From Space to Earth and Back Again
The sheer scale, complexity, and urgency of climate-change action is nothing short of overwhelming for anyone to process. We do not typically brace ourselves for uneasy emotions when reading a non-fiction science book; however, the seemingly inevitable arrival of climate change is hard to stomach. While feelings of helplessness are certainly founded, we believe that much of the fear and confusion surrounding climate change stems from the uncertainty that it presents. Knowledge can be a powerful tool to face these uncertainties. Education can help us overcome these fears by equipping ourselves with the science-backed knowledge needed to advocate boldly for ourselves, our communities, and our planet. In this chapter we will take a major step back (actually leaving the solar system!) to gain some perspective on how this whole mess with CO2 and climate change started in the first place.
Back to the Beginning
Where did carbon dioxide originate? This question does not usually arise in discussions around climate change. To answer it, we must go all the way back to the big bang, when the universe was but a miniscule singularity, which then expanded during 13.8 billion years to create the cosmos as we recognize it today.
The start of the universe was marked by a rapid expansion of matter in a state of extremely high density and temperature, followed by a period of cooling, which led to the formation of subatomic particles and eventually atoms and molecules. At 10-43 seconds into the life of the universe, gravity became the first of the fundamental forces to distinguish itself. Before this time all four fundamental forces – gravity, electromagnetism, the weak nuclear force, and the strong nuclear force – are thought to have been unified under a single fundamental force. At 10-36 seconds, the temperature of the universe dropped sufficiently to differentiate the electroweak and strong nuclear forces. The electroweak transition followed at 10-11 seconds, whereupon electromagnetic and weak nuclear forces became distinct from one another. At one microsecond, protons and neutrons formed in the event known as the quark-hadron transition, and at three minutes, nucleosynthesis occurred, at which point light elements such as deuterium, helium, and lithium formed, defining the period of nuclear fusion. At around 5,000 years was the onset of gravitational collapse, characterized by the domination of matter throughout the universe. At 400,000 years, atoms began to form, and at 700 million years, the earliest visible galaxies emerged. It was not until 9 billion years into the life of the universe – that is, 4.6 billion years ago – that the collapse of a dense cloud of matter, whose swirling motion formed a rotating disk of gas and material, created our sun and solar system. Not long after the creation of our solar system, roughly 4.5 billion years ago, Earth and the other planets were formed by the culmination of matter in the outer reaches of the solar system. Although the earliest fossil evidence for life on earth dates to 3.7 billion years ago, the first life forms are estimated to have appeared as early as 4 billion years ago.
Hydrogen, helium, and lithium, the lightest elements, were formed during the early stages of the big bang, but other elements, such as the constituents of carbon dioxide – carbon and oxygen – were formed within stars by a process known as nuclear fusion. All atoms are composed of a positively charged nucleus containing protons and neutrons, which are surrounded by negatively charged electrons. The high pressures inside stars are enough to fuse the nuclei of lighter elements together to form heavier elements. These reactions release energy and push material outward, counteracting the star’s gravitational force, which is simultaneously pulling matter toward the star’s center. As the fusion reactions continue, the nuclei become increasingly large and, at some point, become too large and stable to fuse further. With no more nuclear reactions forcing material outward, the star collapses under its own gravitational force, resulting in an enormous release of matter and energy, known as a supernova. This is the process by which elements are released into space.
As mentioned, the planets of our solar system are the result of accretion of solar nebula – that is, the collision and accumulation of the dust of particles spinning around the Sun and leftover from its formation. The earliest atmospheres of all planets were also made of solar nebula, which consisted mainly of hydrogen. However, the planetary atmospheres evolved over time, becoming incredibly diverse, and range from the thin and tenuous to the dense and powerful. The planets’ atmospheres may consist of everything from hydrogen and helium to oxygen, nitrogen, carbon dioxide, ammonia, and methane. Interestingly, the only planets containing CO2 in their atmospheres are Earth, Mars, and Venus. Mars has a thin atmosphere composed mostly of carbon dioxide, with smaller quantities of argon and nitrogen, along with trace amounts of water and oxygen. Venus, however, has a dense, carbon-dioxide-rich atmosphere with small of amounts of nitrogen and traces of methane and ammonia. It is therefore no surprise that Venus is the hottest planet in our solar system, with a mean surface temperature of 462°C, because its atmosphere creates a particularly potent greenhouse effect.
Earth is believed to have been formed around five billion years ago. A dense atmosphere emerged in the first 500 million years from the vapors and gases that were expelled during the degassing of the earth’s interior. This early atmosphere consisted mainly of carbon dioxide, carbon monoxide, water, nitrogen, and hydrogen and was entirely free of oxygen.
Around one billion years ago water vapor in the atmosphere condensed, creating the oceans, which gave rise to the conditions that spawned the earliest aquatic organisms. These early life forms began to use energy from the sun to combine water and carbon dioxide photosynthetically into organic compounds and oxygen. Part of the oxygen that was created photosynthetically combined with organic carbon to re-create carbon dioxide. The remaining oxygen that accumulated in the atmosphere initiated a massive ecological disaster, at least from the perspective of these early existing anaerobic organisms.
At this time the oxygen in the atmosphere increased while carbon dioxide decreased. Some of the oxygen, located high in the atmosphere, absorbed the ultraviolet rays from the sun and created single oxygen atoms, which, upon reaction with oxygen molecules, formed ozone (O3). Fortunately the ozone so formed was very effective at absorbing ultraviolet rays. It functioned as a thin shield surrounding the planet, absorbing wavelengths from 200 to 300 nanometers (nm), and thus protected the earth from biologically lethal ultraviolet radiation. Ozone is estimated to have come to existence around 600 million years ago.
At this time in the evolution of the earth’s atmosphere the oxygen level was about 10 percent of its present concentration. With less ozone in the stratosphere, life was restricted to the oceans, which absorb sufficient amounts of ultraviolet radiation to allow aquatic life forms to proliferate. However, over time the increase in the concentration of photosynthetically generated oxygen, and the formation of correspondingly high levels of ozone, brought forth the first land organisms, marking the start of the evolution of life on earth.
Excavating Earth’s Carbon Cycle
Carbon is one of the most abundant elements on the earth. Life on Earth is carbon-based. While plants, algae, and certain bacteria metabolize carbon dioxide to provide themselves with energy, other organisms convert it into an exoskeleton through a biomineralization process. This is exemplified by carbonate biominerals, which abound in calcite and aragonite coccolithophores (as shown in figure 5), as well as in sponge spicules, echinoderms, corals, and molluscan shells.81 The shapes and patterns of these exquisitely sculptured biominerals continue to fascinate and deepen our understanding of morphogenesis.
Biomineralization: the process by which a living organism forms a mineral.
Morphogenesis: the processes by which the form or shape of an organism originates and develops.
Figure 5. Emiliania huxleyi, type A coccolithophore.

Reproduced from Young, J. R. et al. A guide to extant coccolithophore taxonomy. Journal of Nannoplankton Research, special issue 1, 1–132 (2003), with permission from Dr. Jeremy Young, University College London.
Carbon pervades all biological materials, is present in geological formations, in the oceans as carbonate, and exists in the atmosphere as CO2 gas. Carbon exchanges between these carbon sinks in a process known as the carbon cycle, as shown in figure 6. Although this process is largely balanced in a natural ecosystem, human activity has led to the addition of billions of tonnes of previously “stored” carbon to the cycle. According to climate scientists, roughly half of the annual production of CO2 generated from fossil fuels is absorbed by the oceans and terrestrial biosphere, while the rest stays in the atmosphere. This is critical because it is a question of not only how much CO2 is released, but also where the CO2 ends up, that matters for maintaining the cycle’s equilibrium.
Figure 6. Schematic showing the carbon pathways between fossil, terrestrial, oceanic, and atmospheric reservoirs under the natural carbon cycle.
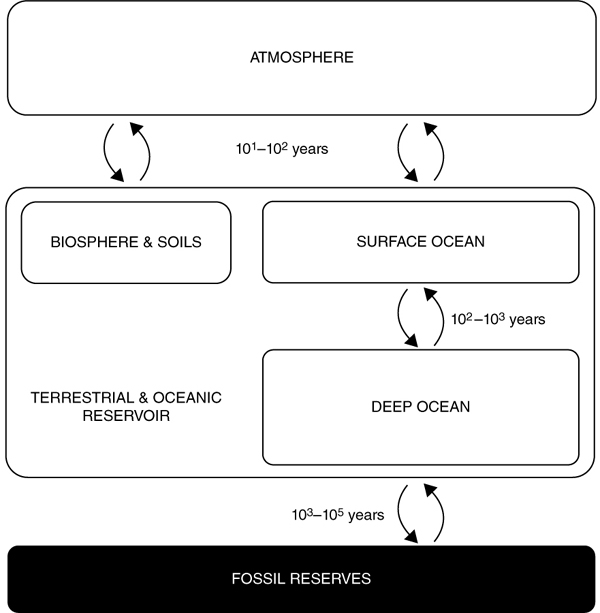
Adapted from Zeebe, R. E. History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu. Rev. Earth Planet. Sci. 40, 141–165 (2012).
The earth’s carbon cycle consists of four principal reservoirs: the atmosphere; the oceans; the terrestrial biosphere, which includes all forms of life on land; and fossil fuels, which are the remains of plants and organisms buried underground. The interactions of atmospheric gases with the oceans and the terrestrial biosphere determine the distribution of CO2 throughout these reservoirs, and the ways in which these interactions change over time ultimately affect global temperature and climate. Understanding the dynamics and loads of various parts of the carbon cycle not only allows climate scientists to predict fluctuations in the composition of the atmosphere, but also helps to identify which mitigation, adaptation, and reduction strategies are most appropriate for addressing the associated climate change effects.
There are multiple pathways by which carbon moves between these reservoirs, with timescales ranging from fewer than seconds to millions of years. Atmospheric carbon is transported to land via rain, by reacting with water to form carbonic acid (H2CO3). This, in turn, reacts with rocks, in a process known as chemical weathering, to produce various ions, notably Ca2+. The ions are then carried away by rivers to the ocean, where they combine with bicarbonate ions (HCO3-) to form calcium carbonate (CaCO3), carbon dioxide, and water. Carbon dioxide is also exchanged between the atmospheric and oceanic reservoirs at the surface of the ocean. Whether the CO2 gas molecules diffuse into or ventilate out of the seawater depends on the relative concentrations of CO2 in the ocean and the atmosphere, as well as the solubility of the water. This interchange pathway is actually more effective at regulating atmospheric carbon dioxide in the short term (that is, on timescales on the order of less than 100,000 years), compared to the chemical weathering of rock pathway, whose feedback loop takes millions of years. Over the past two hundred years oceans have taken up roughly one-third of all anthropogenic carbon emissions. The sudden influx of atmospheric carbon dioxide has severely altered the equilibrium of carbonate species in the oceans, resulting in dramatic acidification and reduced carbon absorption by the oceanic reservoir.82 The reason for this is that, when dissolved in seawater, carbon dioxide produces bicarbonate and hydrogen ions, which cause pH levels to drop. A 0.3–0.4 reduction in pH, equivalent to a 150 percent increase in concentration of hydrogen ions, has been projected for the world’s oceans in the twenty-first century. Moreover, high concentrations of dissolved carbon dioxide shift the chemical equilibrium toward the production of bicarbonate ions (HCO3-) and away from carbonate ions (CO32-), which consequently hinders the formation of calcium carbonate (CaCO3), particularly in the deep sea.83 As a result, shallow waters are becoming saturated with carbonate species, and the oceanic reservoir’s CO2 uptake efficiency is declining. The effect of rapid ocean acidification is expected to have such a dramatic impact on the oceanic carbon cycle and on marine biodiversity, particularly on calcifying organisms, that scientists have dubbed it “the other CO2 problem.”84
The formation of calcium carbonate in the oceans can take many forms: corals and phytoplankton, such as coccolithophores, as well as barrier reefs and carbonate banks. Eventually, when these organisms die, they sink to the ocean floor, where the carbon becomes stored within the layers of sediment. Similarly, carbon originating from organic matter, such as dead plants and animals, both on land and in the oceans, becomes embedded in rock. The sediments are compressed over millions of years and eventually converted into petroleum reserves.
Most of this process took place in the pre-dinosaur age, 286–360 million years ago, during the Carboniferous period of the Paleozoic era. As partially decayed trees and plant material accumulated, they formed peat, which was gradually covered by minerals, sand, and clay, which in turn transformed into sedimentary rock. The growing pressure of the increasing weight of rock turned the peat into coal. Three types of coal were formed: anthracite, bituminous, and lignite. Anthracite is the hardest and contains the most carbon and the highest energy density. The softest and lowest in carbon, yet the highest in hydrogen and oxygen content, is lignite. Bituminous lies between anthracite and lignite. Tiny sea creatures called diatoms, which collected on the sea floor, constituted another source of fossil reserves. The increasing pressure of the overlying sediment and rock gradually converted the organic content of the diatoms into oil and natural gas.
In the pre-industrial world the only mechanism by which these geological carbon reserves could return to the atmosphere was through tectonic activity. During a volcanic eruption, carbon dioxide is released into the atmosphere, and lava and volcanic ash cover the surrounding land to produce a fresh layer of rock. In a business-as-usual scenario, the cycle’s equilibrium would be maintained because the increase in atmospheric carbon dioxide triggers a rise in temperature, leading to more rainfall and subsequent rock weathering.
On a much faster timescale, carbon dioxide is exchanged between the atmospheric and terrestrial or oceanic reservoirs through photosynthetic and respiring organisms. Plants and phytoplankton take carbon dioxide from the atmosphere and, using sunlight, convert it to sugar. The process can be described in its simplest form by the following chemical equation:
6H2O + 6CO2 → C6H12O6 + 6O2
This is a thermodynamically uphill reaction that needs energy to proceed; in nature this energy is provided by sunlight. The light sensitivity of photosynthetic systems results from pigments, specifically chlorophylls and carotenoids, whose chemistry has been optimized over evolutionary history to harvest visible spectral wavelengths from sunlight. Picture a rainbow, in which the range of visible light is broken into its components, starting with red, going to orange-yellow, followed by green roughly in the middle, and ending with blue and dark purple. The green color of grasses, leaves on trees, and other vegetation originates from photosynthetic pigments absorbing blue and red light, both of which are located at the ends of the range of visible light. With very little absorption in the middle of the visible light spectrum, under sunlight, grasses, leaves on trees, and other vegetation appear green.
The sugars produced in the photosynthetic process are the feedstock that enable plants and organisms to survive. Most photosynthetic systems create more sugars than they need to function, and the excess goes on to form carbohydrates, such as sugars, starch, and cellulose. This organic form of carbon can be returned to the atmosphere either through fire or by animals, such as humans, who consume the plant matter and exhale carbon dioxide. The exchange of carbon between the atmosphere and biosphere is so closely tied to photosynthesis that global levels of atmospheric carbon dioxide fluctuate with the seasons. Atmospheric CO2 concentrations drop in the spring and summer months in accordance with plant growth in the northern hemisphere, and concentrations rise again in the fall and winter months, during which time many plants decay or cease to grow.*
The fact that plants feed on carbon dioxide might leave you wondering whether rising concentrations of atmospheric CO2 might actually be to their benefit. Will forests and crops not thrive in a more CO2-rich environment? The effect of changing climatic conditions on photosynthetic organisms is, however, not quite so straightforward. While rising CO2 levels favor photosynthesis, rising temperature levels risk having the opposite effect. The outcome of these competing forces, mixed with the many other factors affecting plant life, such as soil composition and weather patterns, remains challenging to ascertain. A 2019 study, for example, in which researchers modeled forests’ response to climate change, concluded that averting significant drought and reductions in the earth’s biomass was contingent on the ability of plant species to quickly acclimate.85 In other words, whether forests would survive, let alone thrive, under higher atmospheric CO2 concentrations is not clear. Even if plants respond positively to rising CO2 levels, scientists are predicting that any benefits this may yield would be limited. For example, researchers at the University of Gothenburg in Sweden found that crops grown under elevated CO2 conditions contained lower concentrations of nitrogen, potassium, and copper and therefore had significantly reduced nutritional quality.86 Another study, this one focused on North America’s boreal forests, found that, despite making trees use water more efficiently, higher CO2 concentrations did not to lead more rapid tree growth and consequently did not enhance the amount of carbon they stored.87 So, while higher CO2 concentrations might favor photosynthetic processes, climate change, by its nature of interfering with well-evolved ecosystems, poses a serious threat to the quality of forests and crops.
Although we might tend to associate the terrestrial and oceanic reservoirs with plants and ocean water, animals themselves also contribute to the carbon-uptake capacity. Whales, for example, enhance carbon absorption in the oceans simply through the mechanical force of their diving, which carries nutrients up from deeper parts of ocean to the surface, and through their fecal matter, which helps to spread nutrients to new areas of the ocean, thereby attracting the growth of photosynthetic marine plants.88 It is estimated that a single whale has the potential to capture thirty-three tonnes of CO2 over its sixty-year lifetime, or the equivalent of one thousand trees.89 The example of whales illustrates how conservation efforts have a major role to play in a long-term emissions-mitigation strategy. Biodiversity is critical to ensuring the uptake capacity of Earth’s natural carbon sinks and maintaining the flows of earth’s natural carbon cycle.
Recent findings have revealed the carbon-exchange process between the atmosphere and the earth to be more complex than previously thought. The National Aeronautics and Space Administration’s (NASA’s) Geostationary Carbon Cycle Observatory (OCO-2), launched in 2014, has been recording high spatial resolution data on the amount of CO2 in the atmosphere and the uptake of CO2 by the land biosphere. Such high-precision data has allowed researchers to identify, for the first time, the sources, sinks, and seasonal variability of CO2 on both a regional and a global scale. Interestingly, they have found that the interannual variability in the carbon cycle, previously believed to be driven by the net sum of tropical vegetation on the planet, actually results from a combination of regionally specific effects, such as temperature, vegetation species, soil, and rainfall.90–92 Continued monitoring of this kind will provide invaluable information to scientists trying to decipher the subtle complexities of the carbon cycle and ultimately the relationship between carbon and climate.
Tracing the Carbon Trail
With atmospheric CO2 levels ever-increasing, understanding and characterizing the natural pathways of CO2 into the atmosphere is a key task. Simply measuring the quantity of CO2 in the atmosphere, for example, does not reveal whether the CO2 came from the exhaust pipe of a truck or from the smoke of a forest fire. To understand the source of atmospheric CO2, geochemists use a method known as carbon-isotope tracing. Carbon dioxide’s three isotopologues, 12CO2, 13CO2, and 14CO2, all undergo the same chemistry; however, their slight differences in mass result in their having different diffusion rates, as well as different zero-point vibrational energies. Heavier isotopologues diffuse more slowly, leading to variations of relative abundances of isotopes in different environments. The differences in zero-point vibrational energy also influence the energy barriers that must be overcome for certain reactions to proceed; that is, the same chemical reaction will occur at a different rate for different isotopologues. Heavier isotopes, for example, have higher energy barriers for bond-making and bond-breaking processes and proceed more slowly, despite being chemically similar to their lighter counterparts.
isotopologue: molecules having the same chemical composition but differing by an isotope. In the case of the CO2 molecule, the carbon atom can contain either 6, 7, or 8 neutrons.
Zero-point vibrational energy: the characteristic lowest possible energy state of a quantum mechanical system. This is the energy that remains if all other energy sources (i.e., temperature, light) are removed.
Different carbon reservoirs have a distinct affinity toward the three different isotopologues and therefore can be used to trace the movement of carbon dioxide between the sinks. In photosynthetic reactions, for example, the lighter 12CO2 isotopologue reacts more readily than the 13CO2 does, and as a result the terrestrial biosphere has a preference for 12CO2 over 13CO2. Consequently there is a higher relative concentration of 12CO2, compared to 13CO2, in the terrestrial biosphere. The chemistry of the oceanic reservoir, however, does not prefer 12CO2 over 13CO2, resulting in a different relative uptake of atmospheric carbon dioxide isotopologues in the terrestrial and oceanic reservoirs.
Interestingly, while the 12CO2 and 13CO2 isotopologues are stable, 14CO2 is radioactive, with a half-life of 5,730 years, meaning that its concentration changes with time. The signature exponential decay of 14CO2 lies at the heart of radiocarbon dating, an invaluable tool that has allowed archaeologists, paleontologists, and geologists to determine the age of virtually any form of organic matter. Climate scientists can also use this property to identify the source of atmospheric carbon. As fossil fuels are formed over millions of years, they contain no 14CO2 because the radioactive isotopologue has long since decayed to zero concentration. Monitoring changes in 14CO2 in the atmosphere over time therefore offers strong evidence that the combustion of fossil fuels is responsible for the increasing levels of carbon dioxide in the atmosphere.
Half-life: the time it takes for a radioactive substance to be reduced to half of its original amount.
As a result of human use of fossil fuels since the industrial revolution, the total amount of CO2 entering the atmosphere far exceeds that leaving it, as illustrated in figure 7. The carbon cycle’s natural response pathways, particularly those of the oceanic reservoir, simply cannot cope with such pressures, and therefore cannot maintain their previous state of equilibrium.93 Not only have excessive emissions caused the carbon cycle to be thrown out of balance, but climate change itself has already begun to alter the carbon uptake capacity of the other reservoirs.94
Figure 7. The excessive use of fossil fuels since the industrial revolution has tilted the carbon cycle’s natural equilibrium.
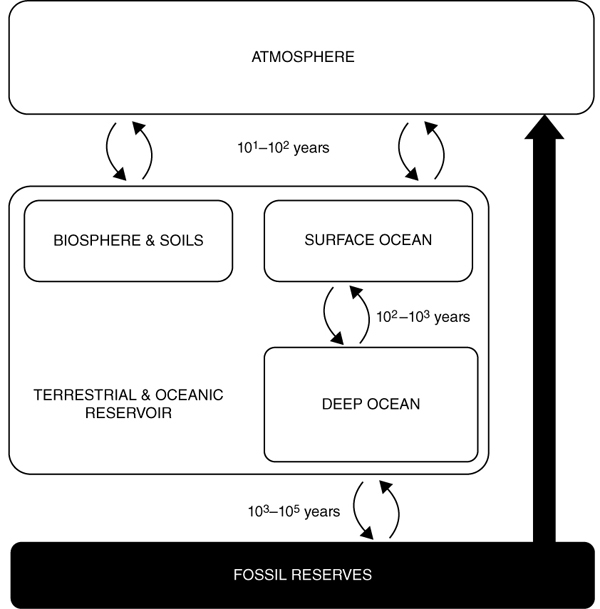
But how exactly is the earth’s carbon cycle related to the planet’s temperature? After all, the carbon cycle certainly is not the only natural system that we have disrupted on earth: human activity during millennia has permanently altered natural landscapes, shifted ecosystems, and destroyed entire species. What is it exactly about the carbon dioxide molecule that gives it so much power over the earth’s climate?
You may have long been aware that carbon dioxide emissions from burning fossil fuels have enhanced the greenhouse effect. Chances are you learned how global warming works from Al Gore’s 2006 film An Inconvenient Truth, which arguably marked a milestone in terms of bringing climate change into the popular consciousness. While Gore’s explanation was correct, it does brush over some finer details pertaining to the chemistry of the CO2 molecule in the atmosphere that may help answer some of these questions.
The Greenhouse Effect and Our Warming Planet
Our understanding of the greenhouse effect has a long history, and, like many scientific developments, its retelling often fails to assign credit to the correct individuals, largely thanks to the social constructs within which science and science history operate. In 1681, almost two hundred years before the world’s first oil refinery was built, French physicist Edme Mariotte was the first to draw a comparison between the earth’s atmosphere and a greenhouse. A century and a half later, in 1838, French mathematician and physicist Claude Pouillet presented the first correct analytical basis for the greenhouse effect, although his contribution is often miscredited to the more renowned mathematician Jean-Baptiste Joseph Fourier.95 Together, Pouillet, Fourier, and English mathematician William Hopkins laid the groundwork that inspired Irish physicist John Tyndall’s pivotal experimental demonstrations of the greenhouse effect. Tyndall wrote: “These speculations were originated by Fourier; but it was to M. Pouillet’s celebrated Memoir, and the recent excellent paper of Mr. Hopkins, to which we were indebted for their chief development. It was supposed that the rays from the sun and fixed stars could reach the earth through the atmosphere more easily than the rays emanating from the earth could get back into space.”96 Although Tyndall is often credited with experimental verification of the greenhouse effect in 1859, it was earlier, in 1856, that American scientist and women’s rights campaigner Eunice Newton Foote first discovered the ability of carbon dioxide and water vapor to absorb heat.* Foote demonstrated the basis of what we now know as the greenhouse effect through a series of experiments using an air pump, thermometers, and two glass cylinders, from which she observed that the heating action of sunlight changed under different gas environments. While Foote’s experiments did not differentiate between direct solar radiation and the infrared radiation that emanates from the earth’s surface – the latter being the primary phenomenon underlying the greenhouse effect – they were the first to draw the link between the composition of the atmosphere and the temperature of the planet. Three years later, Tyndall, who likely was unaware of or had never read Foote’s paper, reconfirmed Foote’s results, using infrared radiation through a series of highly sensitive measurements that were made possible by his newly invented differential spectrometer.98 Tyndall observed: “The solar heat possesses the power of crossing an atmosphere; but, when the heat is absorbed by the planet, it is so changed in quality that the rays emanating from the planet cannot get with the same freedom back into space. Thus the atmosphere admits of the entrance of the solar heat, but checks its exit; and the result is a tendency to accumulate heat at the surface of the planet.”96
Infrared radiation: a type of radiation with energy higher than that of radio waves, but lower than that of visible light. Although it is invisible, we feel infrared radiation as heat.
Tyndall’s description provides an excellent summary of the greenhouse effect: thermal radiation from the earth’s surface, induced by the heat of the sun, is absorbed by GHGs and is radiated back toward the earth’s surface, causing warming.
Nearly forty years later, in 1896, Nobel Prize–winning physical chemist Svante Arrhenius laid the groundwork for quantifying the greenhouse effect, and later, in 1938, Guy Stewart Callendar became the first to demonstrate that the earth’s surface was warming; he even suggested that increased amounts of atmospheric carbon dioxide from burning fossil fuels were the cause.
In the operation of a real greenhouse, incident sunlight is transmitted through the glass and absorbed by the plants inside. As glass poorly transmits any reflected or emitted infrared radiation from inside the greenhouse, and because the heat generated is lost mainly by conduction through the glass, the temperature of the greenhouse rises. The earth’s atmosphere behaves in an analogous way but with the additional heat-balancing effects of convective and evaporative cooling.
Still, what is it exactly that makes carbon dioxide – and not some other molecule – the principal culprit in this matter? The answer to this is surprisingly subtle and lies in the carbon dioxide molecule’s intricate vibrational properties.
You probably encountered the electromagnetic spectrum at some point in science class. Electromagnetic radiation consists of everything from the microwaves that you might use to heat up your lunch to the high-energy X-rays used by doctors and dentists for imaging. It also includes visible light, that is, the part of the spectrum detectable by human eyes. What distinguishes all types of electromagnetic radiation is their energy. Radio waves and microwaves are low energy, whereas UV light, X-rays, and gamma rays lie on the high-energy end of the electromagnetic spectrum.
Electromagnetic radiation: energy- carrying waves that propagate through space, created by the synchronized oscillations of electric and magnetic fields.
To understand the origin and manifestation of the enhanced greenhouse effect, one needs to first understand what happens when electromagnetic radiation from the sun passes through the earth’s atmosphere. The sun can be conceptualized as a blackbody; that is, it emits electromagnetic radiation solely as a function of its temperature. We recognize that the term blackbody is not intuitive; after all, the sun, far from being black in color, is the brightest object we know. However, designating an object as a blackbody simply means that the type and amount of radiation it emits is determined by its temperature. Hotter objects will emit more radiation than will cooler objects. For the physics curious, the relation between a blackbody’s temperature and its spectral radiance is given by Planck’s law.
Blackbody: an object that absorbs and re-emits all incident electromagnetic radiation such that it is in perfect thermal equilibrium with its environment.
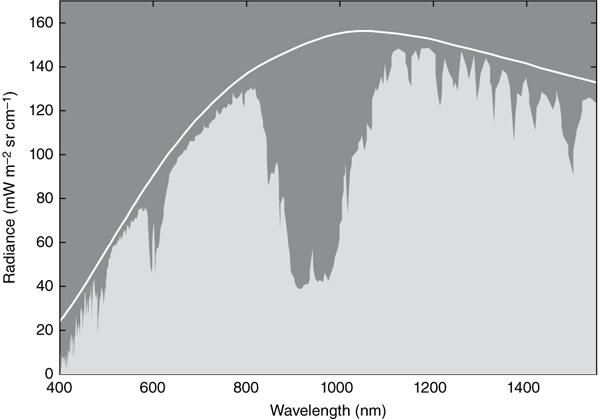
When describing radiation that might be composed of many different energies, such as those that emanate from a blackbody, it is useful to show the object’s spectrum, like the one shown in figure 8. The graph’s horizontal axis corresponds to the frequency of the radiation; that is, how quickly the wave oscillates. The higher the frequency, the more energy the wave carries. The graph’s vertical axis corresponds to the irradiance, which can be thought of as the intensity or the “amount” of wave of a particular energy. The spectra in figure 8 therefore shows the full breakdown of the sun’s radiation: the wave energies of which it is composed, and their corresponding amounts.
Figure 8. Solar irradiance spectrum showing the spectral distribution of radiation emitted by the sun before the radiation enters the earth’s atmosphere (white line), and the spectrum upon the radiation’s reaching the earth’s surface (light-gray area). The dark-gray area enclosed by the white line and the light-gray area corresponds to the incoming solar radiation absorbed by greenhouse gases.

As you may recall, the spectrum of a blackbody depends on its temperature. The sun’s temperature of 5,525 kelvin (5,252°C) results in a spectral irradiance comprising about 51 percent infrared, 37 percent visible, and 12 percent UV, as shown by the white line in figure 8. This is roughly what the radiation spectrum looks like upon arrival at the earth’s atmosphere.
The atmosphere is a layer of gases surrounding the earth; however, it can be further divided into four principal layers: the troposphere, the stratosphere, the mesosphere, and the thermosphere. The troposphere is the inner-most layer, lying closest to the earth’s surface, and holds roughly 75 percent of the atmosphere’s mass, including nearly all of its water vapor. The next layer, the stratosphere, is home to the ozone layer and also marks the highest altitude accessible to jet-powered aircraft. Next is the mesosphere, where meteors entering the atmosphere are typically burned up, giving the appearance of shooting stars. The outermost layer is the thermosphere, with which electrically charged particles collide to produce the stunning aurora borealis, and within which the International Space Station orbits the earth.
Our troposphere comprises mainly nitrogen and oxygen, with trace amounts of water vapor, carbon dioxide, methane, and nitrous oxide. From figure 8 we can see that roughly 12 percent of the solar spectrum is in the form of UV radiation (see the area under the white line to the left of the dotted vertical separating the UV and the visible regions). This is relevant because it is in this region of the electromagnetic spectrum that oxygen molecules absorb light, which causes them to split and form individual oxygen atoms. Other oxygen molecules then react with these oxygen atoms to form ozone (O3), as described by this chemical equation:
O2 + O → O3
The ozone molecules are a crucial component of the atmosphere because they literally shield us from cancer-causing UV rays. Back in 1974, chemists Frank Sherwood Rowland and Mario Molina showed how chlorofluorocarbon (CFC) propellants, under solar irradiation, can decompose in the stratosphere to form highly reactive species that cause the breakdown of ozone molecules back into oxygen.99 In other words, they demonstrated how CFCs could accelerate the destruction of the ozone layer. So compelling were their findings that it prompted governments to fund further research into the problem. Ultimate validation of their theory came in 1985 when scientists Joe Farman, Brian Gardiner, and Jonathan Shanklin reported dramatic losses of ozone above Antarctica, exhibiting a veritable “hole” in the ozone layer.100 The link between CFCs and the depletion of the ozone eventually led to the signing of the Montreal Protocol in 1987, which established an international mandate to phase out the use of ozone-destroying substances. Research has since shown that the treaty and its later amendments have been largely successful in slowly, but surely, remediating ozone depletion.101 Despite this good news, the extent and speed of ozone layer recovery is now being called into question given the increased presence of GHGs in the atmosphere. Monitoring of the status of ozone in the stratosphere has become increasingly obscured by the effects of climate-change-induced changes to its composition and temperature.102 So, although ozone remediation is underway, climate change is certainly not helping researchers track the degree of recovery.
The filtering of incoming solar radiation by the atmosphere can be appreciated in figure 8, in which we can observe a difference between the spectral distribution of solar radiation before it enters the atmosphere (shown by the white line) and that at the surface of the earth (shown by the light-gray area). The dark-gray area in between the white line and the light-gray area in figure 8 therefore corresponds to the combined absorption spectra of the various compounds found in the atmosphere.
The absorptive properties of the atmospheric gases result in roughly 70–75 percent of the sun’s irradiance actually striking the earth. Depending on the type of terrestrial surface, some of the incident radiation is reflected back toward the atmosphere, though the majority is absorbed. The latter causes warming of the earth’s surface, which, in turn, emits infrared radiation back toward the atmosphere. Note that while the sun sends a distribution of different kinds of radiation (UV, visible, infrared) to earth, the radiation that earth re-emits is mainly infrared. About 15–30 percent of this emitted radiation is sent into space, with the remainder being absorbed by the infamous GHGs in the earth’s atmosphere; these in turn re-emit radiation toward both the earth’s surface and space.
It helpful to think of GHGs as the insulation in the walls of a house. If the house is being built to withstand an extremely cold climate, you probably want it to have thick insulation to ensure that the house stays warm and cozy inside (conversely, if it is located in a region with a more moderate climate, thinner insulation will do just as well). The reasoning underlying these decisions is similar to the logic of the greenhouse effect. Sunlight shines through a window to warm the house and the heat slowly escapes through the walls. The insulation in the walls helps to control how much heat escapes back outside. Suppose that you are bracing for an extremely cold winter and decide to replace all the insulation with a thicker grade. The thicker insulation will result in less heat escaping the house. Having the same amount of heat entering, but less heat escaping will cause a shift in the system’s energy balance, and the temperature inside the house will rise.
Just like our house analogy, any changes in the amount of incoming radiation to and outgoing radiation from the earth will cause a shift in its thermal equilibrium. If the rate at which heat enters the atmosphere was suddenly to become greater than the rate at which it escapes, the earth would get hotter, and its temperature would rise until a new thermal equilibrium were achieved. You may have heard of the term radiative forcing used by climate scientists. It is used to describe and quantify the factors influencing incoming and outgoing radiation to provide a measure of how far the planet’s energy is out balance. If there were no radiative forcing, the balance between incoming and outgoing energy could be maintained and there would be no enhanced greenhouse effect. This, of course, is not the case at present, with an estimated 93 percent of the earth’s energy imbalance being accumulated in the oceanic reservoir in the form of heat.103 The continuous rise in ocean temperatures since the late 1950s is a clear indication that the global warming effect is well underway.*
Single-Layer Atmospheric Model
To appreciate better the effect of GHGs on the earth’s temperature, let us take a look at what is known as the single-layer atmospheric model, illustrated in figure 9. Using only this simple diagram (and a little bit of mathematics), we can gain some insight into the impact of GHGs on the earth’s mean temperature. Without going into the details, suffice it to know that the model comprises three main bodies that can radiate and emit heat: the sun, the earth’s surface, and the earth’s atmosphere. Assuming that the earth acts as a blackbody allows us to calculate the number of degrees by which the earth’s surface temperature will shift with a given change in the atmosphere’s emissivity. The emissivity of the atmosphere is a key parameter controlling the temperature of the earth’s surface.
Emissivity: an object’s capacity to re-emit energy as thermal radiation. It is defined as the ratio of radiation re-emitted by an object to that re-emitted by an equivalent blackbody object of the same temperature. An object can have an emissivity value ranging from 0 (zero emission) to 1 (blackbody radiation).
Figure 9. Single-layer atmospheric model.
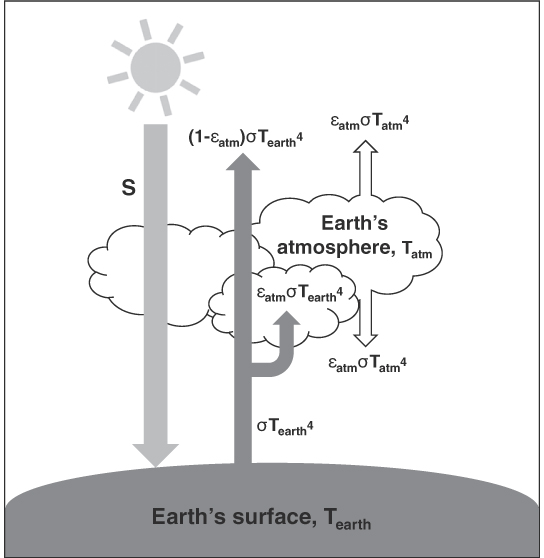
Like most models used in physics, the single-layer atmospheric model rests on certain assumptions. The first assumption is that the atmosphere is transparent to incoming solar radiation, shown by the far-left arrow in figure 9, which is accorded intensity S. The second assumption is that the earth’s surface acts as a blackbody, meaning that it absorbs all the incoming radiation from the sun and then emits it. Some of the earth’s outgoing radiation is absorbed by the atmosphere, while the rest is transmitted directly into space, as shown by the dark-gray arrows in figure 9. Lastly, the model assumes that both the earth and the atmosphere are in thermal equilibrium, thus allowing us to employ an ideal version of Kirchhoff’s law of thermal radiation, in which absorptivity, α, can be equated with emissivity, ε.
Absorptivity: an object’s capacity to absorb radiation, specifically the fraction of incident radiation that is absorbed at a surface.
Recall that an ideal blackbody emits radiation simply as a function of its temperature. If the blackbody is at temperature T, the total energy it emits is given by σT4, where σ is the Stefan-Boltzmann constant. Assuming the earth behaves as an ideal blackbody and that the earth’s surface is at temperature Tearth, the radiation emitted by the earth’s surface can be expressed as
σTearth4
Unlike the sun and the surface of the earth, the model assumes that the atmosphere does not behave as a blackbody; that is, we will assume that it has an emissivity value of less than one. The amount of the earth’s surface radiation absorbed by the atmosphere is therefore be written as
εatmσTearth4
where εatm is the emissivity of the atmosphere. Similarly, the amount of the earth’s radiation transmitted through the atmosphere and back into space is
(1 – εatm)σTearth4
Finally, the radiation emitted by the top surface of the atmosphere, toward space, and the bottom surface of the atmosphere, toward the earth’s surface, can be written as
εatmσTatm4
According to Kirchhoff’s law of thermal radiation, the total energy entering the earth and its atmosphere, must be equal to that emitted back into space. Specifically, the incident energy from the sun must be equal to the energy emitted by the earth and the atmosphere. This can be expressed mathematically using the quantities defined previously:
S = (1 – εatm)σTearth4 + εatmσTatm4 (1)
Kirchhoff’s law can also be applied to the atmosphere. The energy the atmosphere absorbs is equal to that it emits:
εatmσTearth4 = 2εatmσTatm4 (2)
The factor of 2 in the above equation comes from treating the atmosphere as a slab that possesses both a top and a bottom surface. The atmosphere emits εatmσTatm4 toward space, and εatmσTatm4 toward the earth’s surface, resulting in a total emission of 2εatmσTatm4. Solving equations (1) and (2) yields an expression for the earth’s temperature in terms of the incident radiation from the sun and the emissivity of the atmosphere:
Tearth4 = 2S/σ(2 – εatm)
The total radiation hitting the surface of the earth, which comprises the radiation from the sun and that emitted from the atmospheric slab, can be expressed as:
S + εatmσTatm4
By substituting information from the previous equations into the second term and applying some algebraic manipulation, we get the following simplified expression for the amount of radiation hitting the earth’s surface:
2S/(2 – εatm)
From the expression we see that in the case of an atmospheric slab absorbing all of the earth’s radiation (εatm = 1), the total radiation striking the earth is 2S; in the other extreme, where the atmosphere acts completely transparently (εatm = 0), it becomes S. In reality, the total radiative energy striking the earth is somewhere between these two limiting values. Taking S = 235 W/m2 and εatm = 0 yields a planetary temperature of −19.45°C, while, for εatm = 1, the temperature rises to 28.6°C.
The atmosphere trapping some portion of the radiation emitted by the earth results in the earth’s surface temperature being somewhere between −19.45°C and 28.6°C. These two extremes bracket the global mean surface temperature at 15°C.
◊
From the simple single-layer atmospheric model we can quickly see the impact of the emissivity of the atmosphere on the temperature of the planet. The atmosphere’s emissivity is largely determined by the absorption spectra of the atmosphere’s constituent molecules. Understanding the infrared spectra of the individual GHG compounds is key because they largely dictate how much heat can be “trapped” by the atmosphere.
Greenhouse Gases
Although they constitute only 0.1 percent of the atmosphere’s mass, GHGs play a critical role in determining the temperature of the planet because they effectively control how much heat is transmitted back into space. The main GHGs are water, carbon dioxide, methane, nitrous oxide, ozone, and CFCs. Their absorbing effect is illustrated in figure 10, which shows what the radiation escaping earth looks like before and after passing through the atmosphere. The white line gives the distribution of infrared radiation initially emitted by the surface of the earth, and the light-gray area shows what the distribution looks like after the infrared radiation has passed through the atmosphere. The dark-gray area, enclosed by the white line and the light-gray area, therefore corresponds to the radiation absorbed by the GHGs. From this image we can appreciate the importance of understanding the absorptive properties of GHGs because these dictate the amount of radiation that escapes into space.
Figure 10. Radiation spectrum of the earth as seen from above the earth’s atmosphere. The white line denotes the radiation emitted from the surface of the earth, and the light-gray area shows the radiation that goes back into space after passing through the atmosphere. The area located between the boundary of the white line and the light-gray area is effectively the portion of the radiation absorbed by greenhouse gases.
GHGs are characterized by their capacity to absorb infrared light. As mentioned earlier, the radiation coming to the earth from the sun is a combination of UV, visible, and infrared light. The radiation emitted by the earth, however, is mostly of the infrared portion of the electromagnetic spectrum so only the infrared-sensitive compounds in the atmosphere contribute to the greenhouse effect. Oxygen and nitrogen, which constitute most of the atmosphere’s composition, do not contribute to the greenhouse effect, because they do not absorb in the infrared region. Still, some non-GHGs, such as carbon monoxide, nitrogen oxides, and sulfur dioxides, can affect climate, albeit indirectly. For example, a non-GHG can contribute to warming by reacting with other compounds to produce GHGs, by influencing the lifetime of a GHG in the atmosphere, or by affecting cloud formations that could in turn change the earth’s albedo.
The ability of molecules to absorb and emit radiation originates from the motions of atoms within molecules. For this discussion, it is particularly helpful to imagine the atoms in a molecule as little balls connected by springs. The balls (atoms) can move, causing the springs (bonds) between them to stretch, bend, or twist. These motions are called molecular vibrations. Each vibrational motion has a characteristic frequency. When radiation impinges on a molecule its energy can be transferred to the atoms causing them to oscillate. This is analogous to the way in which the tap of the finger can set a bobblehead to wobble. A vibration is only activated if the radiation’s energy matches that of the vibration’s diagnostic frequency. As different molecules have different geometries (and therefore different characteristic modes of vibration), they absorb different parts of the solar spectrum.
Although all infrared-absorbing gases have the potential to contribute to the greenhouse effect, not all contribute to the same extent. After all, water vapor is a GHG, yet we do not hear environmental campaigns to cut our steam emissions. A common measure used to describe the impact of a GHG on the greenhouse effect is its global warming potential (GWP). This measure attempts to quantify how much a GHG would contribute to global warming, in a given amount of time, compared to CO2 (the GWP of CO2 is one and serves as a reference from which the GWP values of other gases are calculated). A GHG’s GWP value depends on the range of infrared radiation it absorbs, how strongly it absorbs, and the duration it resides in the atmosphere. GWP is expressed for a given period of time; for example, GWP-20 refers to a GHG’s GWP relative to carbon dioxide over a twenty-year period. To give some perspective, methane has a GWP-20 of 84, meaning that would have 84 times more warming effect that carbon dioxide would over twenty years. Nitrous oxide, by comparison, has a GWP-20 of 264, and CFCs have a GWP-20 ranging from 6,000 to 11,000.104 These numbers seem frighteningly high. Why is it that we are so concerned with CO2 when it appears to be so much less potent? The reason lies in the fact that we have added excessive amounts of it into the atmosphere. Remember that the GWP considers the effect of injecting 1 kg of GHG compared to injecting 1 kg of CO2. CFCs may be thousands of times more potent than CO2; however, they only exist in trace amounts in the atmosphere.* The dramatic rise in atmospheric CO2 makes it the single most important enhancer of the greenhouse effect. Methane is arguably the second biggest contributor, with its concentration having tripled since the industrial revolution; although methane and sulfur dioxide emissions are not the principal culprits, reducing them is also considered critical to limiting global temperature rise. Emissions of halocarbons and nitrous oxide also contribute to warming, albeit to a lesser extent.
Global warming potential (GWP): a measure of how much a greenhouse gas would contribute to global warming, in a given amount of time, if 1 kg were injected into the atmosphere compared to if 1 kg of carbon dioxide were injected into the atmosphere.
Another measure by which to compare the effect of GHGs, which has been increasingly adopted in recent years, is the global temperature change potential (GTP). GTP is based on the change in global mean surface temperature at a chosen point in time of a gas, relative to that caused by CO2. Just like with GWP, GTP values specified are for a certain period of time (e.g., 20 years, 100 years) and are with reference to CO2, which is assigned a GTP of 1. Again, to give some perspective, methane has a GTP-20 of 67, nitrous oxide has a GTP-20 of 277, and CFCs have GTP-20 values ranging from 6,000 to 12,000.
Global temperature change potential (GTP): a measure of how much a greenhouse gas contributes to the change in global mean surface temperature, in a given amount of time, relative to that contributed by carbon dioxide.
Interestingly, water vapor is the most infrared- absorbing of all the GHGs. Its wealth of vibrational modes makes it absorb across almost the entire infrared region of the electromagnetic spectrum. Water, however, has been spared the bad reputation suffered by its fellow GHGs because the amount of water vapor in the troposphere, which is in large part governed by the earth’s water cycle, has not been subject to change by human activity. Its exact residence time in the atmosphere is particularly challenging to measure due to the complexity of processes involved in the water cycle.
But water is an important part to this story. Although it absorbs a broad range of infrared radiation, it does leave a small window, located roughly in the middle of the infrared spectrum, through which radiation may escape directly into space. Unfortunately, part of the carbon dioxide molecule’s absorption spectrum covers up part of this window. Carbon dioxide has four main vibrational modes, shown in figure 11, three of which are infrared sensitive.
Figure 11. Vibrational motions of the CO2 molecule. The bending mode shown in schemas C and D are responsible for most of CO2’s greenhouse effect.

The symmetric stretching mode shown in schema A of figure 11 involves both carbon-oxygen bonds lengthening and contracting together, whereas the asymmetric motion illustrated in schema B involves one bond contracting while the other one lengthens. The vibrational mode shown in B is said to be infrared active because it can absorb infrared radiation. The symmetric stretching mode shown in A, however, is not. Schemas C and D show the CO2 molecule’s bending vibrations, both of which are infrared active.
Stare long and hard at the molecule’s bending mode shown in schemas C and D. This is the vibrational motion that enables the absorption of energy at the infrared window that is left open by atmospheric water molecules. This specific vibrational motion of the CO2 molecule is responsible for enhancing the greenhouse effect of our atmosphere and the global temperature rise.
Let us return, for a moment, to the analogy of GHGs as insulation on your house. Recall that adding CO2 to the atmosphere has the same effect on the temperature of the earth as adding thicker insulation to your house. However, increased CO2 concentrations also enhance the greenhouse effect in another way. For each vibrational state of the molecule, there exists a series of rotational states owing to the fact that the CO2 molecule is not just bending but also rotating. As the concentration of CO2 in the atmosphere rises, the probability of particles colliding increases. The additional energy gained upon a collision can activate these rotational modes, thereby increasing the molecule’s capacity to absorb infrared radiation. In other words, higher concentrations of CO2 in the atmosphere widen the range of radiation that can be absorbed. This is equivalent to both replacing insulation with thicker insulation and adding insulation to a wall of the house that was not previously insulated.
From its role in the global carbon cycle to its contribution to the greenhouse effect, CO2 is clearly fundamental to our planet’s chemical and thermal balance. It is rather incredible that such a small molecule could have such complex and far-reaching impacts on virtually all of earth’s processes, especially when one considers that the environmental conditions under which all organisms evolved are the direct product of a specific atmospheric composition and an equilibrated carbon cycle. It is clear that any sudden, large-scale deviation in CO2 levels will have inevitable consequences for all life on earth.
KEY TAKEAWAYS
• Carbon can be found on land in both biological materials and geological formations, in the oceans as carbonate, and in the atmosphere as a gas. Carbon exchanges between these carbon sinks through a process known as the carbon cycle.
• The time scale of the earth’s natural carbon cycle is too slow to regulate the sudden influx of carbon dioxide to our atmosphere that has been occurring since the industrial revolution.
• Excessive carbon emissions caused by human activity and climate change itself are decreasing the carbon uptake capacity of the oceanic and terrestrial reservoirs.
• Scientists use isotope tracing to track the sources of carbon. Specifically, monitoring changes in 14CO2 in the atmosphere offers strong evidence that the combustion of fossil fuels is responsible for the increasing levels of carbon dioxide in the atmosphere.
• The saturation of carbonate in the oceans has led to ocean acidification, which is having dire consequences for marine biodiversity.
• Gases in the atmosphere insulate the earth by controlling the amount of radiation that leaves the planet. Radiative forcing involves any changes in the amount of incoming and outgoing radiation to earth. Shifting the planet’s energy balance will trigger a change in planetary temperature.
• GHGs are characterized by their ability to absorb infrared radiation. The ability of molecules to absorb and emit radiation originates from the vibrational and rotational motions of atoms within molecules.
• The impact of a GHG can be quantified by its global warming potential (GWP) or by its global temperature change potential (GTP).
• Although all GHGs contribute to the greenhouse effect, the dramatic rise in atmospheric carbon dioxide makes it the single most important enhancer of the greenhouse effect.
• Increased concentrations of atmospheric carbon dioxide not only result in greater absorption of infrared radiation from earth but also curtail the atmosphere’s natural infrared-transmission window.
__________________
* There is much more land and hence plant growth in the northern hemisphere compared to the southern hemisphere.
* Foote’s paper, Circumstances Affecting the Heat of Sun’s Rays, was presented in August 1856 at a meeting of the American Association for the Advancement of Science, although Foote herself was not permitted to present the paper. The work was instead read aloud by Professor Joseph Henry of the Smithsonian Institution.97
* The massive proportion of excess heat taken up by the oceans renders ocean temperature a more robust indicator than surface temperature for monitoring the anthropogenic influence on planetary warming because it is less prone to fluctuations and local variability.103
* Although emission of CFCs has stopped since they were phased out after the signing of the Montreal Protocol, they still do contribute to the greenhouse effect because of their very long atmospheric lives.
* Globally the aviation industry generates around 2 to 3 percent of anthropogenic CO2 emissions.
