogen, the most-probable thermal speeds increase by a factor of 3 for a given temperature. Of course, there are significant fractions of molecules that are moving either more slowly or more quickly than the most probable speed, and it is possible for a water molecule on Vesta to happen to be moving sufficiently slowly to remain bound to that object. However, each time the molecule interacts with the surface it will reset its speed, and each time the speed is reset the odds of the molecule remaining below the escape speed are poor. Such a water molecule on Vesta is destined to either escape or, with low probability, randomly find its way to an area that is sufficiently cold that the molecule can stably remain: a “cold trap.”
Fig. 10.4 is a simple schematic from Lucey (2009) of how volatiles might move on the Moon, including some of the loss mechanisms discussed here. The transport and harboring of volatiles is directly connected to the nature of their unique thermal surface environments. These are affected by illumination, rotation, surface roughness, regolith insulation, etc.
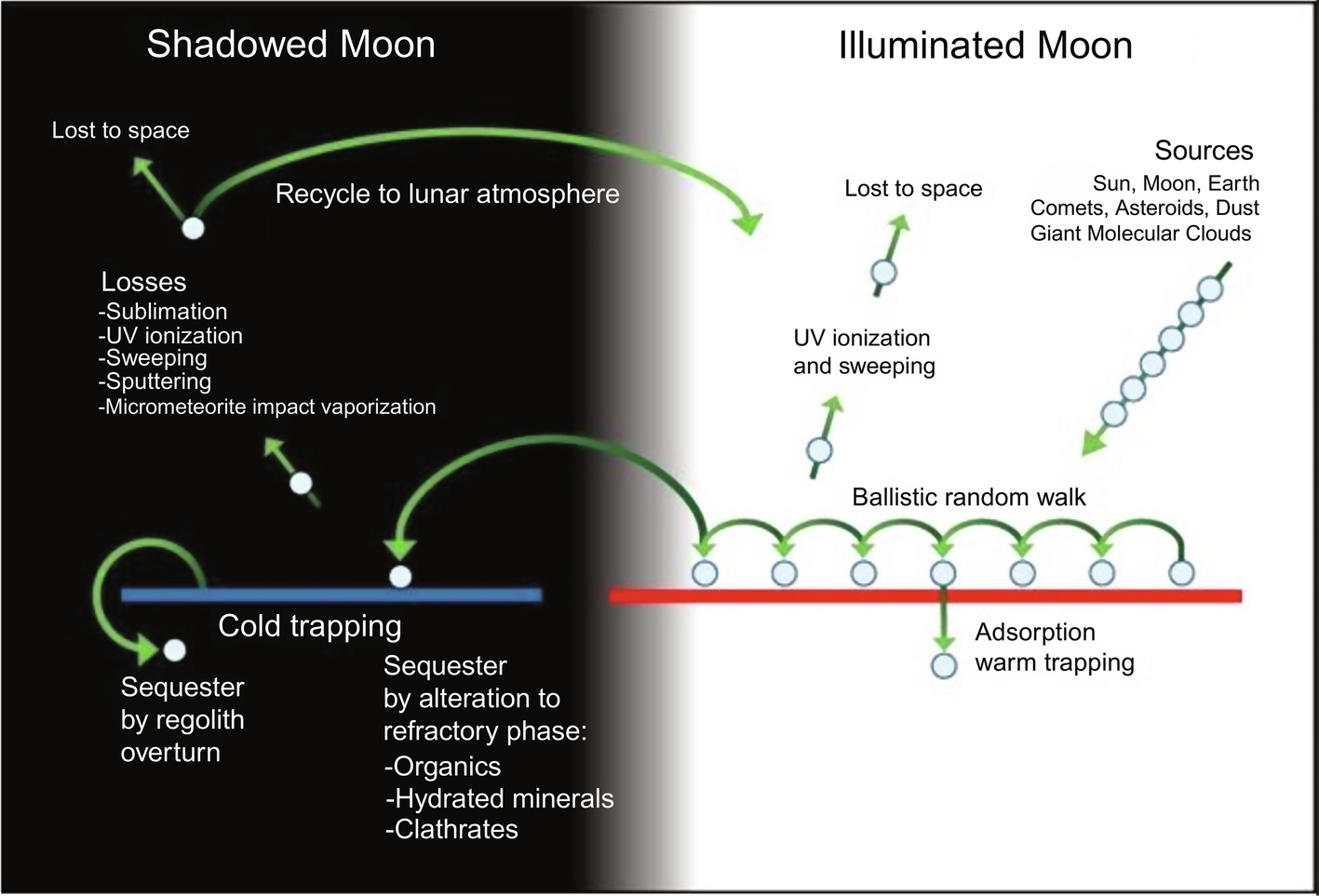
Butler (1997) conducted Monte Carlo modeling of water molecules randomly placed on the Moon and found that 20%–50% reached a permanently shadowed region near the lunar poles after repeated ballistic hops, where they could undergo continued evolution. Crider and Vondrak (2003) performed a similar calculation on incident solar-wind protons, finding that 0.04% of those protons reach polar cold traps as part of a water molecule. Recent work (Prem et al., 2018) has shed light on the importance of small-scale surface roughness in sequestering volatiles on airless bodies. Such roughness affects both the stability of volatiles and their transport. Given the insulating nature of regolith (see later), small-scale surface roughness can result in large temperature variations on airless bodies, providing friendly cold traps on small scales to harbor volatiles.
Retention of Volatiles: How Do Airless Bodies Keep Volatiles?
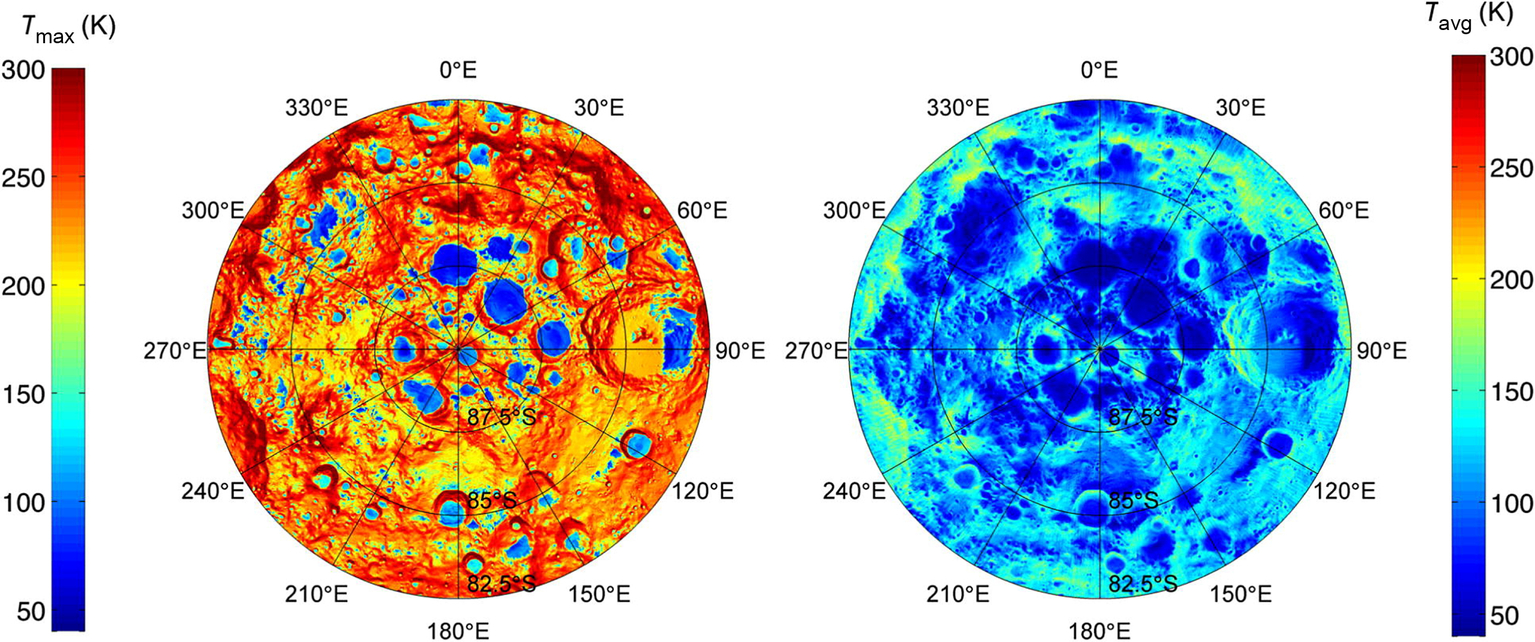
As stated earlier and touched upon in Chapter 2, cold traps are localized areas where the temperatures and conditions allow volatiles to stably remain. On the Moon and Mercury, relatively low obliquities mean the Sun does not stray high above the horizon in polar regions even during the summer, and craters do not have to be terribly deep to create areas that are always in shadow. These permanently shadowed regions increase in size as craters get deeper and closer to the pole. While they are not typically observable in reflected light, save for very concerted effort like the imaging of mercurian PSRs illuminated by scattered light from crater walls (Chabot et al., shown in Chapter 2), permanently shadowed regions can be probed by techniques that provide their own illumination like laser altimeters and radar, or measured by means other than reflected light such as via infrared emission or neutron detection.
Within PSRs, evolution of volatile content can still occur. While the interiors of PSRs are shielded from direct sunlight, they are not shielded from galactic cosmic rays or solar wind plasmas. They are also potentially subject to changing conditions from elsewhere, such as nearby impacts altering the landscape or depositing ejecta in the region.
The gardening of regolith inside PSRs is an important factor in considering the lifetime and nature of volatiles within them. Schorghofer (2008) noted that the loss rate of subliming ice decreases linearly with the thickness of an insulating, ice-free regolith layer, calculating that the retreat rate of subsurface ice at 145 K beneath ~ 1 m of regolith is similar to the retreat rate of ice at 120 K in vacuum: roughly 10 m/Gy. If kept below ~ 145 K, burial by a modest amount of impact ejecta could maintain subsurface ice for very long periods. In the case of Ceres or other icy asteroids, the implication is that an original icy interior could retain ice to this day beneath a shallow lag deposit/regolith (Fig. 10.5).
Volatile Losses: How Do Airless Bodies Lose Volatiles?
Airless bodies lose volatiles through a number of paths. As discussed earlier, the airless bodies are lower in gravity than those objects with substantial atmospheres and they cannot hang on to volatile elements and compounds as effectively. Aside from Mercury, which possesses a magnetic field, they are also subject to direct bombardment by any ionized source such as the solar wind. Paths of loss include sublimation, UV ionization, sputtering, and micrometeorite impact vaporization (Fig. 10.6).

The sublimation rate of ice is extremely temperature sensitive. The loss rate of crystalline ice into vacuum is directly related to the equilibrium vapor pressure (Pvap), which is a function of temperature:
where ptp and Ttp are the pressure and temperature of the triple point of water (611 Pa and 273.16 K, respectively), ΔHs is the enthalpy of sublimation (51.06 MJ/kg), and R is the universal gas constant. Changing the temperature from 100 to 120 K changes the vapor pressure by a factor of nearly 30,000, and the retreat rate in vacuum from < 1 mm/Gy to the ~ 10 m/Gy mentioned earlier. Increasing the temperature to 150 K changes the vapor pressure by another factor of ~ 30,000 and the retreat rate in vacuum to ~ 30 km/Gy.
As noted earlier, the presence of an insulating regolith layer slows sublimation considerably. Ice beneath a 5-m layer of 100-μm particles at 150 K would only retreat at ~ 2 m/Gy. Increasing the temperature to 180 K would increase the retreat rate to ~ 1 km/Gy, but a retreat at that rate would quickly increase the insulating layer and make the path to escape much more tortuous, reducing the actual retreat rate. Nevertheless, reiterating the last section, we expect sublimation to effectively remove ice from the near-surface of airless bodies at temperatures above ~ 145 K. In principle, sublimed volatile molecules can resume ballistic hops around the surface until a cold trap is found, the molecule is destroyed, or escapes.
Photoionization is the process whereby energetic UV photons can essentially knock an electron out of orbit around an atom. This creates a net charge on the atom and the molecule to which it belongs. Given the plasma environment on airless bodies (Chapter 8) and the solar wind magnetic field, this can quickly lead to removal of the volatile molecule. Photodissociation is a related process, breaking molecules into their constituent atoms. Given the very low mass of hydrogen atoms, they quickly escape even the most massive objects we are studying in this volume. Photodissociation is the limiting factor for water molecules at 1 AU, occurring on the timescale of a day (Stern, 1999). If a water molecule cannot find a cold trap on that timescale, it will most likely be destroyed.
The process of sputtering occurs when an ion impacts a mineral crystal structure and ejects a particle either by imparting enough energy to break its bonds (physical sputtering) or by starting a chemical reaction (chemical sputtering). This can be a source of significant erosion over time. Sputtering can either create volatiles or destroy them, depending on the specific particles being sputtered. While we focus here on water and hydrogen, sputtering can eject many different types of atoms, and is thought to be an important process in creating the sodium and potassium components of the lunar and mercurian exospheres.
Impacts can create widespread heating, as noted elsewhere, and obviously impacts that are large enough to create impact melt will also drive off volatiles that may be present in the target material. Micrometeorite impacts also can vaporize and jettison volatiles from airless surfaces, and are frequent enough that they can be expected everywhere on a body, including cold traps.
Crider and Vondrak (2003) investigated the evolution of hydrogen (both in water and in other forms) in lunar cold traps, including the loss effects described earlier. They estimate that approximately 6% of the water that arrives at the lunar cold traps survives over the span of 1 billion years. This assumes the ice is in isolated grains, however. Thicker lenses of ice or ice covered in regolith have a higher survival rate since most of the ice mass is protected from many of the loss mechanisms.
While volatile loss is usually envisioned as a very small-scale process, it can change or create landforms that are visible from orbiting spacecraft. Such landforms on Mercury, Vesta, and Ceres are discussed later, and include pitted areas similar to regions seen on Mars.
Volatiles on Specific Airless Worlds
Some of the details of the volatiles on specific airless worlds can be found here. Note that some discussions appear elsewhere in this book given their relevance to multiple processes. The text points to those chapters when appropriate rather than repeating material.
Volatiles on Larger Worlds—Mercury and the Moon
Volatiles: Mercury
Mercury is home to abundant volatiles in spite of its proximity to the Sun and prodigious heat. While the search for ice at the lunar poles was the result of decades of calculation and dedicated searches and experiments, the finding of mercurian polar ice was serendipitous and immediate: it came in 1991 via the first comprehensive radar mapping of Mercury, designed to complement the Mariner 10 flybys of the 1970s (Slade et al., 1992). During those measurements, the polar regions were seen to have radar polarization ratios inconsistent with rocky material but instead consistent with icy satellite surfaces. Calculations demonstrating PSRs in the interiors of mercurian polar craters would be cold enough to maintain ice if they existed were released as a companion paper to the ice discovery paper (Paige et al., 1992).
Continued radar measurements combined with imaging by the MESSENGER MDIS cameras (see Fig. 2.7) led to a series of results that have greatly improved our understanding of ice at the poles of Mercury (Chabot et al., 2012, 2016, 2018). It has been determined that all radar-bright areas are within PSRs, that these radar-bright areas have low-reflectance surfaces with albedos that vary in concert with the maximum temperatures they experience, but that only roughly 43%–46% of PSRs have radar-bright deposits. Taken together, this is interpreted to mean that the radar-bright areas are mostly water ice but could have some additional volatile components, and that rather than being a continuous process, delivery of material to the PSRs was in a single event or perhaps a small number of occasional events (Chabot et al., 2018). The sharp edges of the PSR deposits as seen by the MDIS images suggest the event was sufficiently recent that subsequent mixing with later impact ejecta has not occurred. The low-reflectance surfaces are thought to be lag deposits protecting ice, as discussed in previous sections.
In addition to water ice (and perhaps other volatiles) in polar deposits, Mercury has a very large sulfur abundance. Nittler et al. (2011) reported an average S abundance of 4% on its surface, roughly 20 times what is seen for other inner planet silicates. Interestingly, this stands in particular contrast to Eros, which was found by the NEAR Shoemaker XRS to be sulfur depleted (Lim and Nittler, 2009). In the case of Eros, this was thought to be because space-weathering processes preferentially drove sulfur from minerals (Loeffler et al., 2008). Given the more intense space-weathering environment expected at Mercury, the expectation was that even less sulfur would be found, rather than more. The high sulfur abundance is currently attributed to Mercury's formation in a more reducing environment than the other inner planets (Nittler et al., 2017).
Volatiles are also associated with “hollows,” enigmatic landforms primarily associated with Mercury's low-reflectance material (LRM) (Fig. 10.7). These were first reported by Blewett et al. (2011) as “irregular, shallow, rimless depressions,” and were thought to be very recent given their appearance. Because it is thought the LRM is graphite-rich, Blewett et al. (2016) proposed that hollows are formed via sputtering of carbon or loss of methane created by solar-wind proton implantation, similar in concept to the hydroxyl creation discussed in an earlier section. However, other formation theories exist, including the exhumation of subsurface volatile layers by impact or mass-wasting processes followed by sublimation of the volatile component. In any case, Blewett et al. suggested that hollows formation ends with the development of a sufficiently thick volatile-free lag deposit, a common feature in many of the situations we discuss in this chapter.

Volatiles: Moon
It is natural to compare and contrast the Moon and Mercury to one another in terms of their volatile retention and transport. Even before the first lunar landers, it was realized that the polar regions of the Moon could have volatiles (Watson et al., 1961). There was great controversy over the nature of lunar rilles and whether they were water cut (Urey, 1967; Peale et al., 1968), but the Apollo landings and the return of lunar samples demonstrated the Moon was a much, much more water-poor object than the Earth, a fact that has subsequently been used as a key constraint for lunar formation theories. More recent work has suggested that rather than retaining no volatiles after its formation, the Moon may have retained a small amount of water and other volatiles (McCubbin et al., 2010; Hui et al., 2013; Boyce et al., 2014) but nevertheless, there is no firm evidence from lunar samples or remote sensing of widespread, common lunar minerals that experienced aqueous alteration.
The type of radar measurements that provided early, unequivocal evidence for ice on Mercury gave much more ambiguous results when made for the Moon. Despite repeated attempts, Earth-based radar measurements have not conclusively detected lunar polar ice, though they have placed important constraints on its properties (Stacy et al., 1997; Campbell et al., 2006). Instead, the definitive detection of polar ice and measurements of its abundance has come from orbiting radars and neutron spectrometers (Nozette et al., 1996; Feldman et al., 1998; Lawrence et al., 2006; Spudis et al., 2013). The most dramatic experiment to study lunar polar ice involved the Lunar Crater Observation and Sensing Satellite (LCROSS), which impacted a PSR in Cabeus crater in late 2009. Before its own impact, it was preceded 4 min earlier by the upper stage of the rocket that carried it. LCROSS observed the upper stage impact using multiple cameras and spectrometers (Fig. 10.8), with the team calculating, based on detections of OH in the plume in the infrared and ultraviolet, that water ice made up 5.6 ± 2.9% of the mass of the regolith where the upper stage impacted (Colaprete et al., 2010). Measurements of the plume (including by other observatories) also found evidence of H2, H2S, NH3, SO2, C2H4, CO2, and CH3OH at concentrations up to 1% of the water abundance. Any or all of these additional volatiles, save the hydrogen, could plausibly have been delivered by cometary impactors.
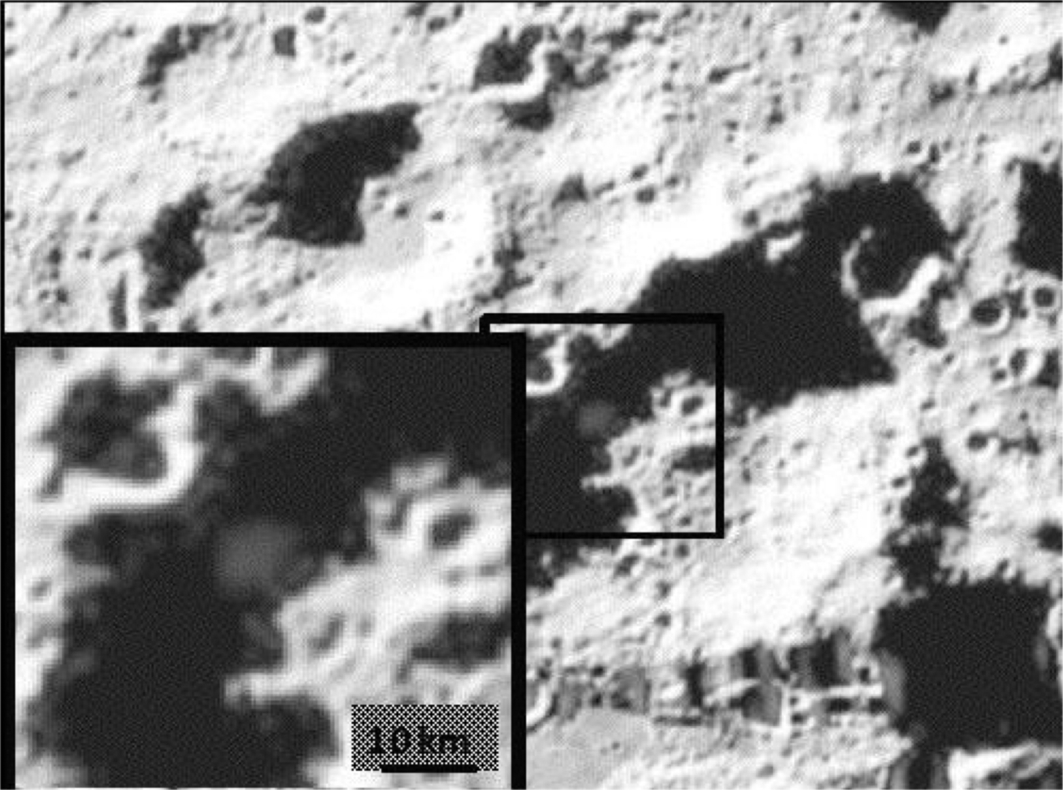
At this point, evidence suggests that the contiguous sheets of ice seen in mercurian PSRs are not present in lunar PSRs, but that ice is instead is found as isolated grains or small patches mixed with regolith, and that lunar PSRs may have only a few percent of water compared to mercurian PSR values with perhaps ten times more water. There have also been orbital measurements of UV reflectance inside PSRs, using illumination from starlight, that show evidence of 0.1%–1% water ice at the surface of some cold traps (Hayne et al., 2015).
It is not currently clear why the lunar PSRs differ so much from the mercurian ones. It is possible that Mercury's more volatile-rich nature leads to capture of more native material in PSRs than on the Moon, or that if the PSRs on Mercury were filled by a recent cometary impact, no such recent impact occurred for the Moon. One additional possibility was posed by Siegler et al. (2016): that the orientation of the Moon in space has shifted with time, a process called polar wander. They suggest that the Moon's pole was once several degrees away from its current position and that a large density anomaly associated with the major episode of mare volcanism led to a reorientation of the Moon. With that reorientation, the original locations of some PSRs might be moved into the sunlight, destroying any volatiles that were in place. If most of the lunar volatiles were emplaced prior to the reorientation, the PSRs may not have had time to refill.
Lunar volatiles, and the PSRs in particular, feature in many future mission plans for spacefaring nations. Planned or proposed missions to the Moon are discussed in Chapter 12.
Volatiles on Smaller Worlds—Dwarf Planets, Asteroids, and Small Satellites
Objects in the main asteroid belt span a wide range of compositions and thermal histories, as we have learned from remote sensing, spacecraft visits, and linkages with meteorites. Some objects, including Vesta, have melted and differentiated and have basaltic surfaces like Mercury and the Moon. Also like Mercury and the Moon, these objects may have volatiles on their surfaces due to interactions with the solar wind or impactors. Other objects have their own native volatiles, bound into minerals or, apparently in some cases, still in the form of ice. As for Phobos and Deimos, it is not yet clear which of these two categories of small bodies they are most like, and whether the detections of hydrated species on their surfaces represent native or delivered material.
Volatiles: Vesta
Like the Moon, Vesta was long thought to be practically volatile-free based on samples. And like the Moon, hydroxyl has subsequently been found on its surface. Detections were first made by ground-based astronomers in the 3-μm region (Hasegawa et al., 2003; Rivkin et al., 2006a). Because the band was shallow, no firm conclusions could be made about the nature of the band in terms of its origin. With the arrival of Dawn at Vesta, the VIR spectrometer confirmed the presence of the 3-μm band and the GRaND neutron spectrometer quantified the hydrogen abundance. The hydrated minerals on Vesta appear to be concentrated in low-albedo regions, which in combination with the hydrogen abundance has been used to argue that they have been delivered via impactor rather than created by solar wind implantation. This is discussed further in Chapter 11.
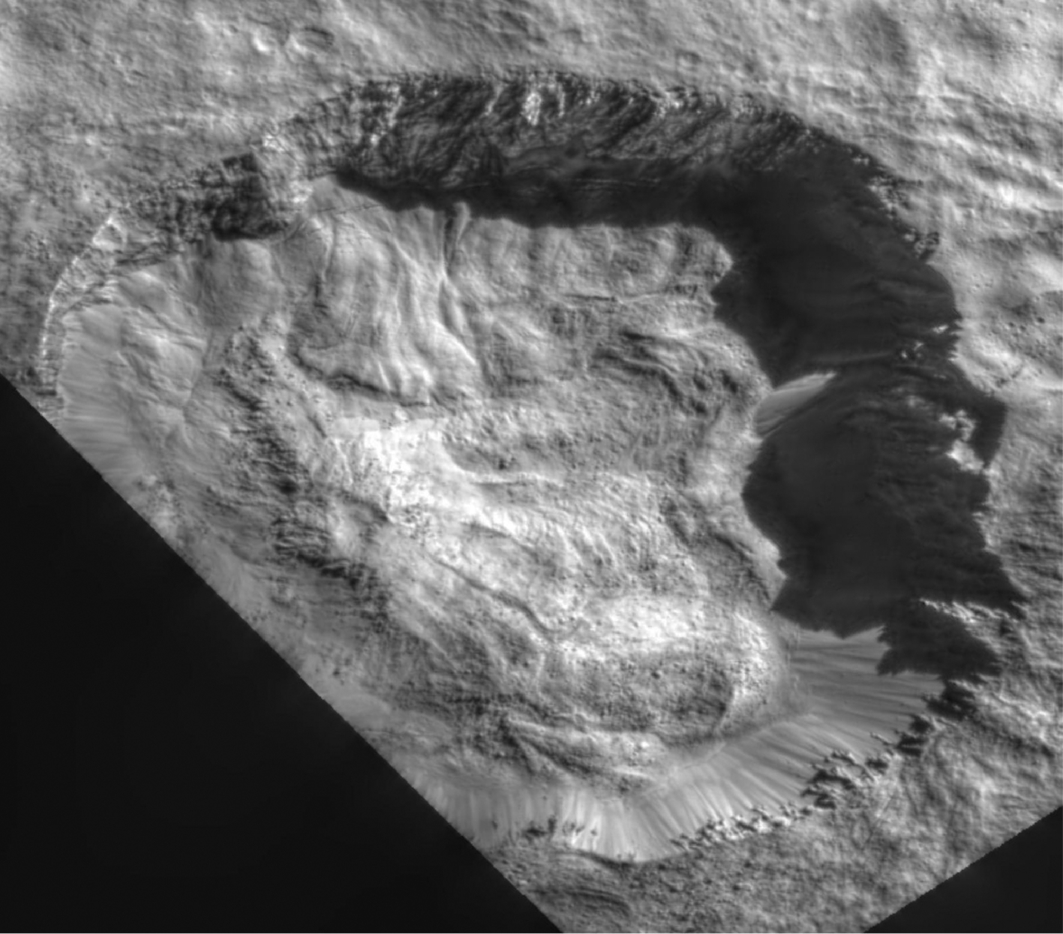
Vesta also has something in common with Mercury with regard to volatiles: landforms that involve volatile escape. On Vesta, these are called pitted terrains, and close similarities to terrains on Mars have been identified by Denevi et al. (2012) (Fig. 10.9). Pitted terrain on both Mars and Vesta is found in conjunction with impact craters. The terrain comprised semicircular depressions without rims. Such pits on Mars are speculated to have formed through the degassing of volatiles from the heat of the associated impact. If the pits seen on Vesta are derived through similar processes, then this implies an abundance of volatiles in the underlying material. As noted earlier, impactors have been interpreted as the source for the detections of OH/hydrogen on Vesta on regional scales. Denevi et al. suggest that the pitted terrain may have formed in multiple stages, with hydrated material brought in by impactors relatively early in Vesta's history, with some areas getting a significant amount of material. Later impacts buried that hydrated material in a second stage, with nearby impacts during a third stage providing the energy to devolatilize the buried hydrated material and create the pits. They also considered single-stage formation during cometary events but found that less likely.

Volatiles: Ceres
Ceres is unusual among the objects discussed thus far in this chapter because it is the only one (at this writing) explored by a spacecraft that accreted significant amounts of water ice and experienced pervasive aqueous alteration. The C-complex asteroid (253) Mathilde was briefly visited by NEAR Shoemaker but only imaging data were taken, and at this writing we are looking forward to the visits of Hayabusa 2 and OSIRIS-REx to the C-complex asteroids (162173) Ryugu and (101955) Bennu, respectively, but as of now Ceres is our sole source of in situ data.
We have known Ceres’ surface to be covered in hydrated minerals since the 1970s. Ground-based measurements of Ceres’ infrared spectrum in the 3-μm region have been made since then, with successive measurements consistent with one another as data quality has improved (Lebofsky, 1978; Lebofsky et al., 1981; Jones et al., 1990; King et al., 1992; Vernazza et al., 2005; Rivkin et al., 2006b). All of the measurements find a band minimum on Ceres near 3.07 μm, a secondary minimum near 3.35 μm, and the more recent measurements find another, shallow absorption near 3.9 μm. What has differed is the interpretation of the 3.07-μm absorption. It was first thought to be evidence of water ice frost by Lebofsky, but it was recognized that ice was not stable on Ceres’ surface and since resupply was considered impossible that interpretation went out of favor. In the early 1990s King et al. reinterpreted the band as due to  in phyllosilicates, but dynamical models in that period did not anticipate much mixing between inner and outer solar system material, which was inconsistent with the idea of Ceres accreting much ammonia. Dawn's visit to Ceres allowed the 2.5–2.85 μm region to be measured for the first time on Ceres, and mixing models of its surface spectrum favor ammoniated phyllosilicate for the 3.07-μm band (De Sanctis et al., 2015a), though in between the work of King et al. and De Sanctis et al. alternative compositions like iron-rich clays and brucite were also offered (Rivkin et al., 2006b; Milliken and Rivkin, 2009). By contrast, the 3.9-μm band was first identified by Rivkin et al. (2006b) and interpreted along with the 3.35-μm band as carbonates, which has remained the accepted interpretation. The question of how Ceres has enough nitrogen to uniformly provide ammoniated minerals as an important mineral on a global scale is a matter of ongoing debate, and it is not clear whether a significant input of material from the outer solar system, or whether Ceres itself must have been created in the outer solar system and transported inward, is necessary to explain it or if aqueous alteration and other processes could have created
in phyllosilicates, but dynamical models in that period did not anticipate much mixing between inner and outer solar system material, which was inconsistent with the idea of Ceres accreting much ammonia. Dawn's visit to Ceres allowed the 2.5–2.85 μm region to be measured for the first time on Ceres, and mixing models of its surface spectrum favor ammoniated phyllosilicate for the 3.07-μm band (De Sanctis et al., 2015a), though in between the work of King et al. and De Sanctis et al. alternative compositions like iron-rich clays and brucite were also offered (Rivkin et al., 2006b; Milliken and Rivkin, 2009). By contrast, the 3.9-μm band was first identified by Rivkin et al. (2006b) and interpreted along with the 3.35-μm band as carbonates, which has remained the accepted interpretation. The question of how Ceres has enough nitrogen to uniformly provide ammoniated minerals as an important mineral on a global scale is a matter of ongoing debate, and it is not clear whether a significant input of material from the outer solar system, or whether Ceres itself must have been created in the outer solar system and transported inward, is necessary to explain it or if aqueous alteration and other processes could have created  in situ. The identification of other large objects with Ceres-like spectra (Rivkin et al., 2011; Takir and Emery, 2012) means that Ceres’ history and composition are probably not best explained as the results of a low-probability event.
in situ. The identification of other large objects with Ceres-like spectra (Rivkin et al., 2011; Takir and Emery, 2012) means that Ceres’ history and composition are probably not best explained as the results of a low-probability event.
As is noted elsewhere, Ceres has a significant ice fraction. The Dawn GRaND instrument was used to map hydrogen across its surface, finding it to vary with latitude (Prettyman et al., 2017). This is interpreted as the result of ice being stable near the surface at high latitudes but the temperature near the equator being too warm to maintain ice within the top meter or so of the regolith. The hydrogen concentration near the equator is equivalent to ~ 17% of H2O, similar to what is seen in the hydrated C chondrites, while near the poles the water equivalent is ~ 27%, with ice likely within a cm of the surface. Prettyman et al. therefore modeled the surface of Ceres in terms of its hydrated minerals as regolith broadly similar to hydrated C chondrites mixed with 10% ice near the poles, with the ice table retreating toward the equator until it is below the level detectable with GRaND.
While the surface of Ceres in general is too warm to maintain ice, it has nevertheless been identified spectroscopically in localized exposures. Combe et al. (2017) report its presence at 9 locations, all poleward of ± 30° and all limited in extent (Fig. 10.10). Given the apparent ubiquitous nature of ice within Ceres’ regolith and near surface it is likely no surprise that Ceres, like Mercury and Vesta, has landforms that appear related to volatile loss. Sizemore et al. (2017) report four pitted areas, with three additional candidates. They conclude that ice sublimation rates on Ceres are too slow to have created these terrains and that they need rapid devolatilization to form, perhaps pointing to impacts as instigators.
Volatiles: Other Asteroids
The carbonaceous chondrites, mentioned at the start of this chapter, are associated with the C-complex asteroids (Table 5.3). The water bound into hydrated minerals in meteorites gives spectral absorptions in the 3-μm region, and similar band shapes are seen in many C-complex asteroids, as well. An additional absorption near 0.7 μm attributed to iron oxides in phyllosilicates is seen in CM chondrites, and has been used to tie the Ch class of asteroids to that meteorite group (Rivkin et al., 2015). It has been estimated that 30%–50% of main-belt C-complex asteroids have the 0.7-μm band (Rivkin, 2012; Fornasier et al., 2014), and that roughly half of the objects in the main asteroid belt are C-complex, implying ~ 20% of main-belt asteroids may have CM-like compositions.
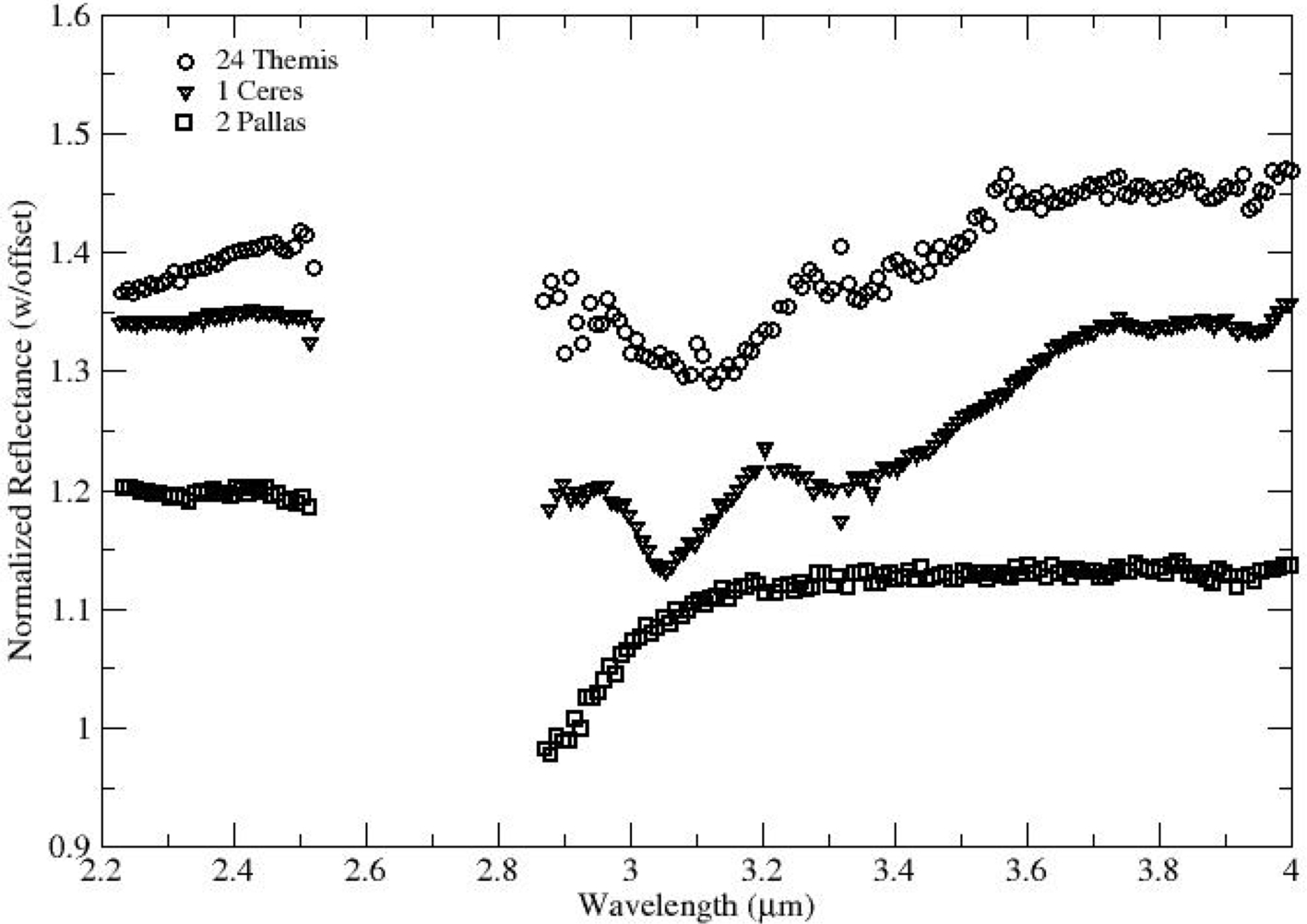
Measurements in the 3-μm region show additional band shapes beyond what is seen in the meteorites. Ceres, as noted earlier, has a significantly different surface spectrum than typical meteorites, and that band shape is shared by at least a few other objects (Rivkin et al., 2011; Takir and Emery, 2012, Fig. 10.11). The asteroid (24) Themis was determined to have a band shape consistent with water ice frost (Rivkin and Emery, 2010; Campins et al., 2010), quickly followed by similar determinations for other outer-belt asteroids like (65) Cybele (Licandro et al., 2011) and (90) Antiope (Hargrove et al., 2015), the latter of which is a member of the Themis family. Ice on the surface of these objects was not expected, for thermodynamic reasons discussed earlier, leading to the suggestion that another mineral was responsible (Beck et al., 2011) or alternately that any surface ice is replenished by slow outgassing from asteroidal interiors, perhaps similar to the diurnal cycle seen on comet 67P (De Sanctis et al., 2015a,b). An additional factor favoring the presence of ice is the existence of so-called active asteroids or main belt comets (MBCs) in the same region of the main belt as Themis and Antiope, including some members of the Themis family (Hsieh and Jewitt, 2006). These objects have orbits that are indistinguishable from typical main-belt asteroids, but exhibit dust comae similar to comets. Some of these objects are thought to have experienced an impact (Bodewits et al., 2011), and others may be disrupting due to YORP torques (Chapter 9), but several exhibit repeated activity near perihelion. Jewitt (2012) argues that sublimation of water ice is the only plausible driver of activity on these objects.
There are other low-albedo asteroid classes besides those in the C complex, as described in Chapter 5. Members of those classes, primarily the D class and P class, become more common as solar distance increases, and they dominate the populations beyond the main belt. There are no known meteorites from these groups, and there are many spectral similarities between them and cometary nuclei, with recent dynamical models favoring their origin among today's transneptunian object population. Brown (2016) found that an average of 8 Trojan asteroid spectra with steeper spectral slopes showed no evidence of any hydrated minerals, while an average of 8 Trojan asteroids with less shallow slopes exhibited a band of similar shape and depth to what is seen on Themis. Still, we must wait for future data from JWST and eventually the Lucy mission (Chapter 12) for us to better understand the volatile content on these objects.
At least one ordinary chondrite, Semarkona, has been found with hydrated minerals, demonstrating that at least one OC parent body was able to interact with water (Hutchison and Alexander, 1987). The Zag and Monahans OCs were found to have halite crystals including fluid inclusions (Rubin et al., 2002). These meteorites were collected rapidly after their falls, which is thought to be an important reason why the halite was observed—it would have quickly been altered by terrestrial humidity if allowed to sit for very long on the Earth's surface. It is possible halite is much more common in OC meteorites than we realize.
As a result of these meteorite measurements, if not out of a desire for completeness, higher-albedo asteroid classes have also been studied in terms of their hydrated minerals. Vesta, Eros, and Ganymed are discussed earlier. The most studied of the high albedo asteroids in the 3-μm spectral range is the M class. Two M asteroids, (92) Undina and (55) Pandora, were observed by Jones et al. (1990) to serve as controls in a study of C-class asteroids. Instead, both were observed to have absorptions showing hydrated minerals. Two follow-up studies by Rivkin et al. (1995, 2000) found that roughly 1/3 of M-class asteroids in their sample had evidence of hydrated minerals. This was interpreted as evidence that these objects could not be metallic, contrary to contemporary expectation, though it was not clear what they were. Subsequent radar measurements by Shepard et al. (2015) found that some of the objects seen to have 3-μm absorptions had radar albedos suggestive of high metal content, although others appeared consistent with having metal content more typical of the C or S classes. Landsman et al. (2015) followed the Rivkin et al. work with higher spectral resolution, confirming the presence of absorption bands. It is possible that at least some of the M asteroids could be metallic and have had hydrated minerals delivered by impactors, like what is interpreted for Vesta, but it appears that others are not metallic.
The low gravity on asteroids makes it unlikely that volatile transport can occur on them the way it does on larger objects—the speed distribution of molecules at asteroidal daytime temperatures leaves very few moving slowly enough to remain long enough for even a single bounce. There is an additional factor working against the retention of volatiles in cold traps: unlike the Moon and Mercury, most asteroids do not have low obliquities. As a result, as discussed in Chapter 2, the Sun can be overhead at a wide range of latitudes on typical asteroids. This reduces the possible locations of PSRs on these bodies significantly, and a majority of asteroids likely have no PSRs on their surfaces at all.
Volatiles: Phobos and Deimos
Our understanding of volatiles on the moons of Mars has largely been indirect. Because the spectra of Phobos and Deimos are similar to those of cometary nuclei and outer-belt asteroids, and their origin was thought to be as captured objects from the outer asteroid belt, there has been an expectation that they could share the same organics- and volatile-rich composition of those objects. Ice is not stable on their surfaces, but measurements of their relatively low densities compared to solid rock were first interpreted as indications of ice-rich interiors. This particular indicator has been reconsidered, however, as measurements of asteroid densities showed that high porosities are common in small bodies and that Phobos and Deimos’ densities more likely reflected macroporosity than interior ice.
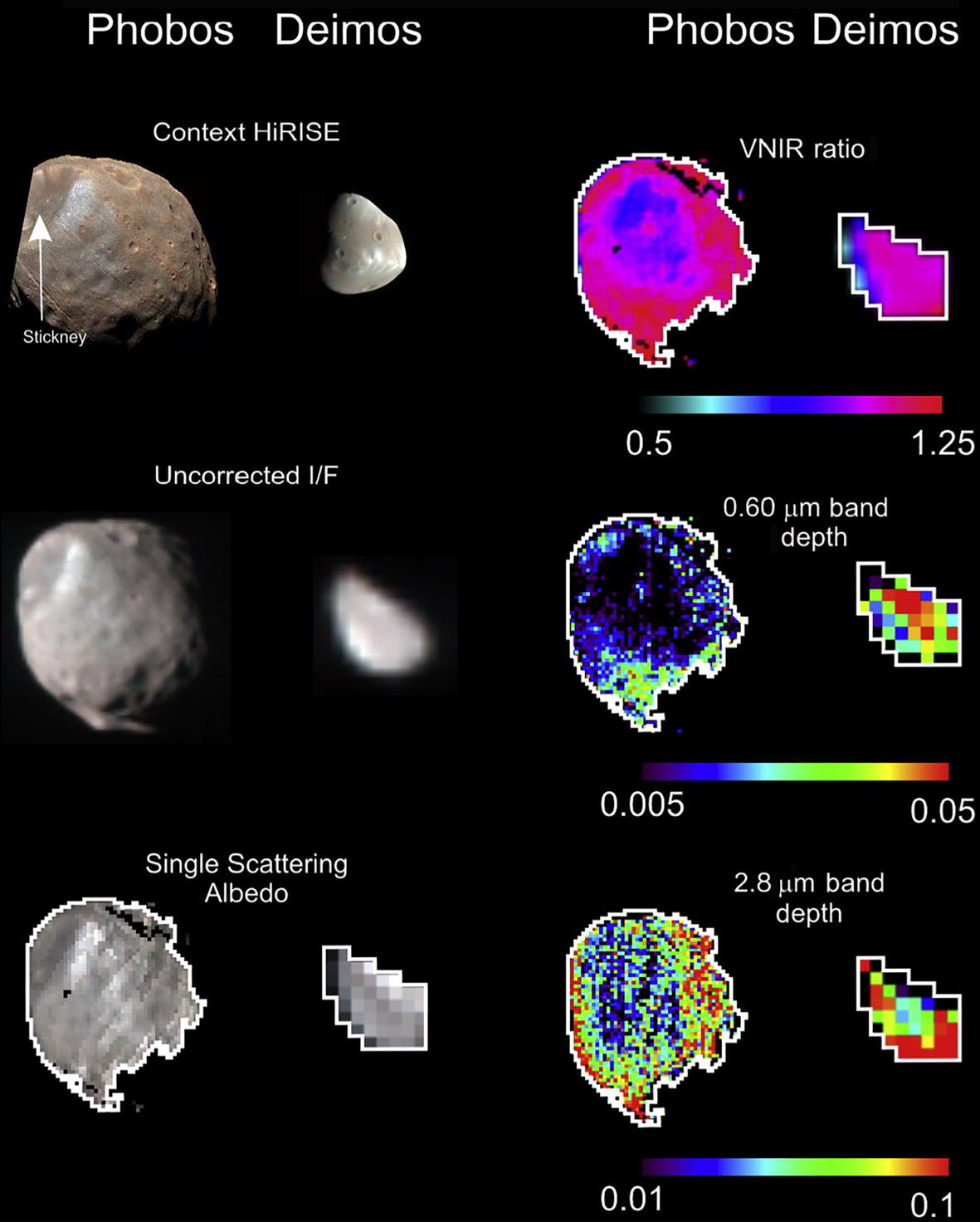
Spectral measurements have also given mixed results. Observations in the 3-μm spectral region from Phobos 2 and ground-based observatories showed no evidence of an absorption due to OH or water (Murchie and Erard, 1996; Rivkin et al., 2002). Additional observations by the Mars Reconnaissance Orbiter (MRO) show a band near 2.8 μm due to OH, but it is not obvious whether that could be caused by solar wind implantation, as with the midlatitudes on the Moon, or if it is native hydrated material (Fraeman et al., 2014, Fig. 10.12). However, its association with only one of the two spectral units on Phobos suggests it is compositional rather than process-related. Midinfrared measurements from Mars Global Surveyor suggest the presence of phyllosilicates, suggesting the MRO measurements are indeed of native hydrated material (Giuranna et al., 2011). Similarly, Fraeman et al. (2012) reported the presence of a 0.7-μm band on Phobos and Deimos from MRO and telescopic data consistent with phyllosilicates seen on CM chondrites and Ch asteroids. However, ongoing studies of the origin of the martian moons are beginning to favor a giant impact formation. Reconciling that origin, or other origin scenarios, with their implications for the presence of volatiles may not be settled until the return of material from Phobos in the decade of the 2020s.
Summary
Even without the atmospheres of Venus, Earth, and Mars, and at temperatures much warmer than the icy satellites of the outer solar system, volatiles still influence the surfaces of the rocky, airless bodies. Some, like Ceres and many asteroids, were “born” with significant amounts of water ice (and perhaps other ices like ammonia) and experienced pervasive aqueous alteration. Others, like Vesta, the Moon, and Mercury, have apparently had much of their surface water and hydroxyl delivered via impact or created in the regolith via reactions with implanted solar wind protons. These processes also may be acting on some asteroids that did not accrete with ice, in addition to “naturally” hydrated asteroids on which we might not recognize added volatiles. Volatile transport and loss also affect airless surfaces, with ice and other species accumulating in cold traps in polar regions that never get direct sunlight, and volatile escape creating unusual landforms. The details of how quickly cold traps fill and how landforms are created, and how factors may differ from object to object, are still active areas of research.