

 Quantum physics, or quantum mechanics, is a branch of science that deals with how nature behaves at the atomic and subatomic levels, the realm of the infinitesimal.
Quantum physics, or quantum mechanics, is a branch of science that deals with how nature behaves at the atomic and subatomic levels, the realm of the infinitesimal.
The foundations of quantum mechanics were established in roughly the first forty years of the twentieth century, from about 1895 to 1935. The big names in this field are Niels Bohr, Max Planck, Albert Einstein, Werner Heisenberg, Max Born, John von Neumann, Paul Dirac and Wolfgang Pauli, Louis de Broglie, and Erwin Schrödinger, with a host of more minor players.
Max Planck stated that energy waves are radiated and absorbed in discrete, or separate, bundles, or “quanta,” and that the amount of energy is related to the frequency (number of vibrations per second) of the waves. He was clever enough to come up with a formula that says so: E (energy) equals h (Planck’s constant) times f (frequency), or E = hf.
We can kind of see quantum mechanics at work in everyday life. Heat up a piece of iron, say, a nail. Keep increasing the heat. The first color of the glow given off is red. Keep on heating it and soon it turns orange, then a tad blue, and finally it is white-hot. Red is the lowest frequency of visible light, so it requires the least heat energy. The glowing white-hot horseshoe is emitting all the colors and even ultraviolet, and that takes lots of energy.
There are three central ideas of quantum physics. First, energy is not continuous but comes in small, discrete units. Second, electrons behave both as waves and as particles. Last, movement of these particles is random and not predictable. One can’t know both the position and the velocity of a particle at the same time.
The obvious question then is: What good is quantum mechanics? Much of our modern technology rests on quantum physics. Lasers, transistors, microchips, LEDs, MRIs, electron microscopes, USB flash drives, and superconductors all depend on quantum effects.
And finally, quantum theory settled a centuries-old debate about the nature of light. The Englishman Sir Isaac Newton claimed that light was a particle, but the Dutchman Christiaan Huygens showed that light is a wave. The quantum theory married these two seemingly competing ideas into one, stating that matter can behave as a wave and a wave can act as matter.
 Splitting atoms releases a tremendous amount of energy. This process is called fission. But not just any old atom will work. Fission requires the right kind of atom, and uranium, specifically uranium-235 (U-235), happens to be the atom of choice—it’s the most important atom in the production of nuclear power and atomic bombs.
Splitting atoms releases a tremendous amount of energy. This process is called fission. But not just any old atom will work. Fission requires the right kind of atom, and uranium, specifically uranium-235 (U-235), happens to be the atom of choice—it’s the most important atom in the production of nuclear power and atomic bombs.
That “235” is the sum of neutrons and protons in the nucleus, or center, of the atom. Protons have a positive electrical charge, and neutrons have no charge. The number of protons in the U-235 nucleus is 92; this is matched by the same number of electrons, with a negative charge, that swirl in orbits around the nucleus. Subtract 92 from 235 and you get 143, which is the number of neutrons in the nucleus.
Uranium is a common element on Earth and has been around since the Earth was formed. The U-235 atom “decays,” or changes (transmutes), into a less radioactive element all by itself by throwing off pieces of the nucleus—mostly alpha particles, which consist of two protons and two neutrons. Radioactivity is the streaming or emission of particles or rays from an atom’s nucleus. Sometimes the term “nuclear decay” is employed. This natural radioactivity is happening all the time. A handful of U-235 atoms would take billions of years to completely change into lead, because there are a gazillion atoms in a handful—fairly close to size of our national debt!
But the fact that uranium-235 could undergo changes provided scientists with a tantalizing clue in the late 1930s. What would happen if a neutron hit a U-235 nucleus? Particles with the same charge, such as positive and positive or negative and negative, repel each other. So it has to be a neutron, because a particle with a positive charge (proton) would be repelled by the positive protons in the nucleus, and a particle with a negative charge (electron) would be repelled by the negative electrons already orbiting the nucleus.
Lise Meitner, Fritz Strassman, Otto Frisch, and Otto Hahn in Germany and Enrico Fermi in Italy are credited with first inducing radioactivity artificially. If a neutron strikes a uranium nucleus at high speed, it goes right though the nucleus and comes out the other side. Nothing happens to the atom. But if a neutron can be slowed down sufficiently before it hits the uranium nucleus, it deforms the nucleus, which splits apart. Each part takes a share of the original atom’s protons and electrons, giving rise to two atoms of different elements, typically barium and krypton.
Here’s the wonderful part. Along with the two new elements, at least two neutrons are released. These neutrons can each split two other uranium atoms. And when these atoms split, a minimum of four neutrons split four more uranium atoms. As this cascading effect continues through a mass of uranium, we have a chain reaction. This process continues until all the U-235 atoms are split.
This is what happens in an atomic bomb, which is essentially a runaway nuclear chain reaction (see question 200). In the fission process, a tiny bit of mass is lost, and that lost mass goes into pure energy of blast, heat, and light. The equation that explains the equivalence between mass and energy is Albert Einstein’s famous “energy equals mass times the speed of light squared” (E = mc2). E is energy in joules, m is mass in kilograms, and c is the speed of light in meters per second (see question 201).
Nuclear power plants and nuclear aircraft carriers and submarines use uranium-235, but with a controlled chain reaction, which slows down the rate of the splitting of atoms tremendously.
There is no way to use natural uranium for a bomb. Natural uranium is mostly uranium-238; only 0.7 percent is U-235. That’s another way of saying that one in 140 uranium atoms are of the U-235 variety. The uranium used in power plants is enriched to 3 percent; that is, three in every one hundred atoms is U-235. The uranium used in a bomb must be enriched to about 90 percent U-235. So a nuclear power plant cannot blow up like a nuclear bomb. However, the chain reaction can “get away” from a nuclear power plant; witness the Three Mile Island and Chernobyl meltdowns.
Two commercial methods currently exist for enriching uranium: gas centrifuging and gaseous diffusion. Both require large-scale production facilities. Both also require wads of money and are time-consuming and tedious. The gas centrifuge method uses a series of rotating cylinders in which the heavier U-238 atoms are slung to the outside and the lighter U-235 atoms collect near the center of rotation. It is a fancy version of the old-time farmer’s cream separator. The gaseous diffusion process involves mixing the raw uranium with fluorine gas to get the gaseous compound uranium hexafluoride. This gas bombards screens with tiny holes in them. The smaller U-235 atoms get through, while the larger U-238 atoms do not. This process is repeated thousands of times for any one sample, and each time the uranium is a bit more U-235 and a bit less U-238.
If you have been following the news, you will have noted that Iran is enriching uranium. Their leaders say it is for peaceful purposes, but Western governments consider their intentions suspect; the plutonium from the spent fuel rods of a nuclear power plant can be processed and fashioned into a bomb. Plutonium-239 is one of the by-products of fission in a nuclear power plant. Pu-239 can be chemically separated from the spent fuel rods. Pu-239 is a good choice for weapons builders and was used in the second bomb dropped on Japan in August 1945.
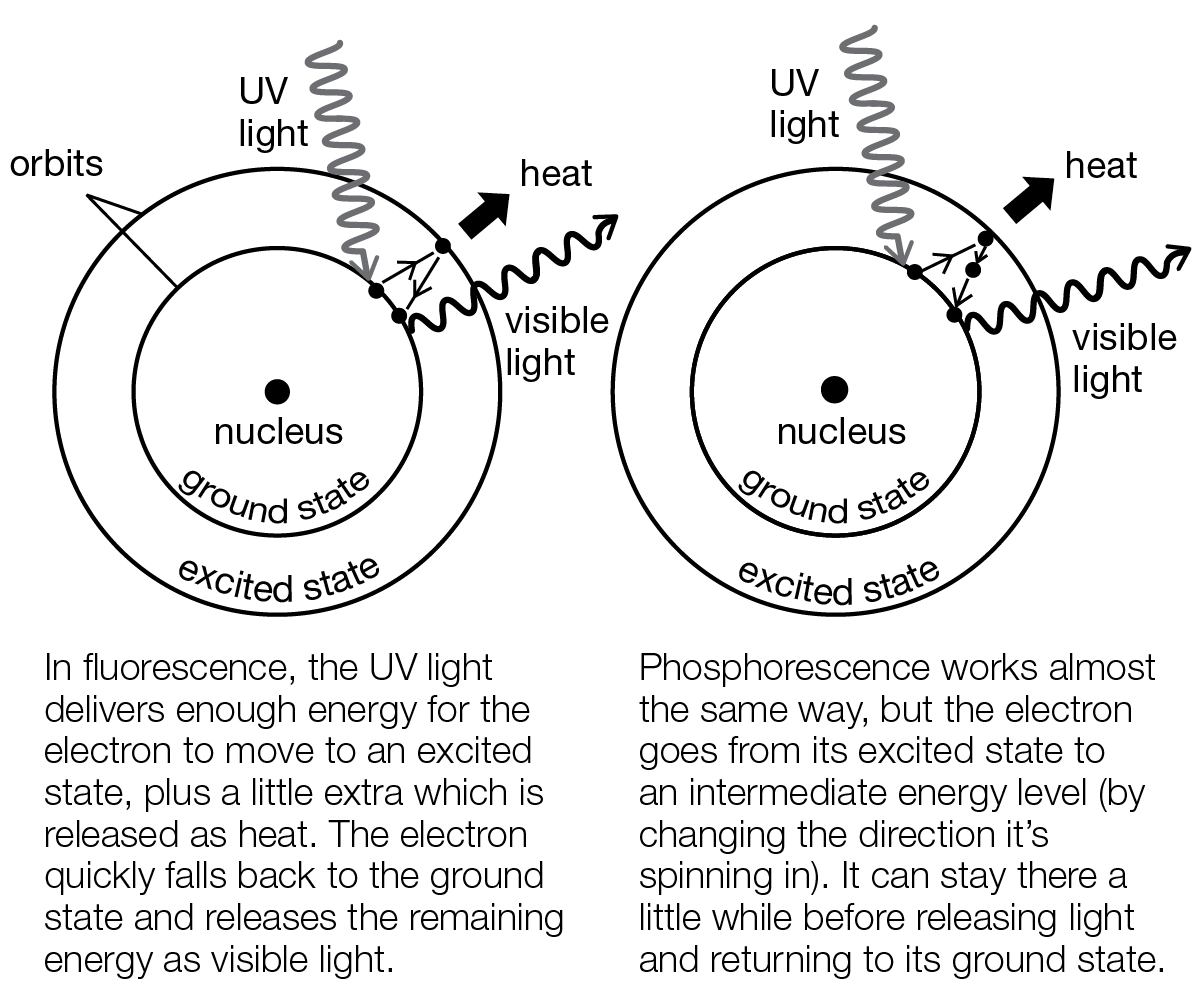
 Phosphorescence is responsible for an object’s glow-in-the-dark quality, but to fully grasp this concept, it would be helpful to first understand a related process, called fluorescence. Fluorescence is the emitting of light of a longer wavelength by an object when hit with light of a shorter wavelength. Simply put, a fluorescent object gives off light that we can see when it is struck by ultraviolet light, which we can’t see, and the visible light is emitted immediately. Some gemstones, like rubies, emeralds, and diamonds, are fluorescent, as are black light posters. A phosphorescent object, on the other hand, emits light over a period of several minutes, even after a light source is taken away; hence the name “glow-in-the-dark.” In most cases, glow-in-the-dark objects can be recharged again and again by exposure to regular light. The so-called regular light can come from sunlight, fluorescent lightbulbs, or incandescent light bulbs.
Phosphorescence is responsible for an object’s glow-in-the-dark quality, but to fully grasp this concept, it would be helpful to first understand a related process, called fluorescence. Fluorescence is the emitting of light of a longer wavelength by an object when hit with light of a shorter wavelength. Simply put, a fluorescent object gives off light that we can see when it is struck by ultraviolet light, which we can’t see, and the visible light is emitted immediately. Some gemstones, like rubies, emeralds, and diamonds, are fluorescent, as are black light posters. A phosphorescent object, on the other hand, emits light over a period of several minutes, even after a light source is taken away; hence the name “glow-in-the-dark.” In most cases, glow-in-the-dark objects can be recharged again and again by exposure to regular light. The so-called regular light can come from sunlight, fluorescent lightbulbs, or incandescent light bulbs.
Keep in mind that those glow sticks that you snap and shake are not phosphorescent materials. Glow sticks, especially popular for Halloween trick-or-treaters, produce light by mixing two different chemicals, which react with each other to produce light (see question 143).
Ultraviolet light striking either fluorescent or phosphorescent materials boosts the electrons orbiting the nuclei of their atoms into orbits farther from the nuclei. It’s something like putting the planet Mercury into Venus’s orbit around the Sun. The atom is said to be in an excited state. When the electron goes back to its original orbit, the atom emits light, only now it is light we can see. In phosphorescent materials, the electrons take their sweet time, going back home to their original orbit a few billion at a time. So they give off light over a time of a few seconds to several hours, depending on the kind of material.
Dating back to the 1930s, zinc sulfide was employed extensively for its luminescent properties. Luminescence is the emitting of light not resulting from heat. Zinc sulfide replaced the dangerous radioactive radium that was placed on watch dials and aircraft instruments so that they could be read in the dark. Later, strontium oxide aluminate was developed, which lasts ten times longer.
Today, such pigments are used on exit signs, pathway markings, and those decorative stars you can stick on ceilings. Timex, the watch company, patented a system they called Indiglo some years ago, which uses a transformer that takes 1.5 volts from the watch battery and multiplies it by a factor of ten, to 150 volts that excite a thin layer of phosphors.
It was the study of phosphorescent materials that led to the discovery of radioactivity in 1896 by the Frenchman Henri Becquerel. The unit for radioactivity, the becquerel (Bq), is named after him. Both the Moon and Mars have craters designated Becquerel in his honor.
 A good nine-volt battery has a high enough voltage to produce a mild shock if you briefly touch the contacts together with your tongue. You might also get a nasty metallic taste in your mouth.
A good nine-volt battery has a high enough voltage to produce a mild shock if you briefly touch the contacts together with your tongue. You might also get a nasty metallic taste in your mouth.
If the battery is completely run-down or depleted, little or no shock would be experienced by the “tester.” That’s the idea or theory of using the tongue as a battery tester. Read on to discover that the tongue procedure is not the best idea in the world.
The other commonly used batteries, AA, AAA, C, and D cells, are all 1.5 volts. So they most likely will not have sufficient voltage to produce this “tongue shock.” A nine-volt battery is actually six 1.5-volt AAA cells wired in series. Voltages over thirty volts may be dangerous.
It may work better if you put one terminal of the battery to your lip and the other end to your tongue. The low voltage and current will not hurt you. The voltage is the electrical force or pressure pushing the electrons (electricity) on a conductor or wire. Current is the flow of the electrons on that same conductor or wire. Voltage is analogous to the water pressure in your plumbing pipes. Current is akin to the water flowing in the pipes.
The body’s resistance to electricity is primarily concentrated in the skin and varies with the skin’s condition. Dry skin has a lot of resistance, so touching a nine-volt battery or a twelve-volt car battery will not produce a shock, because there’s not enough current flowing to overcome that resistance. But if the skin is wet or punctured from a cut, abrasion, or needle, the resistance of the skin goes way down, and more current flows, which raises the likelihood of a shock. Under no circumstances should you touch a battery of any kind using both hands if you have an open wound, sore, cut, etc.; the opening gives the current access to wet and saltier tissue, which transmits it better. You don’t want to put any voltage across your heart from one hand through your body, to the other hand.
The perception of an electric shock is complex. It depends on the voltage, duration, current, and frequency of the electricity and the path it takes. Current entering the hand needs to be about five to ten milliamperes (mA) for a person to feel a shock; currents of under 100 mA can be deadly depending on what parts of the body they pass through.
But let me suggest that there are better ways to check a battery. Discount stores, hardware stores, and auto supply stores sell those cheap little multimeters, or battery testers, for not much more than five dollars. Take care of yours, and it will last a lifetime. Plus you can use the multimeter to measure other voltages, find electrical shorts and open circuits, and measure current flows. In addition, if you have one of those multimeters lying around, visitors will be impressed with your electrical knowledge and handyperson skills. So it makes a good conversation piece.
When I was a kid growing up on that Seneca farm, there were young lads who would test an electric fence in a decidedly different fashion. Instead of touching the fence, they would walk up to the electric fence, pull their trousers down, aim, and . . . Not a good idea!
 The jury is out on that question. The issue came to national attention in 1993, when a Florida man appeared on a national talk show and claimed his wife’s brain tumor was caused by radio frequency radiation from her cell phone. His lawsuit was dismissed in 1995 due to a lack of scientific and medical evidence.
The jury is out on that question. The issue came to national attention in 1993, when a Florida man appeared on a national talk show and claimed his wife’s brain tumor was caused by radio frequency radiation from her cell phone. His lawsuit was dismissed in 1995 due to a lack of scientific and medical evidence.
There is some anecdotal support, but not scientific evidence, of a link between brain tumors and extreme cell phone use. The lawyer who defended O. J. Simpson, Johnnie Cochran, developed a brain tumor on the side of his head that he held his cell phone against. Senator Edward Kennedy of Massachusetts was diagnosed with a brain tumor in May 2008 and died in August 2009. Some believe his brain tumor was caused by heavy cell phone use. One has to be careful about these kinds of stories. Cause and effect can be quite tricky.
Cell phones use radio frequencies (RFs) that are higher than the frequencies of radio and TV waves but somewhat lower than those of radars and microwave ovens. The amount of that RF energy a person using a cell phone is exposed to varies with the distance from the cell tower, the frequency of cell phone use, the cumulative length of their calls, and the age of the cell phone. The older, analog cell phones emit more radiation than the newer digital ones. The radio waves used by cell phones are nonionizing, unlike the radiation from X-rays and radioactive materials, which is ionizing. Ionizing radiation has enough energy to knock an electron out of its orbit around an atom’s nucleus, which causes the atom to become charged, or ionized (see question 206).
The evidence so far indicates that the energy from a cell phone does not contain enough energy to damage genetic material, which could lead to cancer. But the problem is that cell phone use is relatively new. No one knows for sure about long-term use, say, over a lifetime. Scientists have not had the opportunity to carry out long-term studies. Brain tumors often take twenty or more years of exposure to develop, and since most people began using cellphones about twenty years ago, we’re just starting to be able to see long-term effects.
Nor do scientists know enough about frequent use, when people are on their cell phone for several hours a day. Few authoritative people claim that there is a definite cause and effect between brain cancer and cell phone use. But prudent people in the field are saying, “better safe than sorry.”
The European Union has been doing a massive study. European countries have been using cell phones since the early 1990s, so they have a longer timeframe in which to study the problem. The study was completed in 2012. The report indicates that there is no link between cell phone use and cancer, but the information has not satisfied all critics.
 Radiation treatment of cancer, called radiation therapy or radiotherapy, comes in two forms. One kind of treatment uses beams that come from machines, much like X-rays but at much higher energy levels. Indeed, X-rays themselves are used for cancer treatment. The second method uses waves or particles that come from radioactive sources.
Radiation treatment of cancer, called radiation therapy or radiotherapy, comes in two forms. One kind of treatment uses beams that come from machines, much like X-rays but at much higher energy levels. Indeed, X-rays themselves are used for cancer treatment. The second method uses waves or particles that come from radioactive sources.
Cancer is cell division gone wild, or out of control. The idea of radiation therapy is to kill cancer cells. Radiation ionizes atoms and molecules, which means that it knocks electrons out of orbit around the nucleus of the atoms. The atoms are no longer neutral; they have a charge of positive or negative. This ionization affects the nuclei of the cells, and particularly the DNA in the nuclei, which influences the cells’ ability to grow and divide. The hope is that many of the cancer cells will fail to divide and therefore will die. Good cells are also damaged during radiotherapy, but those cells have a better chance of repairing the damage to their DNA and recuperating. Healthy cells rejuvenate on their own.
In addition, doctors restrict the amount of radiation to what is known to be safe for the normal cells. They can also target the therapeutic dose to the site of the cancer so as to minimize the exposure of normal tissues.
In many cases, the radiation therapy is delivered by an external beam aimed at the cancer site, as in the case of the gamma-ray knife, used for brain tumors. A rigid head frame is attached to the patient’s skull so the head cannot move during therapy. Over two hundred beams of low-intensity gamma rays from cobalt-60 converge on a deep-seated brain tumor, adding up to an intense dose of radiation delivered to a small area safely. Treatment can last anywhere from minutes to several hours.
The second method of radiotherapy is to introduce radioactive sources into the body. These may take the form of pellets, or seeds, in or next to the targeted cancer sites. Prostate cancers, for example, can be treated by implanting radioactive seeds or pellets directly into a localized cancer site. It is an outpatient procedure and has a short recovery time. Another approach is to inject or have the patient swallow a radioactive substance that naturally binds to the type of tissue where the cancer arose or has an antibody attached that targets such tissue.
Radiation therapy is often used in combination with surgery (sometimes before it, to reduce the size of a tumor), chemotherapy, and/or hormone therapy, because it can decrease the chances of cancer’s recurring. And even when a cancer cure is not possible, radiation therapy is used to relieve pressure or reduce pain.
Radiotherapy does have side effects. Tissue damage, hair loss, fatigue, infertility, and nausea are some of the most common.
 We’ve all noticed that most liquids and gases are transparent, or clear, and that most solids are opaque, meaning that light can’t pass through them. This is a fundamental difference between solids and liquids.
We’ve all noticed that most liquids and gases are transparent, or clear, and that most solids are opaque, meaning that light can’t pass through them. This is a fundamental difference between solids and liquids.
Solids are materials in which the molecules are held tightly together by bonds that give the matter rigidity. When a solid melts, the strength of those bonds lessens and the molecules begin to align themselves randomly. This movement from ordered to random is the reason that light can pass through liquids and gases. The molecules are not stacked neatly together, and the gaps and holes allow light to pass through (with a few exceptions, like liquid mercury, which is opaque). Think in terms of a brick wall. When the bricks are neatly laid and mortared together, no light gets through. But if bricks are lying in a random pile, light can get through the gaps.
But that doesn’t explain how light gets through glass, which, of course, is a solid. Now we have to go to the atomic level. When light hits a pane of glass, it sets up vibrations in the atoms of the glass. A system tends to oscillate, or vibrate, with a larger amplitude at some rates of vibration (frequencies) than at others. The vibration rate at which a substance responds most strongly is referred to as its resonant frequency, or natural rate of vibration. A bell rings at a certain tone, or frequency. A tuning fork vibrates at a set frequency. So do the electrons in different kinds of matter.
Light in the visible region hitting an atom in glass forces its electrons into vibration. The atom holds the energy for a bit of time, then passes it on to the next atom, causing that one to vibrate a tad and then pass it on to the next, and so on, until finally the light comes out of the glass at the same frequency as the light that went in.
In a material, such as transparent glass, the vibration rate of the atoms is such that the energy is transferred from atom to atom, so that a piece of light hitting the first atoms are able to cause the last atoms to emit light. In a solid material, such as steel, which does not transmit light, the incoming light causes the first few atoms to vibrate a bit, but that vibration dies down quickly because it cannot pass its vibration to the next atoms. Instead, the light energy heats up the material.
This process of absorptions and re-emissions of the light energy through glass takes time. So while light travels through space or air at about 186,000 miles per second, it travels through glass at about 124,000 miles per second.
If ultraviolet light hits glass, there is a strong resonance between the electrons and nuclei of the glass atoms, which causes them to vibrate violently. The atoms collide repeatedly with nearby atoms and give up energy as heat. This transforms nearly all the energy of the ultraviolet light wave to heat energy; very little is left in the form of a light wave to pass through the glass. Infrared light waves, which are much longer than those of visible light and have a lower frequency, vibrate not only the electrons but the entire structure of glass. This vibration increases the internal energy of the glass and makes it warm. The infrared light can’t get through. That is why our car heats up when we leave it sitting in the Sun. Visible light gets through the glass, but the infrared, with its longer waves, can’t get back out and the interior heats up. It is the classic greenhouse effect. In short, glass is generally transparent to visible light but not to ultraviolet or infrared.
 There are two types: fission and fusion. The fission type of atomic bomb works by splitting an atom. The fusion kind brings together, or fuses, lighter atoms. Either type, the fission atom bomb (A-bomb) or the fusion hydrogen bomb (H-bomb), releases a tremendous amount of energy.
There are two types: fission and fusion. The fission type of atomic bomb works by splitting an atom. The fusion kind brings together, or fuses, lighter atoms. Either type, the fission atom bomb (A-bomb) or the fusion hydrogen bomb (H-bomb), releases a tremendous amount of energy.
A fission bomb uses uranium-235 (92 protons and 143 neutrons) or plutonium-239 (94 protons and 145 neutrons) to create a nuclear explosion (see question 203). A neutron splits the U-235 atom’s nucleus into two fragments, with the release of an enormous amount of energy and a loss of two or three neutrons in the process. The released energy takes the form of kinetic energy, heat, and light. Meanwhile, the two or three neutrons given off can then split other U-235 atoms, which give off more neutrons, which split more atoms, and so on—a chain reaction.
Energy is released by splitting the nucleus because the fission fragments and the neutrons together weigh less, or have less mass, than the original U-235 nucleus. The lost mass, or mass defect, is actually turned into pure energy, which follows Einstein’s famous equation, E = mc2. Because c stands for the speed of light and c squared is a huge number, just a little bit of mass is equivalent to a fantastic amount of energy. A pound of uranium, for example, has the same energy as three hundred thousand gallons of gasoline. The critical mass is the minimum mass of fissionable material needed to sustain a chain reaction. Reports indicate that as little as thirty pounds of U-235 or twelve pounds of Pu-239 can be fashioned into an atomic bomb. In any fission bomb, the nuclear material must be kept in separate subcritical masses that don’t, on their own, support fission.
There are two general methods of making an A-bomb: gun and implosion. The first A-bomb, used on Hiroshima on August 6, 1945, and dubbed Little Boy, was the gun-type, while the one used on Nagasaki on August 9, 1945, and named Fat Man, was the implosion kind.
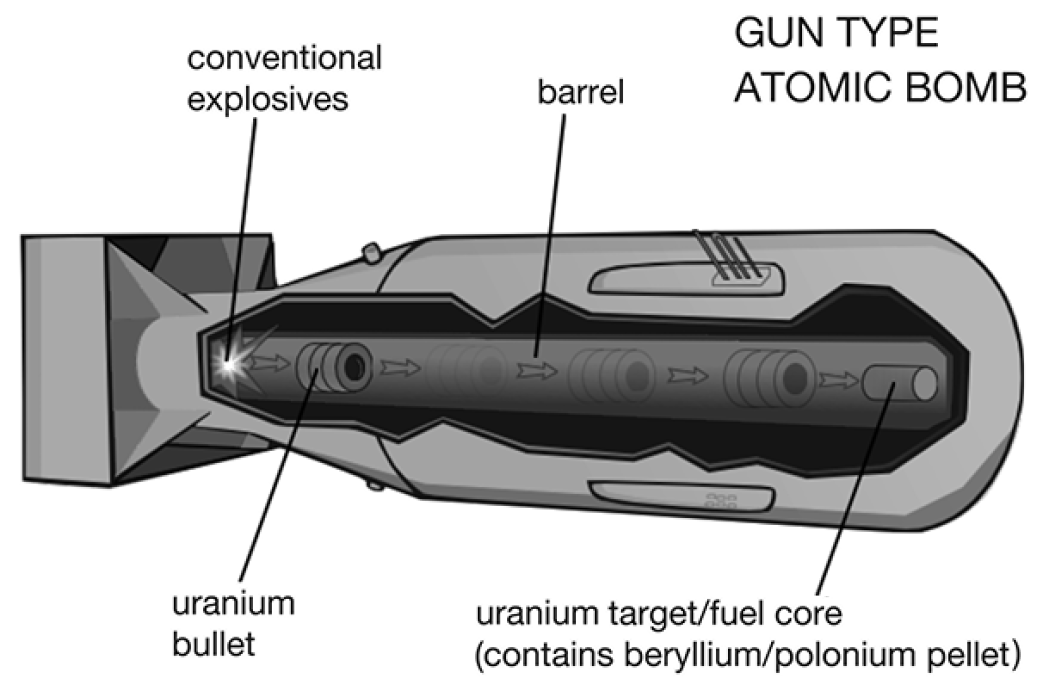
In the gun type, two subcritical masses of enriched U-235 are kept separate from each other in a sealed cylinder like the barrel of a gun until the time of detonation, when explosives propel one piece down the barrel like a bullet into the other piece. Neutrons, needed to start the chain reaction, are produced by using a small pellet of polonium and beryllium within the fuel core. A piece of foil keeps the two elements separated. When the masses of uranium come together, the foil is broken, and the polonium emits alpha particles, which collide with the beryllium to produce the neutrons needed to initiate the chain reaction.
Because the gun type is necessarily long, the first bomb, Little Boy, looks like an overgrown cigar. Scientists were so sure it would work back in 1945 that they never tested it beforehand. At the time, enriched uranium-235 was so scarce, there wasn’t enough to drop another Little Boy–type bomb for many months.
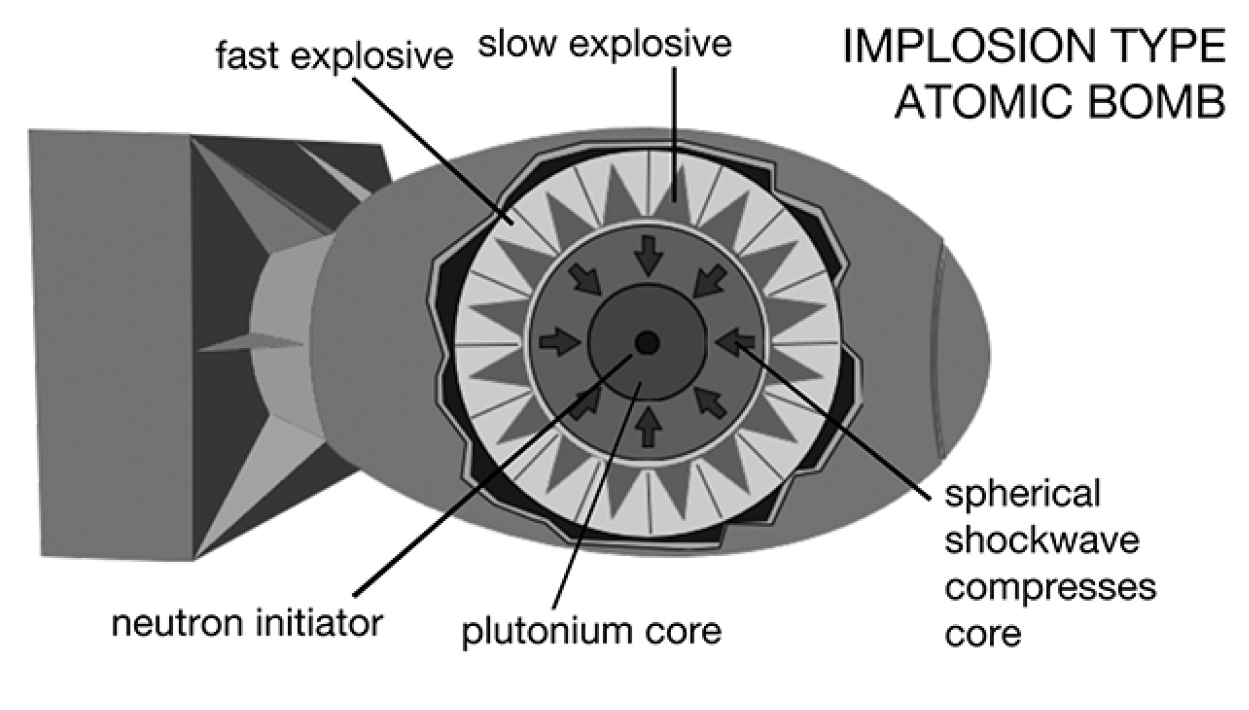
The implosion type weapon is one in which the fissile Pu-239 is surrounded by high explosives that compress the material sufficiently to make it detonate. It is similar to packing loose snow into a snowball.
Some excellent movies have been made about the making of the atomic bomb. Day One is a 1989 TV movie with big-name stars like Brian Dennehy, Hume Cronyn, and Hal Holbrook. Fat Man and Little Boy is a big-screen movie, also made in 1989, with Paul Newman playing General Leslie Groves.
 Not a whole lot, really. Albert Einstein wrote his famous equation practically as an afterthought to his 1905 theory of relativity. He discovered that there is an intimate relationship between mass and energy. Energy is the ability to do work, and mass is the amount of substance of something—how much stuff there is. They may seem very different, but mass and energy are interchangeable aspects of the same thing. Scientists refer to this connected unit as mass-energy. The equation tells us that even a tiny bit of mass contains a huge amount of energy. The letter m stands for mass and the letter c for the speed of light. The speed of light is a huge number (186,000 miles per second), and its square is even huger. Mass multiplied by the square of the speed of light yields an unbelievably large figure. So even a tiny bit of mass holds a tremendous amount of energy.
Not a whole lot, really. Albert Einstein wrote his famous equation practically as an afterthought to his 1905 theory of relativity. He discovered that there is an intimate relationship between mass and energy. Energy is the ability to do work, and mass is the amount of substance of something—how much stuff there is. They may seem very different, but mass and energy are interchangeable aspects of the same thing. Scientists refer to this connected unit as mass-energy. The equation tells us that even a tiny bit of mass contains a huge amount of energy. The letter m stands for mass and the letter c for the speed of light. The speed of light is a huge number (186,000 miles per second), and its square is even huger. Mass multiplied by the square of the speed of light yields an unbelievably large figure. So even a tiny bit of mass holds a tremendous amount of energy.
We don’t experience this in everyday life. We don’t see that every gram of water or soil or soap contains the energy to run whole cities. It’s like a rich person who doesn’t spend any money; no one can tell how rich they are. In everyday life, our energy-producing activities are chemical processes, from metabolizing food to burning wood in a fireplace, gasoline in an engine, or coal in a power plant.
Want energy? The name of the game is to lose mass. Chemical processes do cause a loss of mass, but it is so tiny it can hardly be measured. That’s why we don’t get much energy from chemical processes, even dynamite, or TNT. A pound (454 grams) of TNT exploding loses only half a billionth of a gram of mass.
Nuclear processes are quite different from chemical processes. Chemical reactions involve the transfer, loss, gain, and sharing of electrons. Nothing takes place in the nucleus of the atom in chemical reactions. “Nuclear” means working with the inner core of an atom, the nucleus, rather than with the electrons going around the nucleus. Nuclei of uranium atoms, for example, can split into two pieces, and when they do, the two pieces don’t add up to the original whole. That seems odd at first and defies commonsense thinking. Cut a loaf of bread into two pieces, for instance, weigh the two pieces on a scale, and you will find the two pieces add up to the weight of the whole loaf that you started with.
That does not happen at the nuclear level. If an atom of uranium is split, the two pieces together weigh less than the whole uranium atom, by about a tenth of 1 percent. The lost mass turns into pure energy. How much energy? Einstein’s E = mc2 tells us how much. A little bit of mass turns into an enormous quantity of energy.
Split a uranium-235 atom, then particles (neutrons) from that atom split two more atoms, which in turn split four more atoms, then eight more atoms, and you have a chain reaction (see question 194). If that happens in a split second, it’s an atomic bomb. If you slow down that chain reaction and control it, it’s a nuclear power plant. A nuclear power plant cannot explode like an atomic bomb. The fuel is different. Most reactors use uranium enriched to 3 percent U-235. An atomic bomb is uranium enriched to 90 percent U-235. The Iranian government is accused of trying to do such enrichment.
Isotopes are two or more forms of the same atom that have the same number of protons but a different number of neutrons. For example, uranium-235 and uranium-238 both have the same number of protons, namely 92. But U-235 has 143 neutrons and U-238 has 146 neutrons.
So Einstein’s famous equation is not needed to build an atomic bomb. However, the equation does measure the size of the blast.
 Superman actually provides a good example of the effect of lead on X-rays. Superman, Clark Kent, was “more powerful than a locomotive,” “able to leap tall buildings in a single bound,” and “faster than a speeding bullet.” And with his X-ray vision, he could see through solid objects, but not lead.
Superman actually provides a good example of the effect of lead on X-rays. Superman, Clark Kent, was “more powerful than a locomotive,” “able to leap tall buildings in a single bound,” and “faster than a speeding bullet.” And with his X-ray vision, he could see through solid objects, but not lead.
There is good physics behind Jerry Siegel and Joe Shuster’s 1930s cartoon character. X-rays are a form of electromagnetic radiation, just like light, microwave, cell phone, radio, and television waves. All these energy waves are bouncing up and down and sideways as they all move at the speed of light, and X-ray waves do this at a higher frequency than the rest. The higher the frequency (vibrations per second), the higher the radiation energy. The higher the energy, the deeper a ray will penetrate matter, which is the reason why dentists and doctors use X-rays to see images of our teeth and our bones. The X-rays go right through our flesh, but some are absorbed by the denser bones and cast a nice diagnostic shadow on a piece of photographic film.
That’s the good news. The bad news is that X-rays, as well as gamma rays, are ionizing radiation. They knock electrons off atoms, leaving behind an ion. An ion is an atom that is missing one or more electrons. These atoms are not playing with a full hand, so to speak. They can change body chemistry in harmful ways. One such bad outcome is cancer.
So what can stop X-rays? Anything that has a lot of atoms with a lot of electrons going around the nuclei, because each time an X-ray knocks an electron out of orbit, it loses energy. The law of conservation of energy is in play here: If the atom containing the electron gains energy, then the X-ray loses energy.
To make your own X-ray shield, you’d need to get a material that has the most electrons per atom and the most atoms per cubic inch possible—something densely packed. Uranium is an excellent choice, because it has ninety-two electrons per atom and is nineteen times as dense as water. Gold works, too: seventy-nine electrons per atom and nineteen times denser than water. Or platinum, which has seventy-eight electrons per atom and is twenty-one times denser than water. But uranium, gold, and platinum are too expensive, and not much better than lead. And that’s why we settle for lead, which has eighty-two electrons per atom and is eleven times denser than water. Lead is cheap, about one dollar a pound.
The X-ray technician drapes a lead-lined vest over the patient at the dental office. Then they stand behind a lead-lined wall and look through a lead-laced window before zapping the patient’s mouth with X-rays. Because of its density of electrons, lead can stop any kind of ionizing radiation, so we find it used in all sorts of medical settings.
 Plutonium is a radioactive metal. Its symbol is Pu, and it is named after the former planet Pluto. Plutonium has ninety-four protons in the nucleus, so we say it has an atomic number of ninety-four. Its most important isotope is Pu-239, which has 145 neutrons in the nucleus. Add those 94 protons to the 145 neutrons and you have an atomic mass, or atomic weight, of 239.
Plutonium is a radioactive metal. Its symbol is Pu, and it is named after the former planet Pluto. Plutonium has ninety-four protons in the nucleus, so we say it has an atomic number of ninety-four. Its most important isotope is Pu-239, which has 145 neutrons in the nucleus. Add those 94 protons to the 145 neutrons and you have an atomic mass, or atomic weight, of 239.
Pu-239 is valuable as a fuel in nuclear power plants and in atomic- bomb making.
Plutonium has a half-life of 24,100 years. If you start with a pound of plutonium, you will have a half-pound of plutonium left after 24,100 years. The other half-pound will have changed (transmuted) to some other element.
Plutonium is silvery white in pure form, but turns yellowish and greenish when oxygen hits it. It’s very heavy stuff, having about the same density as gold. When a plutonium nucleus fissions, or splits, it releases a tremendous amount of energy in the form of kinetic energy and heat.
Virtually no plutonium exists in nature. In 1940, a team of scientists led by Dr. Glenn T. Seaborg and Dr. Edwin McMillan bombarded uranium with neutrons to produce neptunium, which transmuted to plutonium. During the Manhattan Project, large reactors were set up at Hanford, Washington, along the Columbia River, to produce plutonium to build an atomic bomb. Other Manhattan Project scientists at Los Alamos, New Mexico, discovered that plutonium fissions too quickly to be used in a gun-type weapon. So they designed an implosion-type weapon, one with a symmetrical shock wave that would compress the plutonium into an explosive critical mass. It’s something like taking a handful of loose snow and squeezing it into a snowball. Plutonium pieces are arranged symmetrically around a beryllium-polonium core trigger. Explosives are packed on the outside, and, upon detonation, the explosives create a shock wave that compresses the Pu-239 onto the core, fission begins, and the bomb explodes. The whole fission reaction occurs in about six hundred-billionths of a second.
The first atomic bomb using plutonium was tested at Trinity Site in New Mexico in July 1945. A plutonium-239 implosion-type atomic bomb, named Fat Man, was dropped on Nagasaki on August 9, 1945.
Terrible as they were, this bomb and Little Boy, dropped on Hiroshima, arguably shortened the war, saved thousands, if not millions, of lives on both sides, and kept the Russians out of the area. An invasion of Japan, scheduled for November 1945, would have been so very bloody and devastating.
It was revealed after the war that the Japanese High Command had hoped to make the American invasion so costly in men and material that they could sue for peace and maintain their islands, government, and military infrastructure.
Not all plutonium is associated with atomic bombs. Another isotope of plutonium is Pu-238, which has a half-life of eighty-eight years. It emits alpha particles, so it is valuable as an electric power source for our space probes sent to the Moon, Jupiter, Saturn, and Mars. Artificial heart pacemakers using plutonium-238 have been around for about forty years. The NASA robotic rover to Mars, Curiosity, carried 4.8 kilograms, or about 10.5 pounds of Pu-238. Curiosity landed on the Red Planet in August 2012.
 When you buy a clock or watch labeled “atomic clock,” you are buying one that you can synchronize to the United States official atomic clock in Colorado. These clocks and wristwatches can pick up the shortwave radio transmissions of broadcast stations on several frequencies. The National Institute of Standards and Technology (NIST) and the US Naval Observatory air time signals from powerful transmitters on radio stations WWV, and WWVB from Fort Collins, Colorado, and WWVH, from Kauai, Hawaii. The frequencies are 2.5, 5, 10, 15, and 20 MHz. Reception of any radio signal can be affected by weather, location, time of day, time of year, atmospheric and ionospheric conditions, and other factors. Using so many frequencies ensures reception anywhere in the world for at least part of the day.
When you buy a clock or watch labeled “atomic clock,” you are buying one that you can synchronize to the United States official atomic clock in Colorado. These clocks and wristwatches can pick up the shortwave radio transmissions of broadcast stations on several frequencies. The National Institute of Standards and Technology (NIST) and the US Naval Observatory air time signals from powerful transmitters on radio stations WWV, and WWVB from Fort Collins, Colorado, and WWVH, from Kauai, Hawaii. The frequencies are 2.5, 5, 10, 15, and 20 MHz. Reception of any radio signal can be affected by weather, location, time of day, time of year, atmospheric and ionospheric conditions, and other factors. Using so many frequencies ensures reception anywhere in the world for at least part of the day.
The radio receiver is tiny, about the size of the tip of a pencil, and can easily be embedded in computer chips and used in GPS units and cell phones. This miniature receiver need only pick up the radio transmission once every few days to correct any error in the receiver’s clock. Generally, clock updating takes place at night, when the signal is strongest.
Many automated home devices, including computers connected to the Internet, are continually and automatically updated to the correct time. For example, Windows has a built-in service that allows your computer to reference the atomic clocks operated by NIST. Your current computer time is compared with the current atomic time and an adjustment is made to keep your local computer up-to-date with the exact time. Apple computers have a similar arrangement.
WWVB sends a digital time code on 60 kilohertz (kHz) that modulates, or changes, the power of the carrier signal picked up by the receiving antenna to indicate a 1, a 0, or a separator. The receiver decodes these bits to get indicators for the time, the day of the year, standard or daylight saving time, and regular or leap year. These indicators report what is called Coordinated Universal Time.
A grandfather clock keeps time by the steady back-and-forth oscillations of a pendulum powered by falling weights. Old-time clocks use a balance wheel with alternating backward and forward motion powered by a wound spring. Quartz watches depend on the vibrating oscillations of a crystal. The tiny crystal looks like a tuning fork, and will vibrate at a fixed rate (usually 32,768 times per second) when powered by a small battery.
Atomic clocks, the kind used by NIST, go back to 1945, when physicist Isidor Rabi determined that atoms maintain a steady, unchanging vibration rate even more precise than the pendulum, balance wheel, and quartz crystal.
Early atomic clocks used the vibration of ammonia molecules, but today’s atomic clocks go with cesium. Every atom has several characteristic oscillation frequencies. To turn the cesium resonance into an atomic clock, it is necessary to measure one of the resonant frequencies accurately. This is done by locking a crystal oscillator to the principal microwave resonance of the cesium atom. This signal is in the microwave range of the radio spectrum, and just happens to be at the same sort of frequency as direct broadcast signals.
Estimates vary as to the accuracy of atomic clocks. One scientist claims precision to within one second in 126 years. Another report puts it at one second in 30 million years. Either one will get you to the church on time!
NIST has the big expensive atomic clocks that generate extremely accurate time signals, and you and I can buy inexpensive atomic clocks synchronized to the government’s atomic clocks. We just have to set them to the right time zone.
Listen in to WWV on any one of the frequencies listed on page 278 on any shortwave radio receiver. The 15- and 20-MHz signals are strongest during the day. The 5- and 10-MHz come in best at night. You hear the steady tick, tick, tick, and then a human voice announces the time on the minute. It’s quite amazing!
 No one has ever really seen an atom, and yes, we humans like to see something to believe in it. In order to see something, light has to hit it and reflect some of that light to our eyes. But an atom is thousands of times smaller than a wave of light. So light can’t hit a single atom and bounce off it.
No one has ever really seen an atom, and yes, we humans like to see something to believe in it. In order to see something, light has to hit it and reflect some of that light to our eyes. But an atom is thousands of times smaller than a wave of light. So light can’t hit a single atom and bounce off it.
Most of what we know about atoms comes from having something, like an electron or an alpha particle, hit an atom and then watching where the particle goes after it bounces out of the atom. We can see where particles go by using a cloud chamber or by monitoring them when they hit a specially coated screen. A cloud chamber is a closed box containing a supersaturated vapor of alcohol. A charged particle will cause the vapor to condense on it, leaving a trail of tiny bubbles that are visible to an observer.
The story of the atom goes way back to the Greeks. Democritus (460–370 BC) first proposed the idea of an “atom,” or “iota,” the smallest unit of matter that maintains the identity of the whole. Mendeleyev, with his periodic table of elements, showed in about 1869 that elements have regular repeating properties. John Dalton, an English school teacher, proposed the first modern atomic theory in 1808. In 1897, English scientist J. J. Thomson passed electricity through gases and discovered the electron. In 1911, Ernest Rutherford, a British physicist, shot alpha particles at a gold foil and proved that the atom has a dense central core, termed the nucleus. In 1932, James Chadwick discovered a particle within the nucleus, called the neutron. In the 1930s, particle accelerators, commonly referred to as atom smashers, were used to measure the sizes and masses of atoms and their parts. Today, the most accurate picture of the atom deals with quantum mechanics and discoveries made in the early 1900s by Niels Bohr, Erwin Schrödinger, Louis de Broglie, and Werner Heisenberg.
A new type of microscope was invented in 1981 called a scanning tunneling microscope (STM), which uses a tiny, sharp tip that conducts electricity and a piezoelectric scanning device attached to the tip. A piezoelectric device is a crystal that flexes when a voltage is applied and, conversely, generates a voltage when it is flexed. When the tip meets an atom, the flow of electrons, or current, between the atom and the tip changes. A computer registers the change in terms of x and y coordinates and maps the current’s pattern over a surface. It is much like the old phonograph needle following the grooves of a vinyl record. This STM microscope allows scientists to see the outlines, but not the insides, of atoms.
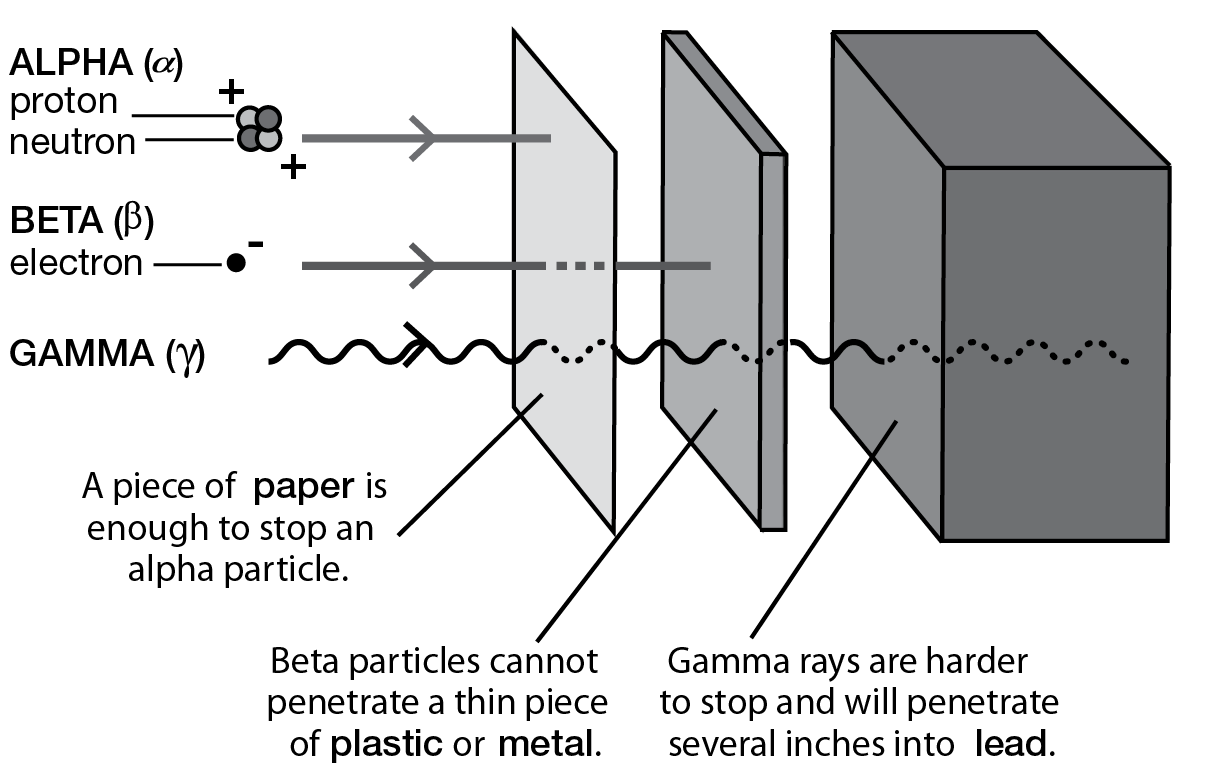
 All electromagnetic waves, like light, radio, and television waves, are considered to be radiation. But when we hear the word “radiation,” we generally think of nuclear radiation. There are three basic kinds of nuclear radiation: alpha, beta, and gamma.
All electromagnetic waves, like light, radio, and television waves, are considered to be radiation. But when we hear the word “radiation,” we generally think of nuclear radiation. There are three basic kinds of nuclear radiation: alpha, beta, and gamma.
An alpha particle is the nucleus of a helium atom. It consists of two protons and two neutrons. An alpha particle is big on a nuclear scale, so it cannot penetrate very far into matter; a piece of paper is enough to stop it. But alpha particles make changes in our bodies by knocking out electrons that orbit the nuclei in our cells (ionization); they’re like bulls in a china shop. If alpha particles are kept outside the body, little harm can be done. If ingested, alpha particles are devastating. Read the account of the polonium-210 poisoning of former Russian spy Alexander Litvinenko to get a sense of how deadly alpha radiation can be if taken internally.
A beta particle is an electron, which is much smaller. Betas bore deeper into matter. Most beta particles can be stopped by thin pieces of plastic or metal. Beta particles penetrate deeper than alpha particles but are less damaging than alpha particles that have traveled equal distances. Alpha particles are huge compared to beta particles and cause havoc in tissue. If alpha particles are like bulls in a china shop, then beta particles are like poodles in a china shop.
Direct exposure of the skin to beta particles can damage and redden it. Inhaling or ingesting beta particles is of greater concern. Inside the body, beta particles can damage tissue and organs and lead to cancer later in life.
Gamma rays are similar to X-rays but have higher energy. Gamma radiation is very penetrating and is often used to treat tumors, but uncontrolled exposure can cause widespread damage in many body tissues. Because gamma rays have no mass, they are not easily stopped or slowed down by collisions with matter.
Radiation can be helpful or harmful, because it has the potential to either kill a person or cure a person. A single large dose can be fatal, but the same dose over a long period, say a month or a year, can have little or no effect. A large dose of radiation distributed over the entire body can cause death, but the same dosage directed to a small area, say, a cancerous tumor, can be delivered safely.
The troubling question about radiation is: What are the effects of very low levels? Is there a threshold, or minimum value, of radiation that has an effect on the human body? Even one hundred years after the discovery of radiation, there is much debate on this topic.
 I know what you mean! I have three acrylic long-sleeve winter shirts. When these shirts come out of the clothes dryer, my black cotton socks have to be pulled off the shirts. A crackling sound can be heard, and sparks can be seen. This is static electricity, where the electrons are not moving on a conductor, such as a wire. Current flow electricity implies moving, not static, charges.
I know what you mean! I have three acrylic long-sleeve winter shirts. When these shirts come out of the clothes dryer, my black cotton socks have to be pulled off the shirts. A crackling sound can be heard, and sparks can be seen. This is static electricity, where the electrons are not moving on a conductor, such as a wire. Current flow electricity implies moving, not static, charges.
Clothes, of course, are ultimately made of atoms, and atoms consist of electrons, protons, and neutrons. Electrons have a negative electric charge, protons a positive charge, and neutrons no charge. An object that has an equal number of protons and electrons has no net electric charge. So it is neutral, that is, not charged, and does not cling to anything.
While tumbling in the clothes dryer, the acrylic shirts acquire a negative charge and the cotton socks get a positive charge. The charges are caused by friction between the two types of material. Opposite charges attract, so the sock clings to the shirt. When the sock is pulled from the shirt, the charge tries to equalize, or become neutral, by producing a tiny lightning bolt to discharge itself. This little lightning bolt, which see as a spark, can be as high as thirty thousand volts but holds hardly any current.
What type of charge a material acquires by friction is based on the triboelectric series, a list first published in 1757 on static charges. Materials are listed in order of the polarity of charge separation when they are touched with another object. A material toward the bottom of the series, when touched to a material near the top of the series, will attain a more negative charge. Acrylic shirts and cotton socks are sufficiently far apart on this list to ensure a very fine exchange of electrons, and a mighty fine spark.