Chapter 8
Soil Fertility and Plant Nutrition
Water, carbon dioxide, and certain chemical elements called plant nutrients are essential for plant growth. Water is supplied by either rainfall or irrigation, carbon dioxide from the atmosphere, and the essential plant nutrients from the soil, fertilizers, or other soil amendments.
Soil Fertility
Soil fertility includes the ability of a soil to hold plant nutrients, the level of plant nutrients present, and the availability of the nutrients for uptake by plants. A soil that has a high level of essential nutrients available for use by plants is usually a productive soil if it also has sufficient soil water and if the crops are well managed. Plant nutrients exist in the soil in several different forms. They include the following:
- Minerals. Examples include the feldspar group, which is the most abundant group of minerals in the rocks of the earth. Some are high in potassium and others in calcium. Nutrients are released from the minerals by weathering.
- Cations or anions. These are plant nutrients that exist on the surface of clay or humus. These surfaces are called the exchange complex and are positive or negative. They attract, hold, and exchange the cations or anions (see Chapter 5).
- Chemical compounds. There are many chemical compounds that form in the soil. An example would be the formation of phosphorus complexes on the surface of calcium carbonate.
- Soluble ions. Numerous ions exist in the soil solution. Plants absorb a large portion of their essential nutrients from this source. This pool of nutrients is small, but can be readily replenished through cation exchange reactions and other buffering mechanisms.
- Organic matter. Plant residues and organic matter contain the elements that plants require for growth. As decomposition (mineralization) occurs, these nutrients are released and can be used by plants.
The availability of nutrients to be absorbed by plants varies according to the form in which they exist (see Table 8.1). As an illustration (Fig. 8.1), phosphorous is readily available to plants as inorganic orthophosphate ions (HPO42− and H2PO42−). The bulk of the soil phosphorous exists as low solubility organic and inorganic phosphorous compounds that are not readily available for plant uptake. Nutrients in soil solution are quite readily available for use by plants. Those on the exchange complex are generally available for absorption by plants but not quite as readily available as those in the soil solution.
Table 8.1 Elements required for plant growth and principal forms in which they are taken up by plants (Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
| Nutrient | Chemical symbol | Form(s) absorbed |
| Macronutrients | ||
| Carbon | C | CO2 |
| Hydrogen | H | H2O |
| Oxygen | O | H2O, O2 |
| Nitrogen | N | Ammonium (NH4+), nitrate (NO3−) |
| Phosphorus | P | Orthophosphate ions (HPO42−), (H2PO4−) |
| Potassium | K | Potassium ion (K+) |
| Calcium | Ca | Calcium ion (Ca2+) |
| Magnesium | Mg | Magnesium ion (Mg2+) |
| Sulfur | S | Hydrogen sulfate (HSO4−), sulfate (SO42−) |
| Micronutrients | ||
| Boron | B | H3BO3 (boric acid), H2BO3− HBO3−2, BO3−3, B4O7−2 |
| Copper | Cu | Copper (cupric) ion (Cu2+), copper hydroxide ion (Cu(OH)+) |
| Chlorine | Cl | Chloride ion (Cl−) |
| Iron | Fe | Ferrous iron (Fe2+), Fe(OH)2+, Fe(OH)2+, ferric iron (Fe3+) |
| Manganese | Mn | Manganese ion (Mn2+) |
| Molybdenum | Mo | MoO42−, HMoO4− |
| Zinc | Zn | Zinc ion (Zn2+) |
| Co | Cobalt | Cobalt ion (Co2+) |
| Nickel | Ni | Nickel ion (Ni2+) |
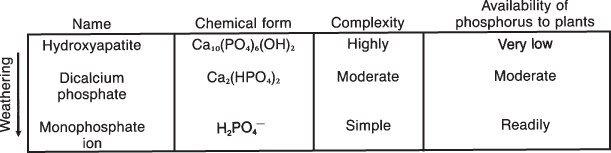
Figure 8.1 Phosphorus exists originally as a complex mineral with very low solubility. Weathering breaks it down into less complex forms, some of which can be used by plants.
Nutrients present as complex chemical compounds, or as precipitated salts as well as those in organic matter are normally only moderately available depending to a great extent on soil water content, soil temperature, and soil pH. Those present as minerals are slowly available. They are released only as the mineral breaks down during the process of weathering (see Chapter 2 for details on weathering).
Most nutrients can be classified as readily available, moderately available, or slowly available. It is desirable to have an adequate supply of nutrients in a readily available form.
Conditions Affecting Level and Availability of Plant Nutrients
Certain soil characteristics influence the availability of nutrients. One is soil texture, the relative amount of sand, silt, and clay. Because clay particles provide a part of the exchange complex, the percentage of clay in the soil influences the capability of a soil to hold nutrients. The percentage of clay determines the size of the “nutrient warehouse.”
The type of clay is also important. As explained in Chapter 5, three of the types of clay in soils in the United States are kaolinite, illite, and smectite (montmorillonite). Each type has a different capacity to hold nutrients (cation exchange capacity, CEC). In general, soils with a high CEC will be more fertile because more nutrient cations can be held on the soil complex.
The CEC is relatively low for kaolinite (3–15 cmolc/kg), moderate for illite (10–40 cmolc/kg), and high for smectite (80–100 cmolc/kg). It follows that a soil with 20% clay as smectite would have a much greater capacity to hold nutrients than a soil with 20% clay as kaolinite. The clay content and the type of clay are both important in soil fertility.
Soil texture can also influence water retention and drainage. Sandier soils tend to drain more quickly and retain less water than do soils with higher clay contents. Sandy soils also have large pore spaces, allowing for more leaching of nutrients. The effects of soil water on nutrient availability are discussed in the following.
Structure is defined as the arrangement of soil particles into aggregates. A good soil structure is essential for water and nutrient movement and retention, as well as root growth. Large spaces between aggregates allow soil water (and the nutrients dissolved therein) to flow more freely, resulting in leaching losses. Small or no spaces between aggregates, especially due to compaction, prevent water from moving through the soil profile, resulting in runoff.
Organic matter is another important soil characteristic that, if high enough in content, can favorably impact the availability of nutrients. It has a threefold effect on fertility. First, the fraction of organic matter that is humus (the colloidal fraction) is similar to clay particles in that it has an exchange capacity ranging from 50 to 200 cmolc/kg (depending on the pH of the soil) and attracts and holds nutrients for plant uptake. Second, as organic matter decomposes (mineralizes), the essential plant nutrients it contains are released and organic acids are formed that increase the availability of most nutrients. Third, an adequate level of organic matter in a soil is generally desirable not only from a plant nutrient standpoint but also because of its favorable effect on soil characteristics such as physical condition, water-holding capacity, and infiltration rate as explained in Chapter 6.
Soil water content also influences nutrient availability. Most nutrients utilized by plants are absorbed from the soil solution. A higher level of soil water normally means a higher level of most nutrients in solution, leading to improved nutrient uptake by diffusion and root interception. Adequate moisture also increases the rate of organic matter decomposition (see Chapter 5), which releases N, P, and S. Soluble and mobile nutrients such as N in the nitrate form may be lost in a process known as leaching as water percolates below the root zone. Poorly drained or very wet soils increase the solubility of minerals such as iron and manganese. Furthermore, nitrate may be lost due to denitrification (see Chapter 4) in flooded soils. Low moisture can result in reduced nutrient uptake due to formation of insoluble compounds. Higher soil temperature usually leads to greater availability of most plant nutrients, as explained in Chapter 7.
Soil pH, which is a measure of the degree of soil acidity or alkalinity, also influences the availability of nutrients through its effects on root growth and nutrient form (see Chapter 5). Most nutrients are at their highest level of availability when the pH is slightly acid to neutral (pH 6.0–7.0). Acidic soils reduce root growth, which is critical to P uptake. The pH is also important in N transformations, such as mineralization, nitrification, and N fixation, as the bacteria involved are sensitive to pH. The different forms of N have different availabilities as they have different leaching capabilities. As a soil becomes more acid, certain nutrients become less available; if a soil becomes alkaline, the availability of certain nutrients decreases (see Fig. 8.2).
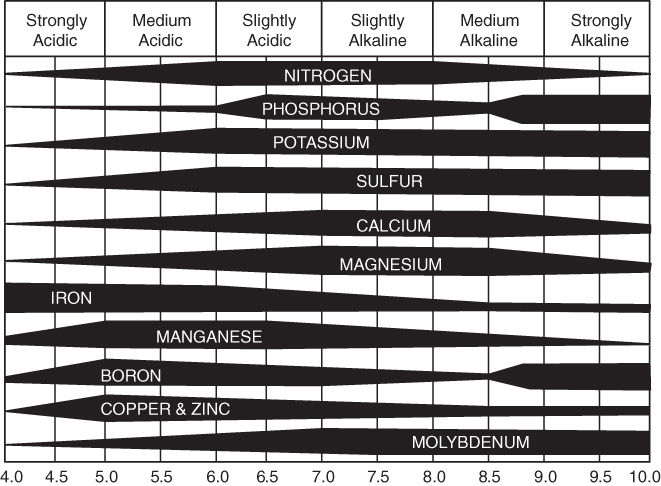
Figure 8.2 The influence of soil pH on nutrient availability. The wider the bar, the greater the availability.
Nutrient Mobility in Soils
Nutrient mobility in the soil affects the ease of its uptake by plants, and the likelihood of its leaching through the soil. Mobility of nutrients within the soil is influenced by soil physical properties such as soil texture and structure, soil chemical properties such as CEC and AEC and pH, as well as soil conditions such as moisture. Calcium, potassium, and magnesium are positively charged ions (cations). Most soil colloids have a net negative charge (see Chapter 5). Since opposite charges attract, these cations are attracted and held onto the cation exchange sites and are released only when excess cations are added to replace their place on the exchange sites. For this reason, these cations are considered less mobile and are slowly available to plants. Their movement and enrichment in waters is rarely an environmental issue, particularly where erosion is controlled.
Sulfur occurs as the anion sulfate form (SO4−), which does not bind to cation exchange sites and is thus mobile in most soils. Nitrogen usually exists in the soil as ammonium (NH4+) and nitrate (NO3−) forms, hence its mobility depends on the form it is in. The NH4+-N can be held on cation exchange sites and is therefore relatively immobile, and is not susceptible to leaching. On the contrary, the negatively charged NO3− ion is not held on the exchange sites, hence it is very mobile in soil water. In addition, nitrates are highly soluble in water and are subject to losses to ground water by leaching, and to surface waters by runoff (see Chapter 4).
Phosphorus normally exists in soils with pH values between 5 and 7 as the ortho-phosphate ion (H2PO4−, HPO42−). Unlike the nitrate anion, the ortho-phosphate ion forms very tight bonds to soil particles. As a consequence, phosphorus is typically immobile in soil, and it does not readily leach out of the root zone. The potential for P-loss is mainly associated with erosion and runoff. The lack of P mobility also reduces the availability of P-fertilizer to plants. Both nitrates and phosphorus when transported to surface waters stimulate algal growth to the point of crowding out other more desirable species through a process called eutrophication (see Chapter 4).
For nutrients to be utilized by plants, they must move to the surface of the plant roots, where absorption takes place. There are three processes by which nutrients move to the root surface. These are root interception, mass flow, and diffusion.
Root interception: Root interception, also known as contact exchange, occurs when the root comes into direct physical contact with nutrients associated with soil colloids as it grows through the soil. Root interception generally increases as the surface area and mass of the root increases, thereby enabling the plant to explore a greater volume of soil. Mycorrhizae also increase the surface area explored by roots thereby enhancing root interception. Root interception is an important mode of transport for calcium and magnesium. However, since the volume of soil occupied by roots is usually less than 1%, root interception is a minor pathway for nutrient transfer.
Mass flow: Mass flow occurs when dissolved nutrients in the soil solution are transported to the surface of roots as plants take up water for transpiration. Mass flow decreases as soil water content decreases. Nitrate, sulfate, calcium, and magnesium are largely supplied by mass flow.
Diffusion: Diffusion is the movement of nutrients to the root surface in response to a concentration gradient. Continued uptake of a nutrients by plants causes its concentration in the soil solution adjacent to the root surface to decrease. This creates a concentration gradient from the bulk soil to the root surface that causes nutrients to move to the root surface, where they can be taken up. Diffusion is largely responsible for supply of phosphorus and potassium.
Methods to Increase the Availability of Added Nutrients
There are a number of ways to increase the availability of nutrients. For example, potassium fertilization prior to application of ammonium fertilizers can be used to reduce NH4+ fixation in soils with vermiculite and illite types of clay. Early season uptake of phosphate ions by crop roots can be facilitated by placing phosphorus-containing fertilizer in or close to the seed-row at planting. In this way, phosphate ions are taken up by the roots before they react with cations dominating under acidic (e.g., Al3+ or Fe3+) or alkaline (e.g., Ca2+ or Mg2+) soil conditions. Under alkaline soil conditions, the phosphate fertilizer can be applied in bands with a fertilizer that generates ammonium (NH4+) ions. This allows slight acidification of the soil adjacent to the fertilizer band. Alternatively, compound nutrient fertilizer granules that contain nitrogen (N), phosphorus (P), and/or elemental sulfur can be applied to alkaline soils. In this case, the soil adjacent to the granule will be acidified and P uptake will be enhanced. Addition of lime to acidic soils can also enhance availability of some nutrients.
In high pH soils, soil applied iron (Fe) fertilizers often do not successfully correct Fe deficiencies. This is because the Fe3+ ions from the Fe fertilizer react so fast with soil that the nutrient is tied up and rendered unavailable to plants. In these soils, Fe enhanced can be corrected through foliar application of soluble iron fertilizer compounds. By avoiding the soil and applying the Fe directly to the leaves, the small amount of Fe required by plants is successfully introduced into the crop.
Plant Nutrition
Essential Elements
At least 17 elements, called plant nutrients, are essential for plant growth (Table 8.1). The first group includes three elements—carbon, hydrogen, and oxygen—that are the basic building blocks of all plant compounds. These three are needed in much larger quantities than all others combined. The initial product of photosynthesis is the simple sugar C6H12O6. The carbon and oxygen come from carbon dioxide, and the hydrogen comes from water. The oxygen in the water is given off by plants and goes back into the atmosphere. This process assures us of a continuing source of oxygen.
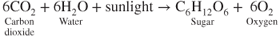
The second group of essential elements, called macronutrients, consists of nitrogen, phosphorus, potassium, sulfur, calcium, and magnesium. They are classified as macronutrients because they are used in relatively large quantities by plants.
Another group of eight elements is called micronutrients because they are normally used in smaller quantities. This group includes iron, zinc, manganese, copper, boron, molybdenum, nickel, and chlorine.
Some scientists contend that some other elements may also be essential for plant growth. Included in this group are silicon and sodium. These two elements plus vanadium, cobalt, and iodine are often called beneficial elements because they can be used by plants as substitutes for nutrients that are essential.
Approximately 90% of the dry weight of a plant is made up of carbon, hydrogen, and oxygen; the balance consists of the other essential elements. Most of this remainder consists of the elements classified as macronutrients, whereas less than approximately one-tenth of this 10% is in the micronutrient group. Several elements not known to be essential may also be included in the 10%.
Natural Sources of Plant Nutrients
Plants obtain carbon, hydrogen, and oxygen from soil water and atmospheric carbon dioxide and assimilate them into glucose during photosynthesis. The remaining 14 elements are normally absorbed in ionic form from the soil (Fig. 8.3, Table 8.1), although small amounts can be taken in through the leaves if placed there in solution by precipitation, foliar application, or sprinkler irrigation. Nitrogen comes originally from the atmosphere, which is nearly 79% N, and is in a form that plants cannot use.
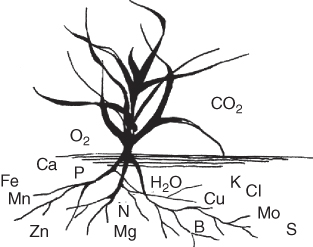
Figure 8.3 Carbon and oxygen come from carbon dioxide in the air, hydrogen from water in the soil, and other elements are absorbed by plants from the soil.
Nature has several methods by which atmospheric N is converted into forms that plants can use. They include the following:
- Bacteria and leguminous plants join together in a process called symbiotic nitrogen fixation, which, when combined with other steps in the nitrogen cycle, provides nitrogen in a form usable by plants (see Chapter 4).
- Fixation of nitrogen by soil bacteria without the help of legumes called nonsymbiotic fixation.
The action of lightning discharging in the atmosphere causes nitrogen oxides to be formed, which are then brought to earth by rain. These latter two sources of nitrogen provide relatively small quantities for plant growth.
Within soils, most N is in the form of organic matter. While this pool of N is very important, N in the organic form generally is not available to plants. As discussed in earlier chapters, the organic N must undergo mineralization to be made available for plants.
The remaining 13 essential elements are naturally derived from the weathering of rocks and minerals of the earth. Phosphorus, for example, comes principally from a mineral called apatite, whereas magnesium comes from minerals such as serpentine and dolomite. When rocks and minerals undergo weathering, elements are released and become part of the soil system.
Sulfur is often emitted into the atmosphere by coal-burning facilities as sulfur dioxide, which is moved by air currents and then carried to the soil by precipitation (Fig. 8.4). This can also be true for a small portion of the nitrogen used by plants. Plant nutrients can also be added through irrigation water.
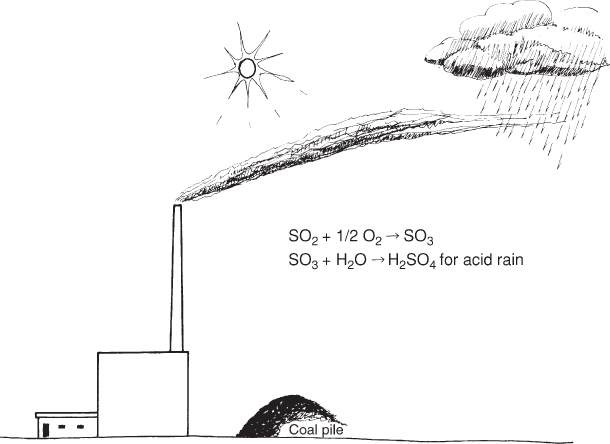
Figure 8.4 The sulfur in fossil fuels such as coal is the source of sulfur dioxide (SO2) emission into the atmosphere when it is burned.
Role of Essential Plant Nutrients
Each plant nutrient plays one or more special roles in plant growth. Table 8.2 lists each nutrient, one of its functions in plant growth, and some deficiency symptoms. A nutrient may be the essential part of a plant compound, thus providing it with a structural base. Calcium, for example, is part of calcium pectate, which is a compound that is a part of the plant cell wall.
Table 8.2 Essential plant nutrients, function in plant growth, and deficiency symptoms (Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
| Plant nutrient | Function in plant | Deficiency symptom |
| Nitrogen | Essential part of amino acids, protein, and chlorophyll | Yellowing of midrib of lower leaves |
| Phosphorus | Part of energy transfer compounds | Reddish-purple color of leaves of young plant |
| Potassium | Regulation of osmosis and water use and transportation system | Browning of outer edges of lower leaves |
| Calcium | Formation of calcium pectate used in cell walls | No development of terminal buds and apical tips of roots |
| Magnesium | Central atom of the chlorophyll molecule | Interveinal chlorosis of middle or lower leaves |
| Sulfur | Essential part of three amino acids essential for protein formation | Uniformly chlorotic upper leaves and slow growth |
| Iron | Component of chlorophyll and cofactor for enzymatic reactions | Interveinal chlorosis in young leaves |
| Zinc | Involvement in auxin metabolism and part of dehydrogenase enzyme | Spotted white or yellow areas between veins of upper third of leaves; also lack of terminal growth |
| Manganese | Electron transport and part of enzyme system | Interveinal chlorosis in young leaves |
| Copper | Part of oxidase enzyme system | Yellowing and stunting of young leaves |
| Nickel | Involved in enzyme converting urea to ammonium | Interveinal chlorosis in young leaves—progressing to necrosis |
| Molybdenum | Part of nitrate reductase enzyme | Yellowing of midrib of lower leaves |
| Boron | Growth and development of a new meristematic cells | Pale green young leaves; leaves die and terminal growth ceases |
| Chlorine | Osmotic and cation neutralization | Partial wilting and loss of leaf turgor when moisture is adequate |
Other nutrients may be essential for making compounds involved in plant growth processes, such as phosphorus as a part of adenosine diphosphate (ADP) and adenosine triphosphate (ATP), which are two compounds involved in the transfer of energy within a plant. The compounds used as storage of plant foods such as protein require nitrogen and sulfur.
Another group is involved in the regulation of certain enzymatic processes. Enzymes in plants such as catalase and lactase act as catalysts or activators. They often contain micronutrients such as iron and copper.
Determining Nutrient Needs
To be able to produce top yields, all essential plant nutrients must be present in adequate quantities. The most common element to be deficient for most crops and lawns is nitrogen. Phosphorus is normally the second most common element to be deficient. Potassium, calcium, and magnesium are often lacking in soils in the eastern half of the United States where rainfall normally exceeds 25 in. (625 mm) per year; whereas in the western United States, these elements are well supplied in most soils.
Several methods are available to determine if a nutrient is deficient and the quantity needed to correct the deficiency. These methods include chemical analyses of the soil and the plant, nutrient deficiency symptoms, and growth tests. In order for chemical analysis of the soil and plants to be of value, soil and plant samples need to be representative of the area in question.
Soil Sampling
The method most often used for determining nutrient need is chemical analysis of soil. To determine nutrient need most accurately, two things are required: (1) a soil sample that truly represents the field in question, and (2) the chemical method that has been adequately researched and properly correlated/calibrated for the crops and soils in question.
For soil sampling, there are three common approaches. They are sampling (1) by soil type, (2) on a grid basis, or (3) on a management zone basis, which determines sampling locations based on yield maps, remote sensing, past management history, and so on. The same basic principles apply to sampling lawns and gardens but on a smaller scale.
To sample by soil type, diagram a field by soil type (such maps are available in soil surveys) and obtain a composite sample from each soil type (see Fig. 8.5). For each composite sample, systematically or randomly take 10–15 individual cores of one soil type using a sampling tube or a shovel. A soil core covers one inch square area and may extend to varying soil depths, depending on the type of the crop to be grown (shallow rooted vs deep rooted), the field (pasture, no-till, tilled), and type of test (mobile nutrients vs immobile nutrients) to be performed. Usually, soil cores are taken to a depth of 6 in. (15 cm) in pasture, turf, no-till land, tilled fields, or gardens. Testing for mobile nutrients, such as nitrates and sulfates requires deeper sampling. Take samples with stainless steel or chrome-plated sampling tools and clean plastic buckets to avoid contaminating the samples with micronutrients, particularly copper and zinc. Do not use brass, bronze, or galvanized (zinc plated) tools.

Figure 8.5 Proper collection of soil samples is extremely important. Tests made on carelessly taken samples can be misleading and costly.
(Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
Thoroughly homogenize the cores and remove approximately 1 pint (0.5 l) or 1 lb. (0.4 kg) to submit for testing. Repeat the same process for each soil type in the field. For fields larger than 12 acres (5 ha), proportionately more cores should be taken. The more the cores taken, the more reliable the measure of fertility of the field. One sample should not represent more than 25 acres (10 ha). If one soil type area in a field is too small to be fertilized separately (e.g., less than 5 acres, or around 2 ha, in size), do not sample any of the area.
If the samples are taken systematically, the area sampled should be traversed in a zigzag pattern (See Fig. 8.5, step 2) to provide a uniform distribution of the sampling sites in the entire area. High variation within the field being sampled will decrease the accuracy and reliability of a soil test recommendation. For this reason, a uniform soil should not differ in soil type, color, slope, drainage, texture, past management, and natural vegetation, and any parts of the field showing these differences should be sampled separately. Areas with recent lime, manure, composts, and fertilizer additions should not be sampled. Severely eroded sections, manure or lime stockpile areas, wet spots, old building sites, fence rows, and areas adjacent to gravel roads should also not be sampled. Soil samples taken from these locations would not be typical of the soil in the rest of the field, hence including them would produce misleading results.
To sample on a grid basis, divide a field into 3–5 acre (1.2–2.0 ha) squares as a grid. Take one composite sample (of 8 single-sample core samples) from each square (see Fig. 8.6.). The grids may be based on yield maps, and so on, as discussed previously. These sampling areas are not necessarily rectangular.
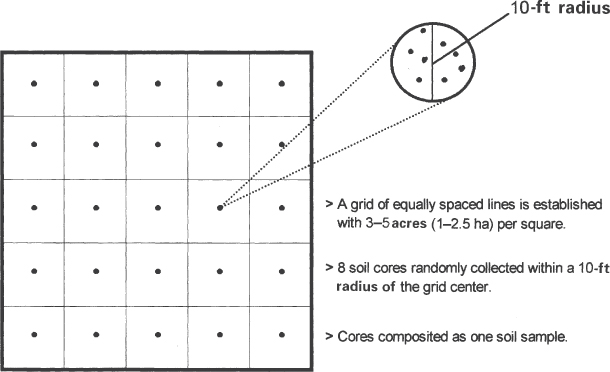
Figure 8.6 Grid sampling is an alternative method of sampling where soils are quite variable.
(Courtesy of Plant Food, Fall 1994, Potash and Phosphate Institute, Atlanta, GA.)
For grid sampling, various patterns of sampling can be used by shifting from the center of the grid to randomize the sites. This approach to sampling is being used for the computerized application of nutrients or variable-rate application, often called precision or site-specific nutrient management programs.
Ideally, soil samples should be taken as close as possible to planting or to the time of crop need for the nutrient (2–4 weeks before planting or fertilizing the crop). This is because nutrient concentrations in the soil fluctuate with the season. However, to give sufficient time to plan and implement land management decisions before the busy planting season, it is more practical to collect soil samples 3–6 months prior to planting time. Do not collect samples when the soil is too wet or too dry as it will be difficult to mix the cores. As a rule, if the soil is too wet or too dry to plow, it is too wet or dry to sample.
To ensure accurate results and minimize changes in nutrient levels caused by soil organisms, soil samples should be handled with great care. Moist soil samples should be stored in a cool box during sampling and in a refrigerator after sampling. If it is not feasible to refrigerate or freeze the samples soon after collection, take them to the soil testing laboratory or air dry them by spreading the soil on a plastic sheet. Break up all clods or lumps and dry at room temperature. A circulating fan may be used to facilitate drying.
Take soil samples at the same time of the year, once every 2 or 3 years. Sampling at the same time of year minimizes the effect of seasonal variations on soil test results.
Soil Test Methods
Composite samples should always be placed in a clean container to avoid contamination. It is best to use containers provided by the soil-testing laboratory, if available. Be sure to provide the producer's name, address, and field number for each sample. Also provide information on cropping history for at least 2 years, previous fertilizer use and manure applications, and yield levels, crop to be fertilized, and anticipated yield potential for the next crop. Be sure to choose a well-qualified laboratory whose agronomist is familiar with the soils and crops in the producer's locale. State-operated soil-testing laboratories vary in their specific instructions for soil sampling so it is advisable to check with the local county agricultural extension agent.
A soil test is a chemical assessment of the nutrient-supplying ability of a soil at the time of sampling. Many methods of soil testing are available. Some measure the total content of a nutrient in the soil, while other tests attempt to measure the “available” nutrient levels. Most testing that is done to predict fertilizer needs for a crop is in the second category, which is to provide an index, or an estimate of the nutrient-supplying ability of the soil. During testing, this fraction is separated from the soil using an extracting solution that is mixed with the soil for a specified period of time. The nutrient in the extractant is then analyzed after filtration.
For any method to work, the soil tests have to be evaluated on the basis of actual crop response. Without locally applicable crop response data, a soil test is useless. Each test must be correlated/calibrated with long-term field experiments and fertilizer trials so as to assess the likelihood of a yield response to addition of fertilizer. For the calibration to be complete, these soil test correlation trials must be conducted for several years on a specific crop growing on a specific soil type.
Soils that contain a high amount of available nutrients as indicated by high soil test values require less fertilizer input than do soils that contain a low amount of available nutrients. A low soil-test value for a particular nutrient indicates that the crop will not obtain enough of that nutrient from the soil to produce the highest yield under average soil and climatic conditions. Supplementation through fertilizer addition will be necessary to correct nutrient deficiency. In general, there is a high chance of getting a response to a nutrient if the soil test is low.
The test is then calibrated to determine the amount of each nutrient needed to maximize profit from fertilizer application. The soil test level above which crop yields remain the same even though soil fertility continues to increase is known as the critical soil test. In other words, no response is expected above the critical soil test level and a response is expected below it. Nutrient guidelines for fields testing below the critical soil test value are determined by conducting yield trials on a number of fields, across a full range of soil test levels. The treatments on each field are selected to represent the full range of rates of the nutrient in question (e.g., 0, 30, 60, 90, and 120 lb or 0, 12, 24, 36, and 48 kg of P205/acre). The results from these tests indicate how many pounds of fertilizer are needed at a given soil test level to reach economically optimum yield, that is, the fertilization level that brings the most profit per acre (i.e., the value of extra yield vs the cost of extra fertilizer) at a given soil test level. Therefore, by using the best soil-testing procedures and sound fertilizer recommendations based on adequate field research, laboratories are able to predict the optimum economic rate of fertilizer that is environmentally sound.
Potential yields vary; hence, the relationship between soil test values and crop response will vary. This is because yield is affected by climate, disease, and weeds as well as soil fertility. The interpreter of the soil test results should also consider (and should be knowledgeable about) potential yield levels for any given area or even specific farms, if possible. Previous yield history, cropping systems, and fertilizer practices, if known, are needed for best recommendations.
Fertilizer Recommendations: Approaches and Philosophies
Although soil testing is the basis for determining the adequacy of many nutrients, soil tests do not provide the final answer of what fertilizer rate needs to be applied to an individual field in a given year. There are two approaches commonly used for giving fertilizer recommendations for phosphorus and potassium. These are the build-maintenance and nutrient sufficiency approaches.
Build-Maintenance Approach
The strategy here is to maintain soil fertility for future years by applying more nutrients than the crop removes, so that yields are not limited by nutrient level in the soil. To this end, enough fertilizer is applied to both meet the nutrient requirements of the immediate crop and to build up the level of nutrient in the soil to a critical soil test level over a few years. The critical soil test level is the soil test level at which near maximum (90–95%) yield is obtained. It is based on yield response curves, which are the result of years of research and trials, and are specific to a particular soil and climatic conditions.
Once the soil test value has been raised to the critical level, the soil is largely capable of supplying crop nutrient requirements in a given year and soil test level is maintained at, or above, the critical level by applying fertilizer rates to replace only the amount of nutrients expected to be removed by the crop. This keeps the soil test level from falling below optimum between soil tests. Once the soil test reaches a level where crop removal will not reduce the soil test level to below optimum, no additional nutrients are added except for the small amounts supplied in starter fertilizer applications.
Since nutrient availability in the soil is increased over time, for future years, more fertilizer is used. While this reduces the risk of nutrient deficiencies related to their availability in soil, profitability in a given year is decreased. This approach also increases the risks of over-fertilizing and negative impacts on the environment.
Since plant nutrients rarely work in isolation, over-fertilizing can also lead to antagonisms, whereby high levels of one nutrient may influence the uptake of another. For instance, excess calcium levels can cause potassium, boron, or magnesium deficiencies in some soils, excess magnesium can reduce potassium uptake and vice versa, and excess phosphorus can lead to reduced zinc uptake. Therefore, balanced fertilization is important for increasing crop yields and improving nutrient use efficiency.
Sufficiency Approach
This approach utilizes the limiting factor concept to make nutrient recommendations. The limiting factor concept states that crop yield increases will cease when a nutrient or factor “runs out;” that is, it cannot promote further increases. When that factor is supplied, yield will increase until another factor becomes limiting. Fertilizers are applied only to meet the nutrient requirements of the crop. The goal is to maximize profitability in a given year, while minimizing fertilizer applications and costs. No consideration is given to future soil test values. When soil test levels are low, crops are likely to respond to additional nutrients and the recommended fertilizer rates exceed nutrient removal by the crop. As soil test levels increase to the critical soil test level, fertilizer recommendation decrease to almost zero as these soils are unlikely to demonstrate any yield response to added fertilizer nutrients. Overall, there is decreased fertilizer usage with this approach leading to a slower increase in soil test values compared to the build-maintenance approach. Most laboratories and universities in the US use this approach for their fertilizer recommendations.
Soil Testing for Nitrogen
Nitrogen exists in organic and inorganic forms in the soil, and interconverts readily among those forms, causing large variations in inorganic N concentrations. For this reason, soil testing for nitrogen has not proven useful, particularly in the more humid climate of the Corn Belt and Southern/Southeastern United States. In these areas, weather-induced variations in inorganic N concentrations greatly affect the ability of a soil test to accurately predict N availability to the crop in a given growing season. So, while a soil test is one of the best methods to determine the right rate of P, K, and several other nutrients in these higher-rainfall areas, N fertilization programs are generally not based on pre-plant soil-testing. Rather, nitrogen management depends on knowledge of nitrogen requirements of the various crop species.
Plant Sampling
The procedure for collecting plant samples for chemical analysis is similar to collecting soil samples. First, determine whether the crop in a field is relatively uniform. Then select a plant part (or parts) from about 15 places in the field. If the grower is concerned about a problem area, take one sample (15 subsamples) of fresh material from the affected area and another sample from the area of healthy/normal growth. Samples should be taken as soon as the problem appears. These two plant samples will provide a comparison of the two areas and an indication of whether nutrient supply is adequate for optimum growth.
Plant part to be sampled depends on many factors—age of plant, type of plant, nutrient to be tested, and so on. Take care not to contaminate the samples with soil as even a small amount of soil will cause the results to be invalid. Contact your local farm advisor or laboratory consultant for information on how to sample a specific crop.
A report from the testing laboratory provides results on the soil and/or plant tests and normally gives recommendations on the type and quantity of nutrients that need to be added.
Nutrient Deficiency Symptoms
Nutrient deficiency symptoms in plants may be seen as poor growth, lack of green color (chlorosis), or browning of tissue (necrosis). The best-known nutrient deficiency is the one caused by lack of nitrogen. On a corn plant, for example, the green tissue along the midrib of the lower leaves turns yellow. The type of symptom and its location on the plant suggest the nutrient that is deficient. See Figure 8.7 for common deficiency symptoms for various nutrients. Table 8.2 also lists common deficiency symptoms for each plant nutrient.
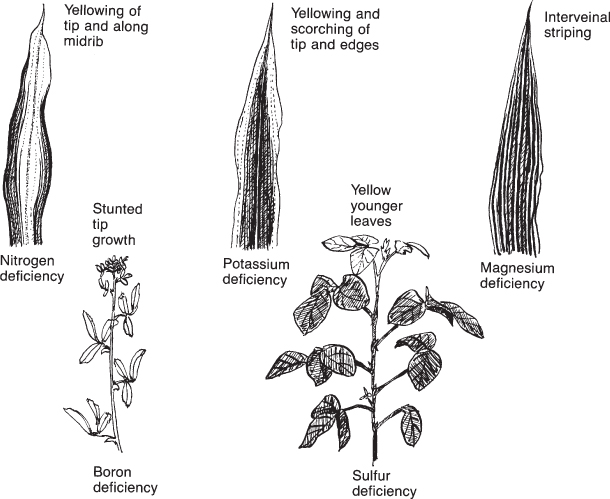
Figure 8.7 Some typical nutrient deficiency symptoms caused by lack of a specific nutrient.
Nutrient Mobility Within the Plant
Certain nutrients, when deficient in the plant tissue, have the ability to move from older leaves to younger leaves where they are needed for growth. Nutrients with this ability are said to be mobile, and include nitrogen, phosphorus, potassium, magnesium, and molybdenum. Other nutrients do not have the ability to move from old to new growth and are said to be immobile. Immobile nutrients include calcium, sulfur, boron, copper, iron, manganese, and zinc. Knowing whether a nutrient is mobile or immobile can provide us with clues when diagnosing deficiency symptoms. If the deficiency symptoms show up on the younger, new growth, we know that the deficient nutrient is immobile. On the other hand, if deficiency symptoms appear in older mature leaves, we know that the deficient nutrient is mobile.
Deficiency symptoms should not be solely relied upon for making fertilizer recommendations. This is because deficiency symptoms may be caused by other factors including insect damage, diseases and many physiological problems. In addition, although the law of the minimum stipulates that the nutrient in the shortest supply will be the first to limit growth, other nutrients may be deficient. Collecting both soil and tissue samples from both “poor” and “good” areas of a field is the best way to diagnose nutrient deficiencies.
There are other diagnostic tools for assessing the nutrient status of crops. These include chlorophyll meters, leaf color charts and on-the-go sensors.
Biological Growth Tests
Biological growth tests may also be used to determine nutrient needs. A simple method is to split a field and apply one type of nutrient on one half and another nutrient on the other half. Or try one rate of a nutrient on one half and double the rate on the other half.
Biological tests can also be used in greenhouses for a short growth period. For such “pot” tests, up to a gallon (4 l) of soil is brought into the greenhouse and divided into small, 1-pint (0.5-l) containers, which receive various rates of the nutrients in question (leaving one untreated). Rapid-growing plants (such as small grains) are planted, harvested in a short time (such as 30 days), and weighed to determine which rate provided the greatest growth.
Adding Plant Nutrients
If the level of essential plant nutrients in the soil is low or their availability is decreased for some reason, nutrients need to be added to achieve good crop yields. Unless nutrients removed in harvested grain and plant biomass are replaced, soil fertility will deteriorate and crop yields will decline.
Fertilizers
Each essential plant nutrient (except carbon, hydrogen, and oxygen) can be applied as a commercial fertilizer. There are many different types and forms—so many, in fact, that it would be difficult to describe all of them here. But a few are discussed next.
Fertilizers come in dry, liquid, and gaseous forms. Some contain only one essential nutrient, whereas others contain two or more. Percentages of the essential nutrients in a fertilizer also vary widely. The percentage of a nutrient or nutrients in a fertilizer is guaranteed to be at a minimum level or above as required by state laws.
A nutrient guarantee is expressed in three numbers, such as 20-10-5. The first number (20 in the example) represents the percentage of available nitrogen (as N). It may be in nitrate (NO3−), ammonium (NH4+), or organic form. The percentage of the nitrogen in each form must appear on the label. The source of any organic nitrogen also must be shown. The form of nitrogen is important in fertilizer timing as most plants use nitrates preferably and the microbial conversion of other forms to nitrates requires time. The second number (10 in the example) is the percentage phosphorus content expressed as phosphate (P2O5). The actual phosphorus content of the material can be calculated by multiplying the P2O5 by 0.44. To convert back to P2O5, multiply phosphorus by 2.29. The third number is the percentage potash (K2O) content (5 in the example), which is an expression of the potassium in the material. To convert to elemental potassium (K), multiply the K2O number by 0.83. To convert back to K2O, multiply the potassium number by 1.20. Other examples of grades are given in Table 8.3. If there are other guaranteed nutrients present, they are listed as additional numbers with the symbols for the elements. For example, if the 20-10-5 given here also contains 2% zinc and 1% manganese, the grade would show as 20-10-5 + 2% Zn + 1% Mn. This grade guarantee is always listed on the fertilizer container (whether it is in a sack, box, or bottle) and also on the invoice. The manufacturers of the fertilizer make the guarantee and it is enforceable by state law. This normally assures the producer of purchasing the correct product.
Table 8.3 Fertilizer grades (Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
| Grade | N | P2O5 | K2O |
| (%) | (%) | (%) | |
| 20-10-5a | 20 | 10 | 5 |
| 0-20-20a | 0 | 20 | 20 |
| 10-30-10a | 10 | 30 | 10 |
| 0-0-60b | 0 | 0 | 60 |
| 32-0-0a | 32 | 0 | 0 |
a Mixed fertilizers with two or more nutrients.
b Straight fertilizers with only one nutrient.
The percentage of a nutrient in a fertilizer is important because it determines the amount to use per acre to obtain a given quantity of a needed nutrient. For example, a fertilizer with a 20-5-10 grade is 20% N, 5% P205, and 10% K2O by weight. If 100 lb (45 kg) of this fertilizer were applied evenly over an acre, the amounts N, P2O5, and K2O applied would be:
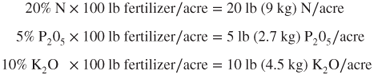
The concentration of nutrients in the fertilizer is multiplied by the amount of the fertilizer material applied per acre to find the actual amount of N, P205, and K2O applied per acre. If crop P guidelines calls for 15 lb (6.75 kg) P205/acre, the amount 20-5-10 fertilizer needed would be:
The amount of 20-5-10 fertilizer material to be applied per acre is calculated by dividing the crop P205 requirement by the concentration of P205 in the fertilizer.
A fertilizer that contains only one nutrient is called a straight fertilizer (or a fertilizer material). An example would be ammonium nitrate, which contains only nitrogen as a nutrient. Mixed fertilizers containing two or more nutrients would be a mixture of two or more straight fertilizers. An example would be mixing ammonium nitrate (33-0-0) with a calcium phosphate (0-46-0) to produce a grade of fertilizer that might be 16-20-0. Other terms often used to describe fertilizers are complete and balanced. A complete fertilizer, a term of little significance from a crop production standpoint, is one that contains all three of the primary nutrients and would be a grade such as 24-10-8 (Fig. 8.8).

Figure 8.8 A complete commercial fertilizer is reported in terms of varying percentages of N, P2O5, and K2O.
A balanced fertilizer contains equal amounts of N-P-K (10-10-10). A balanced fertilizer program is one which provides nutrients based on crop needs and nutrient deficiencies. It might include, for example, only nitrogen if it is the only nutrient deficiency. Or if nitrogen, phosphorus, potassium, zinc, and boron are needed, the fertilizer that would provide the required nutrients for a balanced fertilizer program might be a 15-5-10 + 1% Zn and 0.5% B.
Nitrogen, the fertilizer nutrient used in greatest quantities, comes in all three forms of dry, liquid, or gas. Anhydrous ammonia (NH3) is the principal source of nitrogen used in the United States. It is in a gaseous form when applied to the soil (Fig. 8.9) but is stored as a liquid when under pressure or at low temperatures. It contains only one nutrient and its nitrogen content is 82%. This is the highest nutrient concentration in any commonly used fertilizer. Ammonia is the base for producing other nitrogen fertilizers, such as the examples given in Table 8.4.
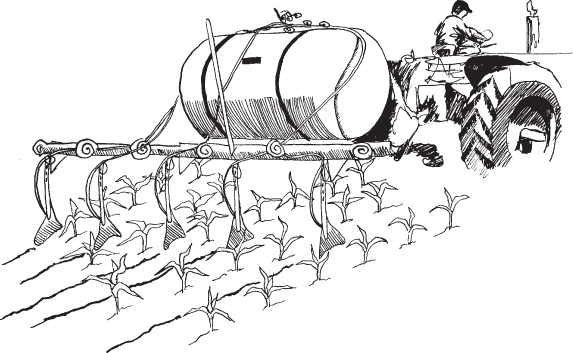
Figure 8.9 Nitrogen may be applied as anhydrous ammonia (NH3) gas fed from a pressure tank through hollow knives that cut into the soil.
Table 8.4 Combination used to produce nitrogen fertilizers (Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
| Combinations | Product | Percentages (N-P2O5-K2O) |
| NH3 + HNO3 | NH4NO3 (ammonium nitrate) | 33.5-0-0 |
| NH3 + H2SO4 | (NH4)2SO4 (ammonium sulfate) | 21-0-0 |
| NH3 + H3PO4 | NH4H2PO4 (ammonium phosphate) | 11-48-0 |
| NH3 + CO2 | (NH2)2CO (urea) | 45-0-0 |
Note: NH3 = ammonia, HNO3 = nitric acid, H2SO4 = sulfuric acid, H3PO4 = phosphoric acid, CO2 = carbon dioxide.
After ammonia is manufactured, it is mixed with various acids in liquid form and the resulting product is then dried to the solid form (Fig. 8.10). While in the liquid state, urea and ammonium nitrate are often mixed to form a solution containing 28 or 32% nitrogen. This is the second most widely used source of nitrogen in the United States (Fig. 8.11).
Figure 8.10 Most nitrogen fertilizers start with ammonia, which reacts with various acids. They exist in gaseous, dry, or liquid forms.
Figure 8.11 Liquid fertilizer may be applied to the soil or, if sufficiently diluted, it can be used as a foliar application.
Phosphorus fertilizers are derived from a mineral called apatite, which is a calcium phosphate and is a form in which the phosphorus is not readily usable by plants. The mineral is mined from deposits just below the surface of the soil in Florida and Idaho in the United States and in Morocco and the former Soviet Union.
Apatite is commonly called rock phosphate (Fig. 8.12). It is treated with an acid (either sulfuric or phosphoric) to produce a calcium phosphate (either 0-20-0 or 0-46-0) in which the phosphorus is in a more usable form than in apatite. Phosphoric acid also can be produced from the apatite and can then be treated with ammonia to produce an ammonium phosphate (Table 8.4). Phosphorus fertilizers are available either in the liquid form or in the dry form. Phosphorus is second to nitrogen in quantity used in the United States.
Figure 8.12 Rock phosphates for making fertilizer are mined from open pits.
Potassium fertilizers are manufactured from minerals such as muriate of potash or langbeinite, which occur in deposits in the earth. Some deposits such as those in Canada are fairly shallow, whereas others such as those in New Mexico are quite deep (Fig. 8.13). Muriate of potash is refined to produce potassium chloride (0-0-60); langbeinite is used to produce potassium-magnesium sulfate (0-0-22 + 11% Mg). Other common potassium fertilizers are potassium sulfate (0-0-50), potassium nitrate (13-0-44), and potassium phosphate (0-26-26).
Figure 8.13 Potash, a potassium compound, is mined from deposits in the earth.
The other fertilizer elements come from various sources. Calcium, for example, comes mostly from limestone (calcite) and gypsum. Magnesium is used either as potassium-magnesium sulfate, limestone (dolomite), or magnesium sulfate. Sulfur is usually applied as elemental sulfur, a thiosulfate, or as one of the sulfate forms such as ammonium or potassium sulfate.
The micronutrients iron, zinc, manganese, and copper are usually used in one of three forms. A sulfate salt such as zinc sulfate is common. Another is called a chelate, which is an organic form that reacts with the micronutrient to make a relatively soluble product. A third form is an oxide, such as zinc oxide.
It is important to use the source of fertilizer best suited for any given crop and condition. A local fertilizer dealer, consultant, or agricultural agent should be consulted for specifics on the best one to use.
Effect of Fertilizers on Soil pH
Different fertilizer materials have different impact on soil pH. Generally, fertilizers with high proportions of total nitrogen and are derived from ammonium sources (such as urea, ammonium sulfate, ammonium phosphate, or ammonium nitrate) can acidify soils with repeated applications. Most fertilizers provide the “Lime Equivalent” on the bag's label. The lime equivalent is the amount of limestone (calcium carbonate) it takes to neutralize the acidifying effects of using one ton of a particular fertilizer.
Some fertilizers can also increase soil pH. These fertilizers are usually low in ammonium, but high in nitrate. Additionally, these fertilizers sometimes contain calcium from calcium nitrate. The lime equivalent for these fertilizers is also given, but it indicates the equivalent liming effect rather than the lime needed to offset acidity.
The following generalizations may be used as a guide:
- Ammonium (NH4+) or ammonium forming fertilizers, such as urea, will cause a decrease in soil pH over time.
- Nitrate (NO3−) sources carrying a basic cation should be less acid-forming than NH4+ fertilizers.
- The presence of Ca, Mg, K, and Na in the fertilizer will slightly increase or cause no change in soil pH.
- Elemental sulfur, ammonium sulfate, and compounds such as iron or aluminum sulfates can reduce the soil pH.
Salt Damage
When inorganic fertilizers are applied to the soil, the concentration of soluble salts increases in the soil solution surrounding the zone of fertilizer application, particularly when there are high rates of evaporation and insufficient rainfall to leach the salts. A high concentration of soluble salts in the soil solution can have harmful effects on plants and germinating seeds. The salt index is a measure of the potential salt damage to the plant. Soluble salts can also originate from manure and minerals in the earth.
Problems associated with salt damage include:
- 1. If the concentration of salt in the soil solution is greater than the salt concentration in plant root cells, moisture availability will be restricted and water will not be absorbed by the plant.
- 2. The high concentration of salts will cause water to leave the plant by osmosis and enter the soil. Excessive water loss causes the protoplasm to shrink away from the cell walls (plasmolysis). As a result, the plant withers and exhibits symptoms similar to those caused by drought.
- 3. High concentrations of soluble salts may also result in elemental toxicities of sodium and chlorine.
Fertilizer Salt index
The fertilizer salt index was developed to classify fertilizers according to their potential to cause salt injury to plants. It is a measure of the salt concentration that a fertilizer material induces in the soil solution and it is measured by placing the material in the soil and determining the osmotic pressure of the soil solution. Sodium nitrate is the standard and has a salt index of 100. Other fertilizers are assigned a salt index value relative to 100, which describes the potential of the fertilizer to cause salt injury relative to the damage caused by an equal amount of sodium nitrate. A fertilizer with a salt index less than 100 has a lower potential to cause salt damage in comparison with a fertilizer with a salt index greater than 100.

Nitrogen and potassium salts have higher salt indices than phosphorus salts, hence they have more damaging effects on germination when placed close to the seed. Salt injury can be avoided by applying N and K fertilizers on the soil surface (broadcast application) or by placing them to the side and below the seed.
Soil Amendments
A soil amendment is a material added primarily to change or enhance the physical, chemical or biological characteristics of soil, rather than as plant food. Examples include liming materials such as lime (calcium carbonate), dolomite (calcium magnesium carbonate), calcium oxide, magnesium oxide, calcium hydroxide, magnesium hydroxide wood ash, and slags, which are used primarily to increase the pH of a soil and make it less acid. Liming materials should not be added with urea or ammonium fertilizers, as N will be lost to the atmosphere. Elemental sulfur is used to decrease pH and make it less alkaline. Gypsum (calcium sulfate) is used primarily as an amendment on soils with excess sodium (sodic soils), which causes a poor physical condition. The calcium in the gypsum replaces the sodium, resulting in a soil with improved structure. The sodium combines with the sulfate and is leached from the soil by irrigation.
These soil amendments also provide plant nutrients if they are needed. For example, limestone provides calcium and in some cases magnesium. Gypsum provides calcium and sulfur.
Animal Manure and Green Manure Crops
Animal manures are excellent sources of plant nutrients. Because manure is often derived from plant material, it is similar to organic matter and therefore contains essential nutrients. When manure decomposes in the soil, its nutrients are released and made available for plant uptake.
Manure not only serves as a source of plant nutrients but also adds organic matter to the soil, which improves its physical condition, water-holding capacity, cation exchange capacity, and other desirable properties. For maximum value, manure should be injected or worked into the soil soon after application (Fig. 8.14). A disadvantage of manure is that it often contains weed seeds.
Figure 8.14 Animal manure improves soil structure as well as supplying nutrients.
The nutrient and moisture content of manure is quite variable, depending principally on the types of feed utilized by the animals and how the manure is handled before it is applied. It should be applied as often as convenient to do so, but in most cases, manure has to be stored and applied later. Alternate wetting and drying in a pile results in the release of ammonia gas into the atmosphere as the manure dries. When rewetted by rain, nitrates leach out and may present a danger if allowed to run off into a water system being used by humans or animals. Many states have laws that restrict the runoff from feedlots and manure piles, and require owners to provide holding ponds or lagoons (Fig. 8.15). If a manure pile dries without subsequent rewetting (in dry areas), loss of ammonia is minimized.
Figure 8.15 Lagoons provide storage and maintain the nutrient value of manure.
The average nutrient value of manure from beef feedlots in Texas is shown in Table 8.5. The nutrient content of manure is also quite variable; thus, only averages are presented in the table. The values of moisture content ranged from a low of 8% to a high of 62%, with an average of 33%. One ton (900 kg) of manure with a moisture content of 33% and average nutrient percentages would be equivalent to 400 lb (180 kg) of an 8-6-11 grade fertilizer.
Table 8.5 Average content of essential elements in beef feedlot manure (based on 30 samples from Texas High Plains feedlots, figured at 30% moisture content) (Eash, Neal S., Cary J. Green, Aga Razvi, and William F. Bennett, eds. Soil Science Simplified. 5th ed. Ames, Iowa: Wiley-Blackwell, 2008. Copyright © 2008, John Wiley & Sons, Inc.)
| Nutrients | Content | Pounds/ton (kg/metric ton) of manure |
| (%) | ||
| Nitrogen (N) | 1.6 | 32 (16) |
| Phosphorus (P2O5) | 1.3 | 26 (13) |
| Potassium (K2O) | 2.2 | 44 (22) |
| Calcium (Ca) | 0.7 | 14 (7) |
| Magnesium (Mg) | 0.2 | 4 (2) |
| (ppm)a | ||
| Iron (Fe) | 1525 | 3.00 (1.50) |
| Zinc (Zn) | 100 | 0.20 (0.10) |
| Manganese (Mn) | 105 | 0.21 (0.105) |
| Copper (Cu) | 7 | 0.02 (0.01) |
| Boron (B) | 15 | 0.03 (0.015) |
a ppm = parts per million or 1/10,000 of a percent.
When animals are confined in barns most of the time, their manure is commonly stored for 6 months or more in lagoons or large tanks and the moisture content is more likely to be around 90%. It is injected into the soil as a slurry from tanks on wheels (Fig. 8.16). This is typical for dairy farms. Under these conditions, the nutrient content is likely to be about one-third of that shown in Table 8.5.

Figure 8.16 A tractor-powered mobile tank and pump unit for injecting liquefied manure into the soil.
The N/P ratio in animal wastes is typically 1:1 to 2:1 (N/P2O5), but most plants require 3:1 to 5:1 (N/P2O5). Therefore, animal waste is relatively high in P. If manures are applied to meet the N needs of plants, excessive amounts of P often result. Since P is relatively immobile in the soil, any P not taken up by plants will accumulate in soil to levels far in excess of amounts needed for optimal crop growth. The excess P in the soil can be lost via leaching, erosion and runoff and can cause nutrient enrichment in ground and surface water sources and cause eutrophication. A more environmentally approach is to apply manure to meet P needs (based on soil testing). This will result in lower N application, but the remaining N needs can be met with fertilizers. This approach requires more land since smaller amounts of manure will be utilized on each field.
The economic value of manure is also variable, depending on the nutrient percentages. In most cases, approximately one-half of the nutrients are released and available the first year. The rate of decomposition, however, varies widely. On this basis, if the analysis is known, a value can be placed on manure. Because manure has relatively low nutrient percentages, the volume that must be handled is relatively high if sufficient plant nutrients are to be applied. Consequently, manure is normally used fairly close to the farm or feedlot where it is produced. Manure application rates are generally 10–15 tons per acre (22–34 Mg/ha [megagram/ hectare]). Manure should normally be applied on an “as-is” basis for crops that have a significant nitrogen requirement.
Crops plowed under soil (Fig. 8.17) to improve fertility and physical condition of the soil are called green manure. The best crops for this purpose are legumes such as alfalfa and clover because they are high in nitrogen content; however, nonlegume crops such as wheat or sudan grass can also be used.

Figure 8.17 Crops can be plowed under as green manure to provide organic matter.
Fertilizer Placement Methods
The major function of correct fertilizer placement is to enhance nutrient availability and plant uptake. Correct fertilizer placement encourages maximum crop yields because it often improves the efficiency of nutrient uptake. This leaves less nutrients in the soil, thereby protecting both surface and groundwater quality.
Numerous placement methods are available but most generally involve surface to subsurface applications before, at or after planting. Prior to planting, fertilizers can be broadcast on the surface or incorporated into the soil, applied as a band on the surface or applied as a subsurface band. At planting, fertilizers can be banded with the seed, below the seed or below and to the side of the seed. After planting, application is usually limited to N as a top-dress or a side-dress.
There is no single best method and several factors need to be considered in making fertilizer placement decisions. These include soil conditions, soil test level, soil P buffering capacity, crop type, salt effect of the fertilizer, convenience to the grower, and equipment availability.
Broadcast Methods
A broadcast involves the application of nutrients uniformly on the soil surface and may or may not be incorporated. A majority of fertilizer used in the USA is applied using broadcast methods. The popularity of broadcast pre-plant applications of solid fertilizers has increased in recent years due to the desire to: reduce fertilizer injury to plants, cut down on the amount of time spent handling fertilizers and the development of bulk blends that enable the application of a large quantity of material at one time.
Surface Broadcast
Surface broadcast is a method by which fertilizer is applied uniformly over the surface of an entire field (Fig. 8.18). It is best suited for high-speed operations and heavy application rates. High capacity fertilizer spreaders are often used to spin dry fertilizer or spray liquid fertilizer on the soil surface or on a growing crop. A broadcast application is referred to as a top-dress application when applied at planting, or when applied to the standing crop (after emergence of crop). Care must be taken in top dressing to ensure that fertilizer is not applied when the leaves are wet or it may burn or scorch the leaves. Broadcasting is the only option for applying N and K fertilizers to existing stands of perennial forage crops such cool season grasses and alfalfa.
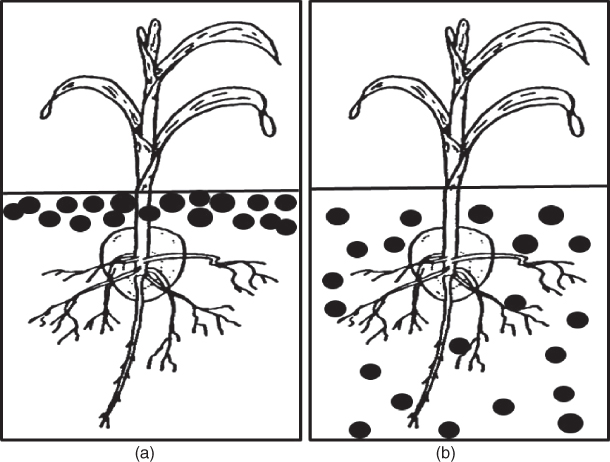
Figure 8.18 Fertilizer distribution using a) broadcast topdressed and b) broadcast incorporated methods of placement.
Advantages:
- 1. It is fast, easy and economical (saves labor and time during planting).
- 2. It results in relatively uniform distribution of fertilizer.
- 3. It requires relatively inexpensive application equipment.
- 4. It can reduce work load at critical times during the year since there are several opportunities for application.
- 5. Large amounts of fertilizer can be applied without the danger of injuring the plant.
- 6. It provides a practical way to apply maintenance fertilizer, especially in forage crops and no-till systems.
Disadvantages. Broadcasting has low efficiency of nutrient use because:
- 1. It enhances N immobilization and losses of N via volatilization in high surface residue systems such as no-till systems.
- 2. N and P losses by erosion as well as N losses by denitrification are higher compared with placement in the soil.
- 3. It leaves more fertilizer available to weeds.
- 4. It requires rainfall or irrigation to move mobile nutrients such as NO3-N and SO4-S into the root zone. If the soil dries out, these nutrients become unavailable.
- 5. It leaves immobile nutrients (P, K, and some micronutrients) on the soil surface, making them unavailable to the plant root system.
- 6. It does not increase the overall fertility of the soil as it does not store fertilizer deep in the profile for later season plant nutrition.
- 7. Uniformity of application can be poor for low rates of fertilizers.
Broadcast Incorporated
A broadcast application is referred to as a broadcast-incorporated when the broadcasted fertilizer is immediately incorporated into the soil by tillage, resulting in fertilizer being mixed throughout the root zone (Fig. 8.18). This improves on the efficiency of surface application. Plowing creates a nutrient-rich zone a few inches below soil surface where young plant roots can absorb it, thereby improving nutrient availability.
Advantages:
- 1. Reduces volatilization and erosion losses compared to broadcast placement.
- 2. Increases uptake of immobile nutrients (i.e., P and K) by increasing the probability of contact between plant roots and fertilized soil.
- 3. Improved plant uptake leaves fertilizer less available for weeds.
- 4. Distribution of nutrients throughout the plow layer encourages deeper rooting and exploration of the soil for water nutrients.
- 5. Incorporation promotes rapid nitrification of NH4+ to NO3+.
- 6. Incorporation with plow mold-board and plow chisel stores fertilizer deep in the profile for later season plant nutrition.
- 7. Reduces the chance of salt injury to seedlings.
- 8. Increases the overall fertility of the soil.
- 9. Maximizes contact between soil and fertilizer thereby creating more potential sites for adsorption and subsequent retention of K and Mg in low CEC (sandy) soils.
Disadvantages:
- 1. Requires more energy to incorporate the fertilizer.
- 2. Results in potentially higher N and S leaching losses than with surface placement.
- 3. Stimulates some weed growth.
- 4. Some tillage implements, such as a mold board plow, may distribute the majority of the fertilizer possibly too deeply for the roots of young seedlings.
- 5. Potential for leaching losses of N and sulfur (S) are higher than with surface placement, particularly in wet years.
- 6. Maximizes contact between the soil and fertilizer, thereby increasing the opportunity for tie-up/fixation and reduced availability of P and K.
- 7. Some implements, such as a chisel, incorporate fertilizer only 2–3 in. (5–7.5 cm) into the soil surface. This leaves nutrients inaccessible to roots when the surface soil dries out.
Nutrients that are generally broadcast include nitrogen, sulfur, calcium, and magnesium (as liming materials), copper, manganese, zinc (but banding is more efficient for all these micronutrients), and boron. Potassium may be applied by either broadcast or band methods. However, broadcasting is preferred in sandy soils that have a low CEC (less than 3 meq/100g) while banding is preferred in soils containing a lot of vermiculite and/or illite where K fixation occurs.
Nutrients that are generally not broadcast include iron (generally applied as a foliar spray) and molybdenum (generally applied as a seed treatment). Phosphorus is broadcast only in soils with moderate to high levels of P (at soil pH values of 5.5–6.5).
Pop-Up Application
Pop-up fertilizer applications refer to placing small amounts of nutrients in direct contact with the seed (Fig. 8.19) to enhance the availability of nutrients to young plants and enhance early seedling vigor. It is also known as starter fertilizer. Nitrogen and P are usually pop-up fertilizer components. The upper limit of pop-up fertilizer that can be used is determined by the relative salt tolerance of the plant. Large amounts of N, K, and S fertilizers cannot be used in pop-up applications due to their high salt contents. Boron cannot be used in a pop-up application as high concentrations of B are toxic to plants.

Figure 8.19 Pop-up or direct seed contact method of fertilizer placement.
Solid fertilizers used in pop-up applications should be highly soluble in water. They should also have a low salt index, a high nutrient content and minimal content of N materials that produce ammonia.
Advantages:
- 1. Both liquid and dry fertilizers can be used.
- 2. Promotes early plant growth and enhances seedling vigor. This early stimulation of crop growth is often termed “pop-up effect.”
- 3. Decreases loss of nutrients by erosion compared with surface placement.
- 4. Positions fertilizer where root systems of seedlings can more readily use nutrients.
- 5. Positions fertilizer so it is more available to the crops than to the weeds.
- 6. Placing P with the seed increases the concentration of available P sufficiently to partially offset the detrimental effects of extremes in soil pH, compaction and low soil temperatures under conservation tillage systems.
Disadvantages:
- 1. Can cause seedling damage if too much fertilizer is applied.
- 2. Retro-fitting planters can be expensive.
- 3. Urea and diammonium phosphate cannot be used.
Total application rate must be kept below 10 lb (4.5 kg) of N + K2O so as to prevent salt burn.
Band Application
Banding refers to placing nutrients below, above, on one side, or on both sides of the seed or seedlings so that developing roots will easily reach the nutrients (Fig. 8.20). Fluid or solid fertilizers can be used. All plant nutrients, with the exception of boron (B), can be successfully banded. High concentrations of B in soils are toxic to plants.

Figure 8.20 Band fertilizer placement method.
Banding can be done before or at the same time with planting/drilling, or after the crop is planted. A low rate of fertilizer is placed in close proximity (at least 2 in./(5 cm) to the side and 2 in. (5 cm) deeper than the seeds or plants). This provides the plants with a concentrated zone of nutrients while preventing salt damage and ammonia toxicity.
Advantages:
- 1. Requires less fertilizer per acre than broadcasting.
- 2. Jump-starts early plant growth by increasing P availability.
- 3. The positioning of fertilizers is such that nutrients are more available to the crops than to the weeds.
- 4. Decreases P and K fixation by limiting surface area of contact of fertilizer with the soil, thereby increasing their availability to plants.
- 5. Absorption as well as movement of P and N to plant roots is much slower at lower soil temperatures. Banding applications of N and P improve plant uptake at lower soil temperatures. This improves growth, thereby promoting winter hardiness.
- 6. Fertilizer application and planting operations can be done simultaneously.
- 7. Reduces nutrient losses due to soil erosion.
- 8. Similar to broadcast – incorporate, banding ammonium-based N fertilizers (e.g., ammonium nitrate, ammonium sulfate, and urea) below the soil surface reduces volatilization losses.
- 9. It slows NH4+ conversion to NO3− (nitrification), reducing the risk of leaching.
- 10. Enhances seedling growth thereby resulting in stronger seedlings that are less prone to suffer from pests and diseases.
- 11. Has high nutrient use efficiency.
Disadvantages:
- 1. There is risk of salt burn to plants.
- 2. There is increased NO3− and SO4− leaching losses compared with surface placement.
- 3. Slows planting if applied with a drill.
- 4. It is costly as it requires expensive equipment or equipment modification.
Side-Dress Application
A surface or subsurface banding treatment after the crop is planted is referred to as a side-dress application. In this case, fertilizer is applied between rows of growing plants to supply N during periods of rapid growth as this is when the crop needs it most. The most common use of side-dress application on farms is the application of N fertilizer between the rows of a growing corn when the plants are 12–24 in. (30–60 cm) tall. The amount of fertilizer N to be applied is determined based on the results of a Pre-Side-dress Nitrate Test (PSNT), which assesses the likelihood of a yield response to the addition of side-dress N. Side-dress application is not recommended for immobile nutrients (P and K) because most crops need these early in the growing season.
Advantages:
- 1. Provides a valuable opportunity to apply the recommended nitrogen throughout the season in smaller amounts (split application), rather than applying all the nitrogen in a single application.
- 2. Applications can be made whenever the equipment can be operated without damage to the crop. This allows a grower more flexibility in application time.
- 3. Split or multiple applications provides N when the plant needs it most, resulting in high nutrient use efficiency.
- 4. Provides crops with additional nutrients if applied during the growing season.
Disadvantages:
- 1. Timing often falls during the wet and busy season; slow process.
- 2. Subsurface side-dress applications with a knife too close to the plant can cause damage to roots by pruning or by fertilizer toxicity.
Point Fertilization
This consists of opening a hole in the soil with a stick or hoe, and placing a quantity of fertilizer into the soil near the crop. This system is commonly used in the production of perennial shrub or tree crops. It is also used in many developing countries, where competition from weeds is minimized by placing small quantities of fertilizer in a hole dug near a hill of sorghum or corn.
Fertigation
Fertigation is the application of dissolved fertilizers and chemicals to the soil through an irrigation system, which applies both water and nutrients to plants. The principal nutrients applied by fertigation are N and S. Fertigation of P is less common due to concerns over precipitation of P in waters high in Ca and Mg.
Advantages:
- 1. Nutrients can be applied close to the time of peak crop demand, thus preventing luxury consumption of nutrients.
- 2. May reduce losses of nitrogen due to leaching and denitrification.
- 3. Provides an opportunity to split nitrogen recommendation into several applications.
- 4. Results in reduced operation costs through elimination of one or more field operations.
- 5. Nutrients may be applied continually throughout the crop growing period. This enables a high degree of flexibility in nutrient management.
- 6. Can be used to correct midseason nutrient deficiencies.
- 7. Combining fertilization and irrigation into a single field operation saves on time and labor.
- 8. High nutrient use efficiency.
Disadvantages:
- 1. Irrigation equipment needed (injection pump, etc.).
- 2. There is risk of uneven distribution of nutrients with low rates of fertilization, in windy situations and under row irrigation.
- 3. Requires a well-managed and equipped irrigation system for uniform, maximum efficiency.
- 4. Nutrients may be leached beyond the root zone.
Foliar Application
This is the application of a small amount of soluble fertilizer or mineral through direct spraying onto the aerial portion of plants. This is a common way to apply micronutrients since micronutrients are required in much smaller quantities than macronutrients.
Advantages:
- 1. Foliar fertilizers supply plant cells with nutrients more rapidly than the soil.
- 2. Can provide a quick way to correct nutrient deficiencies.
- 3. Very effective for micronutrients as these are required in small amounts.
- 4. It is the most effective means of fertilizer application in situations where the problem of nutrient fixation by the soil occurs.
Disadvantages:
- 1. It is expensive.
- 2. It is limited to small and/or repeated applications.
- 3. Has limited capacity to supply macronutrients because of salt hazards.
- 4. The response is usually temporary, hence repeated applications are required.
Injection
Injection is used to place liquid or gaseous fertilizer below the soil near plant roots.
Advantages:
- 1. Reduced volatilization losses through precise application of nutrients.
Disadvantages:
- 1. Slow and expensive (requires specialized equipment).
- 2. Mobile nutrients may be lost via leaching
Timing of Fertilizer Application
Determining the appropriate time to apply fertilizers in the soil is just as important as choosing the correct amounts of plant nutrients and determining the proper zone in the soil in which to apply the fertilizer. Farmers sometimes apply fertilizer soon after the previous year's harvest. While this coincides with availability of equipment and labor, this may not be efficient agronomically as fertilizer applied too far in advance of crop demand may be lost, resulting in negative economic and environmental consequences. Optimal timing of fertilizer application ensures an adequate supply of nutrients during peak and critical crop demand periods. This maximizes nutrient recovery by the crop, thereby reducing the potential for loss of nutrients from the system.
Proper timing of fertilizer applications in the field aims to:
- 1. Provide a sufficient amount of the nutrient when the plant needs it.
- 2. Avoid excess availability of nutrients, especially of N, before or after the principal periods of plant uptake (especially during environmentally sensitive periods of groundwater recharge).
- 3. Make nutrients available when they will strengthen, not weaken, plants.
- 4. Enable field operations to be conducted when it is feasible.
Some basic factors to be considered in fertilizer timing decisions.
- 1. Nutrient element: The greater the potential for loss of a given nutrient from the soil, the greater the importance of timing of application. Proper timing is critical with nitrogen fertilizer, as N is susceptible to loss from the soil through several pathways including leaching, denitrification, volatilization, and runoff/erosion (see Chapter 4). For example, fall application of N for spring-planted crops, such as corn, should not be practiced in humid areas because of high risk of loss. To slow nitrification in the fall and avoid increased nitrate leaching and/or denitrification, fall application of N should only be practiced in late fall after the soil temperature drops to below 50°F (10°C) and expected to continue cooling, even in drier areas with low risk of loss. Time of application is less critical with P and K and fall application is generally considered a reasonable practice as the risk of runoff is small in that season. However, phosphorus application is most efficient when made at or as close to planting time as possible, especially in low P soil and/or soils with high capacity to convert soluble P into less available forms. Application timing is also an important consideration with potassium in sandy, low cation exchange capacity soils in high-rainfall areas, because of the high potential for leaching in these environments.
- 2. The N form: The N form in a fertilizer product can affect the potential for loss and optimal timing. In southern US, the temperatures favor nitrification during a greater portion of the year, hence ammoniacal N applied before planting would be converted to nitrate and lost via leaching. Fertilizer materials containing significant portions of nitrate are not suggested for fall application, because of leaching and gaseous losses. Instead, spring pre-plant and/or side-dress applications are preferable as they typically provide a lower risk of loss and greater profitability. And, even with spring application, materials containing significant portions as nitrate are more susceptible for loss with early pre-plant application.
- 3. Crop and plant nutrient uptake pattern: Crop nutrient demand is not consistent throughout the growing season but it is characterized by an initial stage of slow uptake, followed by a phase of rapid uptake, followed by a period of declining uptake as the crop matures. Therefore fertilizer applications, particularly N, can be timed and targeted at specific growth stages to increase N uptake (crop yield and/or quality) and to reduce the potential for loss to the environment. Timing of fertilizer application can also be targeted to correct specific nutrient deficiencies during the growing period.
- 4. Soil characteristics and environment: Certain site and soil characteristics influence the potential for nutrient loss, nutrient retention, and supply capacity and are important considerations in decisions of fertilizer timing. The characteristics include slope, soil texture, temperature and drainage. The greater the soil's capacity to retain and supply and provide a crop-available nutrient throughout the growing season, the less the need for a critical timing emphasis for that nutrient. For example P and K fertilizers can be applied once on most soils in the Corn Belt to supply crop needs. In contrast, P fertilizer products need to be banded at or near planting time in soils with very high P fixation capacity such as the highly weathered soils in the southern USA and the calcareous soils of the West. Proper timing of N fertilizers is important on sandy-textured soils in areas receiving high rainfall during winter and spring. Nitrogen fertilizer applications on these sandy soils are usually split into two or three applications to increase N uptake and reduce the potential for loss to the environment. Conversely, there is little advantage to splitting application of N between fall and spring in arid environments with low loss potential, hence all N may be applied pre-plant.
Other factors affecting fertilizer application timing decisions include feasibly of conducting the operation, farm size, other field operations, availability of equipment and labor, and fertilizer distribution logistics.
Precision Farming
The computer age has led to many innovations in agriculture. One such innovation is precision farming. Because soils in most farm fields vary considerably in such properties as organic matter, topsoil thickness, texture, structure, and plant nutrient content, it is inefficient for an entire field to receive the same amount of fertilizer when the crop yield potential and soil nutrient level vary from one area of the field to another. It is claimed that fertilizer costs are reduced, the environment is better protected, and crop yields are higher if the proper amount of fertilizer is more precisely applied to each of the various management zones in the field. It can be demonstrated that the variations in soil properties are often more detailed than the soil maps discussed in Chapter 11.
The first step in using precision farming techniques involves sampling the soil by using an ATV (all terrain vehicle) in a grid pattern with each sampling point commonly representing 2.5 acres (1 ha) (see Fig. 8.5). Management zones can also be used. The position of each sample site is monitored and programmed into the computer via the GPS (global positioning system), which relies on a set of satellites about 200 miles (320 km) above the earth. The ATV is equipped with a receiver that collects signals from at least three satellites for accurate positioning by triangulation (Fig. 8.17). With an enhanced system, the sampling sites usually can be pinpointed to within 1 m. Each sampling location appears as a point on a computer screen.
The second step is to analyze the samples for nutrient content and other soil characteristics (such as pH and organic matter). From these results, a pattern of the fertilizer requirements throughout the field can be established and stored on a computer disk.
The third step requires a fertilizer applicator with separate bins for individual fertilizer, each equipped with augers to transfer their contents to the spreading apparatus. The augers are regulated by a computer in the cab of the applicator whose position has been determined by the GPS. In this way, there can be a continuous adjustment in the rate and mixture of fertilizer applied as the truck goes back and forth across the field.
In the fourth step, the combine that harvests the crop is equipped with a device that continuously monitors the yield of the grain harvested from all parts of the field in relation to a previously programmed grid sequence described in the first step. The yield map is used to set the yield goal, which is a factor used to determine the amount of nutrient to apply.
Farmers may contract for the precision farming techniques described previously and many are currently doing so. The extent to which precision farming becomes a common practice depends on how farmers judge its economic value.
Organic Farming/Gardening
Organic farming or gardening involves producing crops without applying commercial fertilizer or chemical pesticides. Organic farming usually includes the slow release of a naturally balanced supply of nutrients from decaying organic matter (Fig. 8.18), such as crop residues and animal manure. Crops of high quality and quantity may be grown by organic methods. In parts of the world where commercial fertilizers are not available, farmers must rely on decaying organic matter for supplying crops with nutrients. Enough food could not be provided for the world's population if the plant nutrients for food crops were to be supplied solely from organic sources. The recycling of carbon and nutrients from plant and animal manures is, however, an important benefit of organic production techniques. Some consumers prefer to eat food grown without chemicals, so they are willing to pay higher prices for crops that are grown using organic methods.
Nutrients from the soil are absorbed by plants mainly in the form of ions (charged particles). Whether these ions come from a weathering mineral, decaying humus, or a chemical fertilizer is of no consequence to the plants. But the nutrients should be in a properly balanced proportion.
Composting
Composting is a planned and managed process to foster the aerobic decomposition of organic matter. Unlike anaerobic decomposition, compositing doesn't produce odors and seeping noxious liquids.
Composting is often used for a source of organic matter for gardeners (Fig. 8.19). The basic ingredients of a compost heap are organic residue, soil, moisture (50–70%), nitrogen fertilizer, and some lime to counteract acidity associated with decomposition. The carbon-to-nitrogen ratio of the material must be close to 30:1. The C/N ratio can be lowered by adding nitrogen fertilizer or by mixing high carbonaceous materials such as straw with low carbon materials such as livestock manure, green grass clippings, and legume hay. Air (oxygen) must be able to diffuse through the pile to the decomposing residue and prevent anaerobic decomposition from taking over. The heap is allowed to decompose (rot) for several months and is then mixed into the soil. The rotting process is carried out by microbial action that is greatly hastened in the presence of an adequate amount of nitrogen. Other materials such as rock phosphate powder, wood ashes, and mixed fertilizers may be added for improved nutrient balance of the final product.
Composting is also becoming more popular for organic farming. A typical system has large rows of organic material (leaves, sawdust, or manure) that is amended with an organic source of nitrogen, and other nutrients if necessary. A machine mixes the material in the rows until the compost is stable (partially decomposed). The heating of the compost, as some of the organic material decays, has an added benefit of killing most of the pathogenic or disease-causing microbes. Finished compost can be bagged for sale in stores or loaded onto trucks or spreaders for application to fields.
Composting is a good way to turn most organic waste products into a reliable and valuable plant nutrient source. However, certain wastes are problematic and they should be avoided. For instance; meat scraps attract rodents and other pests and produce noxious odors as well, cat droppings carry microbes that may be harmful to infants and pregnant women, plastics and glass are non-biodegradable, while plywood may be laden with heavy metals.
Biosolids
Biosolids or sewage sludge, are nutrient-rich organic materials resulting from the processing of domestic sewage in a treatment facility. Biosolids contain nutrients such as nitrogen, phosphorus, and potassium and trace elements such as calcium, copper, iron, magnesium, manganese, sulfur, and zinc. Some biosolids might also be lime-stabilized, resulting in a pH increase when applied to the soil. In addition, the organic matter in biosolids has several benefits (see Chapter 4). Therefore, when used in accordance with existing federal guidelines and regulations, biosolids can be applied as part of a crop nutrient management plan to produce crops for human consumption with negligible risk to both the consumer and the environment. When used to fertilize crops, the nutrient content of biosolids should be tested and their contribution to the total crop nutrient needs should be accounted for. Furthermore, recommendations for the appropriate application rate, method, and timing should be followed.
Since biosolids are regulated byproducts of wastewater treatment, they pose various health and safety risks that must be addressed when they are applied to the soil. These include: presence of disease-causing organisms and heavy metals, odor, and insect problems associated with the application of raw, unstabilized wastes; and surface and ground water contamination by nitrogen, phosphorus, and pathogens.