Power to the CO2
We stated earlier that CO2 can replace fossil fuels because it too contains carbon. Chemically speaking, however, fossil resources are hydrocarbons, meaning that they contain both carbon and hydrogen. Carbon dioxide can offer a substitute for the carbon, but a hydrogen source is still needed. Furthermore, many industrial processes still require a source of energy to make them operate. Large-scale deployment of CO2 capture, storage, and utilization, hydrogen production, renewable energy harvesting, and energy- storage technologies are mutually dependent, and their full integration will be necessary for the sustained operation of an emissions-free economy. In this chapter we delve deeper into the technology and auxiliary systems required to turn waste CO2 into chemicals, minerals, and fuels.
To appreciate how CO2-utilization technology works, we must first understand some particulars about the chemical and physical properties of CO2. The CO2 molecule consists of a single carbon atom attached to two oxygen atoms in a linear fashion, as illustrated in figure 13. The central carbon atom shares two electrons with each oxygen atom, which creates very stable bonds. The stability of a molecule is described by a quantity known as its free energy of formation. In the case of CO2, its free energy of formation is −394 kilojoules per mole (kJ/mol). * The fact that it is negative means that CO2 has a strong preference to exist as the CO2 molecule, rather than as its free elements. Conversely, if the free energy of formation were positive, it would indicate that energy would be needed to create the molecule. The counterpart to CO2 being easy to make is that it is difficult to break. This has consequences for its ability to participate in chemical reactions: the larger the change in oxidation state of the carbon in CO2 on its being converted to product, the more demanding the free energy required to drive the reaction. Simply put, the more the carbon bonds and the electrons that hold them together must be rearranged, the more energy input is needed. The challenge of doing chemistry with CO2 relates to overcoming its exceptional stability.
Free energy of formation: the energy needed to form one mole of a substance in its standard state from its constituent atoms in their standard states.
Oxidation state: the degree of oxidation, that is, the number of electrons lost from an atom.
Figure 13. The structure of the CO2 molecule, consisting of a carbon atom bounded by two oxygen atoms.

Overcoming Barriers
Although CO2 might be hard to break, it is not impossible; it simply requires enough energy and a little help from catalysis. Catalysis is a key concept in the science of CO2 conversion. There exist several approaches, each defined by the reaction conditions and type of energy input. Catalysis is enabled by compounds known as catalysts. They lower the activation energy barrier of chemical reactions, allowing them to proceed faster and to occur under milder conditions. To elaborate further on what this means, let us return to our cooking analogy from the previous chapter. The reactants in a chemical reaction can be thought of as the ingredients of a recipe, and a recipe provides the instructions on how to transform ingredients (i.e., reactants) into bread (i.e., products). Although an energy input, usually heat, is often required to bake bread, bread does not bake itself. A cook is necessary to gather, prepare, and use the ingredients according to the recipe. From this standpoint, a catalyst can be thought of as the cook who puts the recipe into action. And, much like a good cook, a good catalyst should remain intact after the reaction (be careful of that hot stove!).
Catalysis: the enabling of a chemical reaction induced by the presence of a catalyst. The catalyst material itself is left unconsumed by the reaction.
Activation energy: the energy required for a chemical reaction to occur.
Catalysts generally refer to any species that assist in a chemical reaction. Biological catalysts, known as enzymes, play a critical role in the many biochemical processes that keep us alive. Although we will address some examples of enzymes used in CO2-utilization technologies, most of the catalysts we will talk about are non-biological materials. Currently several different types of catalytic CO2-conversion processes are under active investigation, all vying for a stake in the race to utilize CO2 as a supply-chain feedstock. In all cases the discovery of high-performance catalysts is critical to achieving carbon-neutral, efficient, and scalable processes.
All catalytic processes can be classified into one of two main categories: heterogeneous catalysis, in which the catalyst and the reactants are made up of different phases (e.g., a solid catalyst reacting with gaseous reactants); or homogeneous catalysis, in which they are of the same phase (e.g., both the catalyst and the reactants are dissolved and interact in a liquid).
Heterogeneous catalysis: a type of catalysis characterized by the catalyst reactants and catalyst having different phases.
Homogeneous catalysis: a type of catalysis characterized by the reactants and the catalyst having the same phase.
In the case of homogeneous catalysis, the catalytic and reactant constituents are typically interacting in an aqueous or a non-aqueous liquid phase. This results in every catalytic component presenting a unique active site to reactants, which often endows them with higher activity and selectivity. Heterogeneous catalysis, however, most commonly involves gaseous or liquid reactants interacting with the surface of solid catalyst material. In this case, the active sites are limited to the surface of the catalyst such that a catalyst with a larger surface area can pack more active sites. The surface area, or the surface-to-volume ratio, is a key factor in determining the activity of a heterogeneous catalyst compared to that of its homogeneous counterparts. A schematic showing the difference between homogeneous and heterogeneous catalytic processes is shown in figure 14.
Aqueous: characterized by the presence of water.
Active site: the part of a catalyst with which reactant molecules interact.
Figure 14. Schematic illustrating the difference between heterogeneous and homogeneous catalysis. In heterogeneous catalysis the catalyst is of a different phase (typically solid) compared to the reactant species, whereas in homogeneous catalysis they are of the same phase.

To illustrate better the point of surface area and reactant-catalyst interactions, consider the various ways of cooking potatoes. Mashed potatoes, which involves boiling potatoes until they break down and then mixing them with melted butter, can be thought of as a homogeneous approach to making potatoes because the two components (i.e., the potatoes and the butter) interact in a more or less similar phase. Alternatively we can make roasted potatoes, which involves cutting potatoes into chunks and roasting them in the oven with oil. While the potatoes are baking, the oil is likely to interact only with the surface of the potatoes, resulting in the crisp potato skin that so many of us love. The potatoes being in solid form, and the oil being a liquid, limits the interaction between the two, illustrating the concept of a heterogeneous process. We can appreciate, simply from our knowledge of cooking, that different approaches to preparing the same ingredients can lead to excellent outcomes. Similarly, in catalysis, neither homogeneous nor heterogeneous approaches are necessarily better. The choice lies primarily in catalyst performance, scalability, the resources and infrastructure available for the process, and ultimately cost. This said, for reasons of practicality, heterogeneous catalysis is often favored over homogeneous catalysis because its inherent heterogeneity allows easier recuperation of the catalysts from the reaction medium. Specifically, recovery and reuse of a heterogeneous catalyst is generally more straightforward, less energy intensive, and more cost-effective than that of a homogeneous one that would require a more complicated series of distillations and/or precipitations.
Returning to our kitchen analogy in which the catalyst can be thought of as a chef preparing a recipe, we know that, despite his or her set of skills and experience in the kitchen, a chef alone is not sufficient to cook the ingredients. Cooking food requires a source of energy, such as heat in an oven or radiation from microwaves. Similarly, catalysts enable reactions by lowering the activation barrier, that is, the amount of energy needed for a reaction to take place. In other words, they have the capacity to modify the kinetics of a reaction.
Kinetics: the area of physical chemistry concerned with chemical reaction rates.
Consider a mountain pass separating two valleys. A hiker located in one valley wishes to traverse into the neighboring valley; however, to do so, she or he must ascend and descend the mountain separating the two valleys. The height of the mountain can be thought of as the energy barrier that the hiker must overcome to get to the neighboring valley. Getting from one valley to the other is easier if a small hill, rather than a jagged mountain, separates them. Catalysts are conceptually equivalent to small hills: they lessen the energetic barrier that separates those reactants from products in a chemical reaction. Just like hikers (unless they are purposely seeking spectacular views at the highest peaks), reactant molecules will seek the easiest, or lowest-energy, trajectory by which to react.
As previously mentioned, even though the energy requirements are lessened by the presence of a catalyst, most processes still require energy input. Catalytic conversion processes are therefore often characterized by the source of the energy driving the reaction. In the case of electrochemical catalysis, the source of energy is an electric current; for photocatalysis, it is light; and for many processes it is heat. We briefly explore and compare some of the most common catalytic approaches to converting CO2.
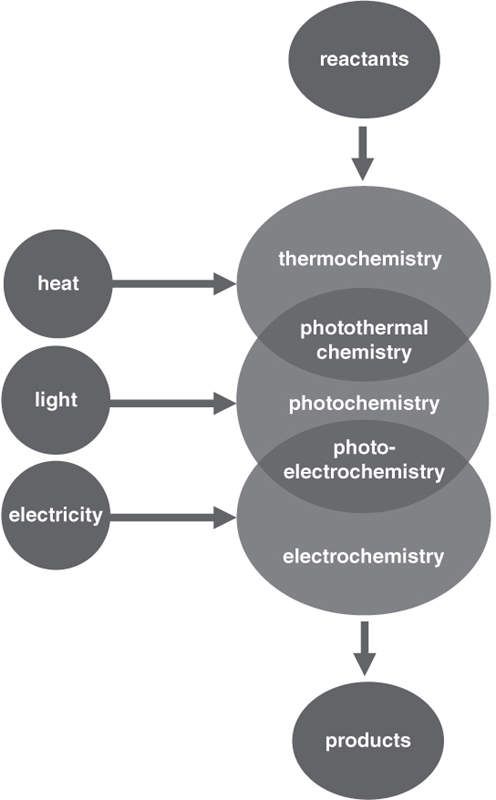
Figure 15 shows the principal types of catalysis that can be used to convert reactants (such as CO2) into products, classified by their energy source (i.e., heat, light, or electricity). Notice that some types of catalysis draw their energy from two sources, rather than a single source. Catalysis based on photothermal chemistry involves processes that are driven by both light and heat, and catalysis based on photoelectrochemical chemistry uses energy from both light and electricity.
Figure 15. Different types of catalysis, classified according to energy source (heat, light, electricity).
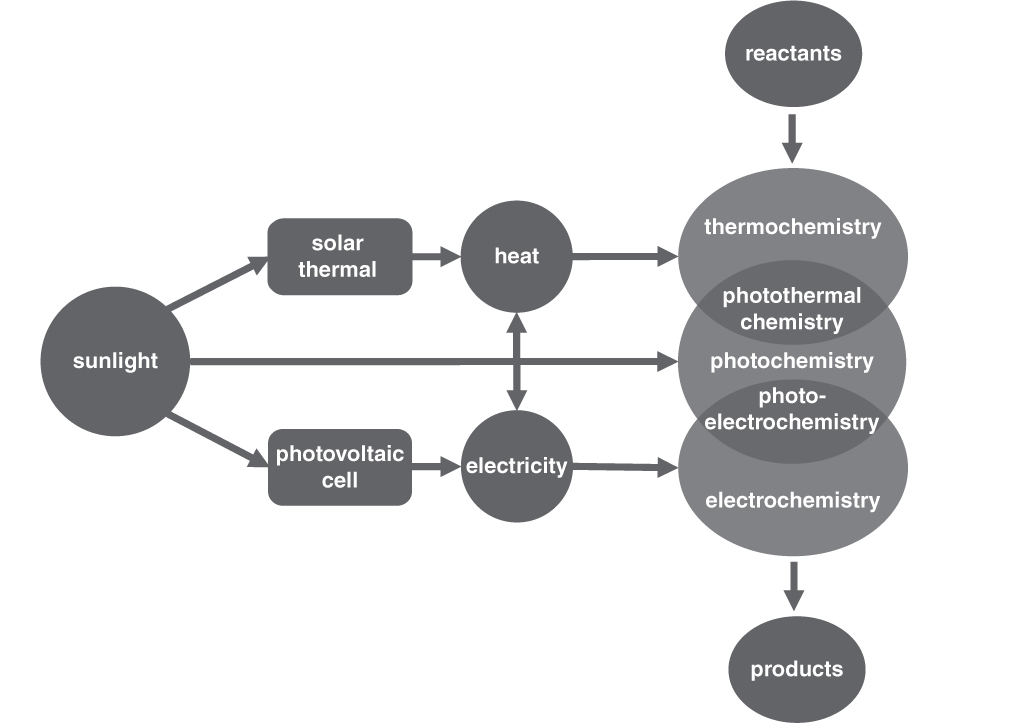
The three energy sources shown in figure 15 can be converted between themselves. For example, light can be converted into electricity, using a photovoltaic device (i.e., a solar cell), or into heat, using a solar thermal device. As well, electricity can be transformed into heat (as in a toaster) and vice versa, using a thermoelectric generator. Although different energy sources are technically interchangeable, doing so is not always favorable, due to reasons of practicality and energy efficiency.
The whole purpose of these processes is to convert CO2 into useful products in a sustainable manner without employing fossil fuels. Ideally these reactions should therefore be powered by clean sources of energy. It would seem counterproductive to burn coal in order to supply the heat to drive these reactions, even if they are consuming CO2 emissions. The most abundant source of energy on earth is provided by the sun; enough solar energy strikes the earth’s surface in a single hour to fulfill all human needs for a year. To ensure that CO2 utilization processes are truly sustainable, it would be ideal if all types could be driven by renewable energy, such as sunlight, as shown in figure 16.
Figure 16. How all types of catalysis can be driven by sunlight.
The Question of Hydrogen
Despite this complete view of the different types of catalytic processes that can convert CO2 into useful chemicals and fuels, there is a missing piece to the puzzle that we have not addressed: the need for hydrogen gas.* As mentioned earlier, if we want to replace fossil hydrocarbons, we need to find alternative supplies of not only carbon but also hydrogen. Hydrogen is key to realizing CO2 refineries and is a missing step to completing a fully carbon-negative or -neutral photocatalytic CO2 process. In fact, the issue is so important that we must pause the discussion on CO2 to focus on our second favorite molecule, hydrogen.
Hydrogen is the lightest molecule in existence and is one of the most important chemical products in our society today. Approximately 50–60 Mt of hydrogen are produced annually in the world, and approximately half of this is used in the production of ammonia via the Haber-Bosch process, for use as a fertilizer. Another 35–40 percent is used in the petrochemical industry to refine crude oil through hydrodesulphurization and hydrocracking processes. The remaining hydrogen is used in smaller but necessarily important applications such as the hydrogenation of fats and oils; the production of chemicals and petrochemicals, such as methanol, ethanol, and dimethyl ether; the refining of metals and steels; food preparation; rocket fuels; and, more recently, the conversion of CO2 to useful products. In addition to its use as a chemical, hydrogen can be used as a carbon-neutral fuel: upon combustion, it produces nothing but water or water vapor.
Haber-Bosch process: an industrial process for converting nitrogen and hydrogen into ammonia, which is used in the production of fertilizer.
The idea of a hydrogen economy has been a global vision since the oil crisis in the 1970s, and although fuel cells were being developed as early as the 1960s, it is only in the last ten to twenty years that the technology has evolved to become a commercial reality. Most major automotive companies, including Hyundai, Toyota, Honda, and Mercedes-Benz, now produce fuel-cell electric vehicles (FCEVs) for commercial use, and many countries have committed to putting more FCEVs on the road in the next five years. Fuel-cell technology is integrated in the vehicle and used in its operation. In this process hydrogen and oxygen produce electricity and water vapor to provide an entirely fossil-free vehicle. Much like electric vehicles, however, they face infrastructural challenges, such as the need to provide refueling stations.
Fuel cell: an electrochemical device that converts fuel, typically hydrogen, into electricity.
Aside from FCEVs, hydrogen fuel-cell technology also holds much promise for the shipping industry. Recently, ABB and Ballard Power Systems agreed to work together on the development of a fuel-cell power system for the shipping industry, based on existing kilowatt-scale fuel-cell technologies.190 The new fuel-cell system is anticipated to constitute a single module that would occupy a space no larger than a conventional marine engine that operates on fossil fuel. Such a technology could have a tremendous impact on the adaptation of the shipping industry to the renewable energy economy.
Although hydrogen may appear to be a strong candidate to replace fossil fuels as the dominant energy carrier of the future, there are issues with its sourcing and cost. Indeed, a sobering 96 percent of the world’s supply of hydrogen currently comes from fossil fuels. Steam methane reforming is the most common way of obtaining hydrogen and accounts for nearly half of its production. As the name suggests, the reaction involves applying extremely high temperature to a mixture of methane (natural gas) and water to produce hydrogen and carbon dioxide. The remaining half of hydrogen production comes from petroleum sources and coal. As a result, a large amount of CO2 is released during its production, with annual emissions topping 500 Mt of CO2. Clearly, there is an urgent need to develop renewable methods of producing hydrogen if we are to curtail CO2 emissions.
There are, fortunately, more-sustainable ways of obtaining hydrogen. Water, which is the most abundant and accessible source of hydrogen on the planet, has been known to produce hydrogen through water electrolysis since 1789. Water electrolysis, which accounts for the remaining 4 percent of today’s global supply of hydrogen, is achieved by passing electrical current through an aqueous solution and producing hydrogen and oxygen gases. If renewable forms of electricity are used to provide the electrical current necessary to split the water molecules, the process can have a net-zero CO2 emission profile.
Industrial-scale water electrolysis using alkaline electrolyzers operates at roughly 75 percent efficiencies, which are similar to those associated with steam methane reformation; however, when accounting for efficiency losses that incur from the compression and transportation of hydrogen, as well as during fuel-cell or gas- turbine operation, the overall efficiency drops to roughly 25 percent. This makes it difficult for hydrogen to compete as a fuel source. In addition, the cost of hydrogen production from electrolysis is six to seven times higher than production from fossil-fuel sources. A large amount of this cost comes from the capital cost of renewable sources of electricity, as well as the cost of electricity from the grid, which can vary throughout the day. The energy required to maintain water electrolysis over extended periods of time can be substantial.
Despite the issues with efficiency and energy losses due to the compression and storage of hydrogen, the principal obstacle to wide-scale electrolytic hydrogen production remains an abundant and cheap source of renewable electricity. With the growth in market share of renewable energy, however, we can hopefully expect that the cost of electricity generated via solar, wind, and tidal technologies will decrease over time.
Direct water electrolysis is not the only alternative means of obtaining hydrogen gas. The copper-chlorine cycle, for example, is a three-step cyclic process for converting water into hydrogen and oxygen. In the first step, copper-chloride salt is reacted with hydrochloric acid to give off hydrogen gas, leaving behind a copper- chloride solution. Next, the copper-chloride solution is reacted with water vapor to produce a solid copper-oxide chloride. Finally, the solid copper-oxide chloride is heated, which transforms it back to the original copper-chloride salt, releasing oxygen in the process. The overall process results in the decomposition of water into hydrogen and oxygen gas, and all the other chemicals and materials involved are recycled. The key to the copper-chlorine cycle is achieving the optimal temperature required at each step. In practice, waste heat from industrial processes and nuclear reactors can be used to power the cycle, thereby reducing its operational carbon footprint. Although the copper-chlorine cycle is cumbersome because it requires three separate steps and substantial equipment, it remains a favored method of obtaining hydrogen due to its relatively low energy requirements, its efficiency, and its potential scalability.
Another possibility for hydrogen production involves the use of a catalyst material to make hydrogen from water and sunlight. Photons from sunlight can transfer their energy to electrons in the catalyst, thereby “exciting” them. In their excited state the electrons can be transferred to the water, causing it to split to form hydrogen and oxygen gas. Metal oxide catalysts are ideal candidates for the job because they tend to be good light absorbers. A second catalyst can be used as the site for the water-splitting reaction. The process is chemically equivalent to the reaction that happens in electrolysis, except in this case the electrons are being drawn from the catalyst by light, rather than by an external electric current.
No matter which water-splitting technique is used, the future of sustainable hydrogen production is intrinsically tied to the future of renewable electricity. In the context of seeking climate-change action strategies that are both environmentally sound and socially equitable, the question of water merits some serious discussion. Every continent is already experiencing freshwater shortages driven by a complexity of issues including population growth, pollution, politics, and climate change itself. The number of people without access to a clean and secure freshwater supply is estimated to be nearly a quarter of the world’s population. Roughly another quarter live in communities that lack the necessary infrastructure to draw water safely from rivers and aquifers. So, while water might appear to be a more carbon-friendly input compared to fossil- sourced hydrogen, one cannot neglect the fact that, as water becomes an increasingly lacking resource in certain parts of the world, its use for energy generation rather than for drinking is ethically debatable.
Desalination of seawater and brackish water seems to be the only feasible way to address water insecurity and has become an increasingly important component of the global water supply. Between 2010 and 2016 the number of desalination installations increased by 9 percent annually.191 Currently, the global desalination capacity is estimated to be 95 billion liters per day and is projected to rise to 170 billion liters per day by 2050.192 Out of necessity, countries in the Middle East have dominated the desalination market. However, the threat of freshwater shortages is increasing around the world, and many countries have opted to install desalination facilities.193 These desalination processes are often energy intensive and costly, not to mention often powered by electricity generated from fossil fuels.
Brackish water: water containing salinity levels in between fresh water and salt water, often resulting from their mixing in estuaries.
Desalination by reverse osmosis and thermal distillation are the two most practiced approaches to obtaining potable water from seawater and brackish water today. In reverse osmosis, water is passed through a semipermeable membrane containing nano-sized pores to remove unwanted molecules and particles. High pressures, created using a pump, drive the water through the membrane. Reverse osmosis is the reverse of the principle of natural osmosis, in which a difference in concentration of ions on either side of a membrane results in flow from the area of high concentration to that of low concentration. Thermal distillation involves evaporating either salt water or brackish water and then recondensing it to obtain a pure product. Both desalination technologies require large amounts of energy. In the case of reverse osmosis, energy is needed to pump water through the membrane, and in thermal distillation, energy is needed to produce heat. In both cases, however, renewable energy can be used to power desalination processes in lieu of fossil fuels. In fact, the use of renewable energy for desalination has increased from 2 percent in 1998 to 23 percent in 2016.194 Direct absorption of heat from the sunlight can also be used to drive thermal distillation. No matter the configuration, the challenge in all cases is to improve the energy efficiency of the process: in other words, the amount of potable water that can be obtained for a given amount of energy input.
Reverse osmosis: a water-purification technique in which water is filtered through a semipermeable membrane.
Thermal distillation: a water-purification technology in which energy is used to evaporate water and then re-condense it.
Technological advancements can help to improve the efficiencies of these processes. For example, in the case of solar-powered thermal distillation, mirrors can be used to concentrate the sunlight in order to promote heat generation right at the interface between air and water. The solar-powered thermal water-heating systems used today for domestic and industry purposes have a long history that can be traced back to 214 BC when Archimedes used mirrors to heat water. But there are ways of concentrating light other than mirrors, which is a cumbersome solution. Another way of efficiently harnessing the heat from sunlight is by using a thin membrane that floats on the surface of the water to facilitate the generation of heat from the sunlight. The membrane consists of a porous hydrophobic material that also displays a strong photothermal effect. The conversion of solar energy to heat in the photothermal membrane causes local heating at the air-water interface. This in turn causes evaporation of the water through the pores of the membrane, whereupon it can be condensed in a cooler region of the desalination system. The key challenge lies in designing a membrane that is simultaneously porous, stable enough to handle the harsh sunlight and salty water conditions, and sufficiently photothermal in nature. Anyone who has made the mistake of wearing a black cotton shirt on a hot summer’s day knows that black materials tend to display a strong photothermal effect. The choice of photothermal materials used in desalination membranes follows this same principle, with typical candidates including copper, aluminum, steel, and silicon and often having different kinds of textured surfaces to optimize the absorption of sunlight.
Photothermal effect: the generation of heat by sunlight.
Recently a thermal distillation system that used as a photothermal membrane an electropolymerized black coating of polypyrrole grown on a stainless-steel mesh was reported to produce fresh water successfully from salt water in a 100 percent solar-driven system.195 The desalination cycle was completed by transferring the evaporated water to a condensing chamber by a solar-powered fan, as depicted in figure 17. A 2019 paper has also demonstrated a means of overcoming the challenges of photothermal membranes by devising a photothermal fabric that hangs over, rather than floats on, the water surface.196 In the design the membrane hangs just over the water such that only two of its edges are immersed, which reduces heat loss from the system and prevents damage to the membrane caused by salt water. Emerging research in the field of photothermal materials will hopefully help push the development of solar-powered water desalination to meet the growing global demand for safe freshwater sources.
Electropolymerization: a coating technique in which a polymer is deposited onto a conducting substrate by means of an electric current.
Pyrrole: a ring-structured organic compound. A polypyrrole consists of a chain of pyrroles.
Figure 17. Illustration of an all-in-one solar distillation system for producing fresh water from salt water.
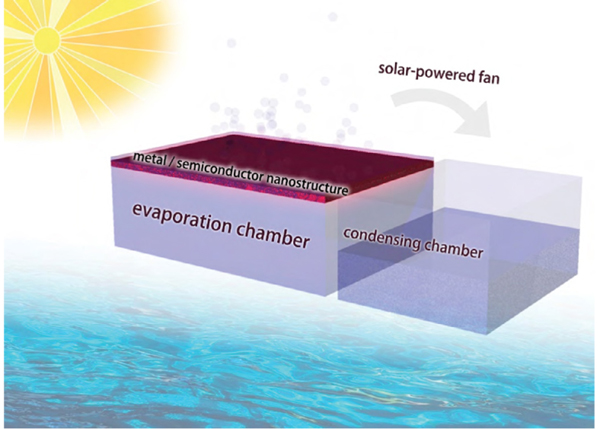
Courtesy of Dr. Chenxi Qian.
While water is arguably the most renowned means to obtain low-carbon hydrogen, it is not the only one, however. Another option, for example, is to incorporate carbon capture technology with conventional steam methane reforming – in other words, to obtain hydrogen from methane but capture the CO2 generated in the process to prevent it from escaping to the atmosphere. If the captured CO2 is sequestered permanently, say in a geological formation, the process becomes a low-carbon route to hydrogen production. Another approach is pyrolysis, which involves obtaining hydrogen gas by heating methane to extremely elevated temperatures in the absence of oxygen. This results in the formation of solid carbon, rather than the release of gaseous CO2 that occurs in steam methane reforming. Both approaches have the obvious advantage of not requiring fresh water; however, they fundamentally involve methane, which at present is still predominantly sourced from fossil natural gas. The most sustainable energy economy of the future would ideally be free of all fossil resources, and therefore it makes sense that we should wean ourselves from natural gas as a source of methane. In this regard, biomass feedstock offers a promising alternative. Hydrogen production from pyrolysis of biomass has been found to be a cost-effective route that is competitive with photovoltaic-driven water electrolysis. Of course, as discussed earlier, the use of biomass as a fuel source is also debatable because the scaling of biomass production risks competing with fertile land that would otherwise be used for food production. At the end of the day there is no “free” source of hydrogen; it must eventually come from somewhere, be it fossil fuel, biomass, or water.
Pyrolysis: a decomposition reaction that involves subjecting a material to extremely high temperatures in an inert gas.
Does CO2 Need Electricity, or Does Electricity Need CO2?
Throughout this book is the recurring theme of the need for electricity generated from renewable sources. The catalytic conversion of CO2 to chemicals, the electrolysis of water to produce hydrogen, and water-desalination technology all require a carbon-neutral source of energy. Although some of these processes have the potential to be powered by sunlight directly, they often still require some degree of electricity. For example, in the case of photocatalysis, electricity is needed to supply hydrogen for the reaction.† The limiting factor to making most of the processes economically viable is the cost of carbon-neutral electricity. Developing and expanding the renewable energy infrastructure is therefore crucial to the success of CO2-utilization technologies, not to mention an imperative step toward decarbonizing the world’s energy and transportation sectors. Switching to renewable electricity as a primary energy source, however, presents its own set of challenges.
The most commonly raised problem with renewable energy is that of intermittency. Unlike fossil-fueled power plants, photovoltaic cells and wind turbines cannot guarantee a consistent supply to the grid. The answer to this intermittency issue is storage; holding excess energy for later use can ensure a robust and reliable power supply at all times. Here, the use of batteries for large-scale storage applications offers a promising solution by storing excess electricity during off-peak hours and releasing power during peak demand.
More than two centuries after its invention by Alessandro Volta in 1800, the battery remains an indispensable part of modern life. A battery is an electrochemical device that converts stored chemical energy into electrical energy. In its simplest form a battery comprises two electrodes (a cathode and an anode) and an electrolyte contained between. The battery operates by the movement of ions between the electrodes, which produces a concomitant flow of charge out of the battery to an external electrical circuit. Many types of batteries exist today, each lending itself to different energy- storage applications – such as lithium-ion batteries found in portable electronics and electric vehicles, and lead-acid batteries used in cars, buses, electric bikes, and industry. Redox flow batteries are one of the most promising candidates for grid-scale storage applications. Their main advantage lies in their power and energy capacity being separately tunable, as well as in their long life cycle. Still, they remain limited by their tendency to discharge and their significant capital costs. Recently lithium-ion batteries, once thought to be limited to vehicles and portable electronics, have also been demonstrated as a viable solution to grid-scale energy storage. The world’s largest lithium-ion energy storage facility, located in Australia, uses Tesla’s Powerpack batteries in conjunction with a wind farm to power tens of thousands of homes with renewable electricity. Tesla’s latest battery storage product, the Megapack, is designed specifically for utility-scale projects. The company has already installed more than one gigawatt hour of electrical storage capacity using its lithium-ion-based battery products.
Redox flow battery: an industrial-scale battery in which charge is stored in the form of liquid electrolytes, allowing for easily scalable storage capacity.
Although certain types of battery and energy-storage systems are technologically mature, many others are being actively explored, developed, and optimized. For example, in January of 2018, researchers at MIT revealed a new design of a rechargeable liquid-sodium battery that could be used for grid-scale storage.198 Although the battery itself is not new (it was designed in 1968 but quickly abandoned for reasons of impracticality), researchers discovered new potential when they found that integrating a novel type of metal mesh membrane could render it robust enough for use in utility-scale applications that require low-cost storage solutions.
Until grid-scale battery storage becomes an established component of the electricity infrastructure, the jury is still out on what to do with excess electricity. A contemporary view is that excess electricity could be stored in the form of chemical bonds. For example, this could involve using the electricity to convert CO2 to chemicals via electrochemical catalysis or to convert water to H2 via electrolysis. Alternatively, the electricity could be converted to heat and then be used to convert CO2 to chemicals via thermal catalysis. Whether it is more efficient to use the excess electricity to power CO2-conversion technologies (either directly or via heat) or to produce H2 remains unclear at this point. In either case, grid-scale electrical-to-chemical energy storage can be employed to control the load leveling of power and demand of the electrical grid, as well as the production of value-added chemicals and fuels.
Compared to flow batteries, chemical storage of electricity via catalysis and electrolysis could allow for seasonal storage benefits. This may be particularly useful to electricity-rich provinces, such as Quebec and Ontario in Canada, which could use their excess electricity for production of chemicals and fuels in the summer, which in turn could be used for consumption in the winter. Storing electricity in the form of chemicals and fuels is also the better solution from the perspective of powering large transport vehicles, such as tankers, cruise ships, aeroplanes, and large trucks that still require fuel. Other advantages include the longer storage times, easy transportation across long distances, and the higher energy density in chemicals and fuels compared to batteries.
A counterargument is that there is simply not enough excess electricity at present to render chemical storage economically significant. Instead, the surplus is more wisely used elsewhere, such as in the charging stations that power the rapidly growing number of EVs. Furthermore, batteries are a well-developed and mature technology, and storing grid electricity in batteries and charging units remains more efficient from an energy point of view compared to converting electricity into chemicals and fuels for later use.
The best option for energy storage is highly dependent on a region’s geography, existing infrastructure, and local industry. Beyond batteries and catalysis, transmission over long distances offers another solution for addressing intermittency, while also bringing renewable energy resources to remote communities. In some countries, the most energy-efficient and cost-effective way of storing megawatt quantities of excess electricity is in the form of potential energy by pumping water up to mountain reservoirs. In other cases, it can be stored as thermal and mechanical energy by compressing air, or as rotational energy in spinning flywheels. Most recently, the Swiss company Energy Vault has demonstrated a groundbreaking gravitational-energy-storage technology. The process involves using cranes to pick up and stack concrete blocks: during periods of excess solar or wind power, the electricity-driven cranes lift the cement blocks and stack them such that they are higher off the ground, eventually creating a tower of blocks. During periods of intermittency the blocks are lowered by the force of gravity and, in the process, power a generator. The system can potentially store 20 MWh of energy – enough to power over a thousand homes for a day. While such examples can certainly inspire one to reflect on the potential of diverse technologies, we should point out that these electricity-storage methods combined do not yet offer the scale of storage capacity needed. Still, they challenge conventional ideas about what energy storage can look like.
When it comes to imagining the renewable energy infrastructure of the future, the single most important change to consider is the anticipated increase in load on the grid. With previously fossil- powered technologies being replaced by electricity-powered ones, we can only expect the demand for electricity to rise dramatically. A recent paper estimated that carbon capture and utilization technologies would require upwards of 18.1 petawatt hours of low carbon electricity.199 Grids will need to be upgraded, managed, and maintained to accommodate the large increases in consumption that are to be expected by new sources drawing power. A recent study in Germany revealed that grid-relieving measures, such as major grid expansion and the integration of flexible loads, would be necessary to accommodate the larger rates of consumption.200,*
We began this chapter by explaining the various ways to power CO2-utilization technologies. Just like cooking food, making products from CO2 and H2 via catalysis takes energy. Whether this energy takes the form of heat, electricity, or direct sunlight, the whole process should ultimately remain carbon-neutral and be free of fossil fuels. The same goes for the production of H2, which requires the development of an extensive water-desalination and electrolysis infrastructure. The renewable energy needed to power all these processes demands that we rethink our electricity grids and address the issue of intermittency. The point is that, far from being stand-alone solutions, CO2 utilization, H2 production, water desalination, renewable-resource harvesting, and energy storage are all mutually dependent systems whose intentional integration will be necessary for the creation of a sustainable, emission-free energy economy. And so, does CO2 need electricity, or does electricity need CO2? We leave it to you to decide.
KEY TAKEAWAYS
• The challenge of converting CO2 to chemicals and fuels lies in overcoming the high chemical stability of CO2.
• Catalysts help to accelerate the rate of chemical reactions and allow them to occur under milder conditions (requiring less energy).
• Converting CO2 to chemicals and fuels requires energy (i.e., heat, electricity, or sunlight) and hydrogen (H2). Renewable energy and fossil-free H2 must be used to guarantee carbon neutrality.
• The most common way of producing H2 today is from fossil fuel via steam methane reforming; however, it can be produced from the electrolysis of (pure) water, using renewable energy.
• Water scarcity is growing worldwide; water-desalination techniques, such as reverse osmosis and thermal distillation, can help to address the demand for potable water.
• The cost of renewably generated electricity is a limiting economic factor in the catalytic conversion of CO2 to chemicals, the electrolysis of water to produce H2, and water-desalination technology.
• Developing and expanding the renewable energy infrastructure is critical to the success of carbon-neutral technologies.
• Batteries can help to address renewable energy’s intermittency problem by storing excess electricity during off-peak hours and releasing power during times of peak demand.
• Energy can alternatively be stored in the form of chemical bonds by using excess electricity to convert CO2 to chemicals, or water to H2, or sea water to potable water.
• The best option for grid-energy storage is highly dependent on a region’s geography, existing infrastructure, and local electricity demand.
• The rise in demand for renewable energy, to be expected as we transition away from fossil fuels, will require major grid expansion and development.
• Large-scale deployment of CO2 capture, storage, and utilization, H2 production, water desalination, renewable-resource harvesting, and energy-storage technologies are mutually dependent. Their integration will be necessary to achieve a sustainable, emissions-free economy.
__________________
* The kilojoules per mole refer to the amount of energy needed to create a compound from its constituent atoms.
* The term hydrogen can refer to either elemental hydrogen or hydrogen gas. In this chapter we will use the terms hydrogen and hydrogen gas interchangeably.
* Fresh water used in agriculture should have a total dissolved-solids level of around 500 ppm, whereas acceptable levels for residential consumption are less than 100 ppm.
† Emerging research is making a case for the possibility of hydrogen production from direct sunlight; however, this remains the exception, and, at present, the electrolysis of water is still the most reliable method of making carbon-neutral hydrogen at scale.
* The case study analysis estimated that in an aggressive electrification strategy for Germany, electricity-consumption rates in 2030 would be 40 percent higher than those in 2015.
