
ATOMS, MOLECULES & COMPOUNDS
ATOMS, MOLECULES & COMPOUNDS
GLOSSARY
alkali metals The column of metals (group IA) on the far left of the periodic table that includes lithium, sodium, potassium, rubidium, cesium and francium.
atomic number The unique number assigned to each element that corresponds to the number of protons in the element’s nucleus.
atomic theory The idea that all matter is composed of tiny particles called atoms.
classical physics Physics before the advent of quantum mechanics.
covalent bonding The joining of atoms by the sharing of one or more electrons.
electron A subatomic particle with a negative charge and a mass of 0.00055 amu (atomic mass unit).
element A fundamental substance that cannot be divided into simpler substances. There are 91 naturally occurring elements.
Heisenberg’s Uncertainty Principle The quantum mechanical principle that certain quantities, such as position and momentum, cannot be simultaneously specified to arbitrary accuracy.
ionic bonding The joining of two atoms by the transfer of an electron from one to the other.
ionic compound A compound, usually composed of a metal and one or more non-metals, that contains atoms joined by ionic bonds.
isotope An atom that has the same number of protons as another atom, but a different number of neutrons.
mass number The sum of the number of protons and neutrons of an atom.
molecular compound A compound, usually composed of two or more non-metals, that contains atoms joined by covalent bonds.
neutron A subatomic particle with no charge and a mass of 1 amu.
noble gases The column of gases (group 8A) on the far right of the periodic table that includes helium, neon, argon, krypton, xenon and radon.
nuclear fusion The joining of two lighter nuclei to form a heavier one.
nuclear model A model for the atom in which most of the mass of the atom is contained in a small dense nucleus composed of protons and neutrons. Most of the volume of the atom is occupied by the electron cloud.
nucleosynthesis The process by which elements form within stars.
proton A subatomic particle with a positive charge and a mass of 1 amu.
quantum mechanics The realm of physics, developed in the early twentieth century, that deals with the very smallest particles that exist.
Schrödinger’s Cat thought experiment A thought experiment involving the application of the uncertainty principle to a cat in a box with a radioactive substance that has a 50 per cent chance of decaying. If the atom decays, then the cat dies, so the cat is in a strange state of being both dead and undead, with a 50 per cent probability of each. Schrödinger used this experiment to show that quantum mechanical ideas are not applicable to large scale objects (such as cats).
valence electrons The highest energy electrons (and therefore the most important in bonding) in an atom.
velocity A measure of how fast (and in what direction) an object is moving.
MATTER IS MADE OF PARTICLES
the 30-second chemistry
The Ancient Greek philosophers believed that matter was infinitely divisible – that matter had no fundamental particles. Subsequent thinkers followed suit for more than 2,000 years. It was not until the eighteenth and nineteenth centuries that early chemists used careful measurements – primarily the relative weights of related samples of matter – to determine otherwise. And it wasn’t until the early twentieth century that the question was definitely settled: the 1926 Nobel Prize for Physics was awarded to Jean Perrin for settling the matter. The Greeks were wrong – matter is particulate (it is made up of particles), and those particles are called atoms. And that turns out to be among the most important ideas in all of human thought. Why? Because the idea that matter is made of particles has enabled us to understand nature from the bottom up. What we found was remarkable: as far as we can tell, the particles that compose matter – their composition and structure – determine the properties of matter. Matter does what the particles that compose it do. Water boils at 100°C (212°F) because the three atoms that compose a water molecule bond together in a certain order, at a certain angle and at certain distances. Change any of these characteristics and water would be a different substance.
3-SECOND NUCLEUS
Matter is composed of particles. The nature of the particles – especially their structure – determines the properties of matter.
3-MINUTE VALENCE
Humans have wondered about the fundamental composition of matter for 2,500 years. The basic question is this: if you divide a sample of matter into smaller and smaller pieces, could you go on forever or would you eventually get to fundamental particles that are no longer divisible? For most of civilization, humans got the answer to this question wrong.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
JOHN DALTON
1766–1844
English chemist who articulated the atomic theory of matter
JEAN PERRIN
1870–1942
French physicist who studied the motion of tiny particles suspended in liquid to experimentally settle the question of the particulate nature of matter
30-SECOND TEXT
Nivaldo Tro
Jean Perrin won the Nobel Prize essentially for proving the existence of atoms.

THE STRUCTURE OF THE ATOM
the 30-second chemistry
In 1897, J. J. Thomson discovered a new type of particle – the electron – that was much smaller than the atom itself. Thomson demonstrated that electrons were negatively charged, that they were present in all different kinds of matter and that their mass was 1/2,000th the mass of the lightest atom. Thomson’s discovery implied that the atom itself was composed of even smaller particles. Based on his discovery, Thomson developed a model for the atom called the ‘plum-pudding model’. In this model, even the lightest atoms were composed of thousands of electrons held in a sphere of positive charge. In 1909, Ernest Rutherford (pictured) set out to confirm Thomson’s model, but he proved it wrong instead. Rutherford accelerated particles (8,000 times more massive than electrons) at a thin sheet of gold atoms. Most of these particles were not deflected by the gold atoms, but a few bounced back. Rutherford claimed that his results were ‘about as credible as if you fired a 15-inch [38-cm] shell at a piece of tissue paper and it came back and hit you.’ Rutherford developed a new model for the atom – the nuclear model – in which the mass of the atom is concentrated in a small space called the nucleus. Most of the volume of the atom is empty space.
3-SECOND NUCLEUS
An atom consists of a tiny nucleus – containing protons and neutrons – with electrons in a diffuse cloud surrounding the nucleus.
3-MINUTE VALENCE
Matter is particulate – it is made of particles. But what are those particles like? What is their structure? The earliest models implied that the distribution of matter within an atom was fairly uniform, but later experiments suggested otherwise. The atom itself is mostly empty space with nearly all of the mass contained in a small space called the nucleus.
RELATED TOPICS
See also
THE DUAL NATURE OF THE ELECTRON
3-SECOND BIOGRAPHIES
J. J. THOMSON
1856–1940
English physicist who discovered the electron
ERNEST RUTHERFORD
1871–1937
New Zealand physicist whose famous Gold Foil Experiment established the nuclear model for the atom
30-SECOND TEXT
Nivaldo Tro
The nuclear atom, with the nucleus enlarged to be visible. If drawn to scale, the nucleus would be a tiny dot, too small to see.

INSIDE THE ATOM
the 30-second chemistry
The number of protons in the nucleus of an atom is called the atomic number (Z) and it determines the identity of the atom and its corresponding element. For example, helium (Z=2) has two protons in its nucleus and sodium (Z=11) has eleven protons in its nucleus. The number of known elements ranges from Z=1 to Z=118 – as shown in the periodic table on the facing page. Each element has a name, a symbol and a unique atomic number. The number of neutrons in the nucleus of an atom can vary within atoms of the same element. For example, most helium atoms have two neutrons, but some have three. Atoms with the same number of protons but a different number of neutrons are called isotopes. Since most of the mass of an atom is due to its protons and neutrons, the sum of the numbers of these two particles is called the mass number (A). Scientists specify isotopes with the following notation: 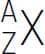 , where X is the chemical symbol, Z is the atomic number and A is the mass number. For example, the helium isotope with 2 neutrons is specified by
, where X is the chemical symbol, Z is the atomic number and A is the mass number. For example, the helium isotope with 2 neutrons is specified by 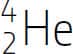 .
.
3-SECOND NUCLEUS
An atom is composed of protons, neutrons and electrons. The number of electrons in a neutral atom always equals the number of protons in its nucleus.
3-MINUTE VALENCE
All atoms are composed of the same three subatomic particles: protons, neutrons and electrons (see table below). So what makes one atom different from another? The numbers of those particles. Incredible as it may seem, both sodium (a reactive metal that explodes in water) and helium (an inert gas that reacts with nothing) are made of the same subatomic particles, just different numbers of them.
RELATED TOPICS
See also
3-SECOND BIOGRAPHY
JAMES CHADWICK
1891–1974
English physicist who discovered the neutron
30-SECOND TEXT
Nivaldo Tro
Subatomic Particles
|
Mass (amu) |
Charge (relative) |
Proton |
1.0 |
+1 |
Neutron |
1.0 |
0 |
Electron |
0.00055 |
-1 |
The periodic table lists the 118 known elements (91 naturally occurring and 27 synthetic) according to their atomic number (top left in each element box).

WHERE DID ATOMS COME FROM?
the 30-second chemistry
According to the big bang model, our universe began as a hot, dense collection of matter and energy that rapidly expanded and cooled. During the first 20 minutes of that expansion, hydrogen and helium (the two most abundant elements in the universe) formed from the soup of subatomic particles. Then nucleosynthesis stopped as the universe continued to expand and cool. Eventually, after about 500 million years, the first stars formed. Stars are the nurseries in which all other elements are made. As stars burn – through a process called nuclear fusion – they fuse together the nuclei of smaller atoms to form larger atoms. Young stars fuse hydrogen atoms to form helium. This fusion gives off tremendous amounts of heat and light and can power a star for billions of years. As a star ages, and if it is large enough, fusion can continue to form larger atoms such as carbon and oxygen – all the way up to iron. The formation of elements beyond iron requires the input of energy, and only happens in the supernova stage of a star’s life. A supernova is essentially a large exploding star. The energy emitted by a supernova can power the nucleosynthesis of elements up to uranium, the heaviest naturally occurring element.
3-SECOND NUCLEUS
Atoms form through nucleosynthesis, which began in the first few minutes after the big bang and happens to this day within the core of stars and supernovae.
3-MINUTE VALENCE
Our planet naturally contains about 91 different elements. Where did the atoms that compose these elements come from? How did atoms form? They formed through a process called nucleosynthesis, which began about 13.7 billion years ago at the very birth of our universe.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
ARTHUR EDDINGTON
1882–1944
English astronomer and physicist who first suggested that stars are powered by fusion
FRED HOYLE
1915–2001
English astronomer who formulated the theory of nucleosynthesis within stars
30-SECOND TEXT
Nivaldo Tro
In stars, smaller atoms fuse together to form larger atoms. All atoms beyond helium were born in the core of stars and supernovae.

THE DUAL NATURE OF THE ELECTRON
the 30-second chemistry
An electron travelling through space behaves very differently from a baseball flying towards the outfield. A baseball has a definite trajectory – a deterministic path that it follows. A good outfielder can predict where a baseball will land. This prediction requires the outfielder simultaneously to know two properties of the flying baseball: its position (where it is) and its velocity (how fast it is going). If the outfielder only knew one of these two properties, he or she could not predict the baseball’s path. An electron behaves differently because it has a dual nature: a wave nature (associated with its velocity) and a particle nature (associated with its position). The key to understanding electron behaviour is Heisenberg’s Uncertainty Principle, which states that ‘an electron never exists as both a wave and a particle simultaneously’. It is either one or the other, but not both. Although Heisenberg’s principle solved a great paradox (how something can be both a wave and a particle), it implied the death of determinism. If you can’t observe the wave nature and particle nature of the electron simultaneously, then you can’t simultaneously know its velocity and its position, which means you can’t predict its future path.
3-SECOND NUCLEUS
For electrons and other small particles, the trajectories of classical physics are replaced with the probability distributions of quantum mechanics.
3-MINUTE VALENCE
Are the smallest particles that exist, such as electrons, just like those that we can see with our eyes only smaller? Does an electron orbiting an atom behave like a planet orbiting the Sun? No. Electrons behave differently. Electrons, and other small particles, have a wave-particle duality that makes it impossible to predict exact trajectories for them. Instead, we describe their behaviour in terms of probability.
RELATED TOPICS
See also
WHERE ELECTRONS ARE WITHIN AN ATOM
3-SECOND BIOGRAPHIES
ERWIN SCHRÖDINGER
1887–1961
Austrian physicist central to the development of quantum mechanics and known for the thought experiment ‘Schrödinger’s Cat’
WERNER HEISENBERG
1901–76
German physicist who articulated the ‘Uncertainty Principle’
30-SECOND TEXT
Nivaldo Tro
In an atom, electrons do not orbit the nucleus like planets orbit the Sun. Instead, they exist in clouds of probability.

WHERE ELECTRONS ARE WITHIN AN ATOM
the 30-second chemistry
The electron orbits of early atomic models were later replaced by quantum mechanical orbitals. Unlike a planetary orbit, an orbital is a three-dimensional probability map that shows the probability of finding an electron in a certain volume of space. You can understand an orbital with a simple analogy. Imagine taking a photo every 10 seconds for several minutes of a moth flying around a light bulb, and then superimposing all the photos to make a single image. The result shows the light bulb with dozens of images of the moth. The volume immediately surrounding the light bulb has many moth images, indicating a high probability of finding the moth in that space. Further away from the light bulb there are fewer moth images, meaning that the probability of finding the moth in that space is lower. A quantum mechanical orbital is analogous – the light bulb is the atomic nucleus and the moth is the electron. Just as the early model of the atom had many different orbits at different distances from the nucleus and with different energies, so the quantum mechanical model has many different orbitals, each with different average distances from the nucleus and with different energies. Electrons can be observed in one orbital or another, but never in between.
3-SECOND NUCLEUS
Electrons in atoms exist in quantum mechanical orbitals, three-dimensional probability maps that show the likelihood of finding the electron in a certain volume of space.
3-MINUTE VALENCE
Atoms bond together by sharing or transferring electrons. As a result, the positions of electrons within an atom – where they are – is important because it affects how atoms bond together. In an early model, electrons were thought to orbit the nucleus of the atom much like planets orbit the Sun. However, this model was later proved wrong and was replaced with the quantum mechanical model for the atom.
RELATED TOPICS
See also
THE DUAL NATURE OF THE ELECTRON
3-SECOND BIOGRAPHIES
NIELS BOHR
1885–1962
Danish physicist central to the development of the quantum mechanical model for atomic structure
ERWIN SCHRÖDINGER
1887–1961
Austrian physicist central to the development of the quantum mechanical model of the atom
30-SECOND TEXT
Nivaldo Tro
Early atomic models had electrons orbiting the nucleus like planets orbit the Sun. These have been replaced by the quantum mechanical model.

PERIODIC PATTERNS
the 30-second chemistry
The ancient Greeks thought that matter was composed of only four elements: earth, water, fire and air. By the mid-1800s, however, scientists had discovered more than 50 different elements. Dmitri Mendeleev noticed a pattern in the properties of known elements when he listed them in order of increasing atomic number: certain properties recurred periodically. Based on this observation, Mendeleev constructed a table of elements with atomic number increasing from left to right, and elements with similar properties aligning in columns. His table contained some gaps that allowed him to predict the existence and properties of yet undiscovered elements (which were later discovered). Mendeleev’s table evolved into the modern periodic table, which lists all known elements to date. The elements on the left and middle of the table are mostly metals, and the elements on the right side of the table are mostly non-metals. Each column represents a family of elements with similar properties. For example, the far left column contains the alkali metals, a family of elements that are all solid metals at room temperature and highly reactive. The far right column, by contrast, contains the noble gases, a family of elements that are all gases at room temperature and display little or no chemical reactivity.
3-SECOND NUCLEUS
When elements are listed in order of increasing atomic number, their properties recur in a regular pattern.
3-MINUTE VALENCE
Our Earth contains about 91 different naturally occurring elements, each one with its own distinctive properties. However, certain groups of elements share similarities. The periodic law and the corresponding periodic table allow us to organize the known elements in ways that help us make sense of their properties.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
JULIUS LOTHAR MEYER
1830–95
German chemist who made significant contributions to the periodic table
DMITRI MENDELEEV
1834–1907
Russian chemistry professor who formulated the periodic law and constructed one of the first periodic tables
30-SECOND TEXT
Nivaldo Tro
Mendeleev formulated one of the first periodic tables, which organizes elements according to atomic number and chemical properties.

BONDING ATOMS TOGETHER
the 30-second chemistry
Atoms bond together by either sharing (covalent bonding) or transferring (ionic bonding) the electrons in their highest-energy orbitals to form compounds. Sharing of electrons typically occurs between two or more non-metals, resulting in a molecular compound, so called because it is composed of distinct molecules (groups of atoms bonded together). Transfer of electrons typically occurs from a metal to a non-metal and results in an ionic compound. Ionic compounds do not contain distinct molecules, but rather exist as an array of ions (charged particles) with alternating positive and negative charge. Water is a good example of a molecular compound. We represent a compound with a chemical formula, which tells us the elements present in the compound and the relative number of atoms of each one. For example, the formula for water is H2O, which means that a water molecule is composed of two hydrogen atoms and one oxygen atom, and the formula for sucrose (table sugar) is C12H22O11. Molecular compounds can contain as few as two atoms in a molecule to as many as thousands. Sodium chloride (table salt) is a good example of an ionic compound. The formula for sodium chloride is NaCl, which indicates sodium and chlorine in a one-to-one atomic ratio.
3-SECOND NUCLEUS
Atoms bond together to form compounds. A compound, unlike a mixture of elements, contains two or more elements in a fixed, definite proportion.
3-MINUTE VALENCE
The universe contains 118 different elements, but would be lifeless if these elements did not bind together to form compounds. When two or more elements combine to form a compound, a completely new substance forms with properties much different from the elements that compose it. In this way, our universe’s 118 different elements can form millions of compounds. And this, among other things, makes life possible.
RELATED TOPICS
See also
WHERE ELECTRONS ARE WITHIN AN ATOM
THE LEWIS MODEL FOR CHEMICAL BONDING
VALENCE BOND & MOLECULAR ORBITAL THEORIES
3-SECOND BIOGRAPHIES
JOSEPH PROUST
1754–1826
French chemist who made observations on the composition of compounds
LINUS PAULING
1901–94
American chemist who made significant contributions to our understanding of chemical bonding
30-SECOND TEXT
Nivaldo Tro
Water is a molecular compound, composed of two hydrogen atoms bonded to one oxygen atom.

THE LEWIS MODEL FOR CHEMICAL BONDING
the 30-second chemistry
In the simplest model for chemical bonding, called the Lewis model, atoms share or transfer their highest energy electrons (called valence electrons) to obtain an octet – eight electrons in their highest energy (or outermost) set of orbitals. One important exception is hydrogen, which shares/transfers its one electron to obtain a duet – two electrons in its outermost orbital. When applying the Lewis model, chemists use special symbols to represent atoms and their valence electrons. For example, the Lewis symbols for hydrogen and oxygen are as follows:
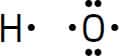
The one dot next to H represents hydrogen’s one valence electron and the six dots around the O represent oxygen’s six valence electrons. The bonding between hydrogen and oxygen to form water involves the sharing of the valence electrons, and we draw the Lewis symbol for water as follows:
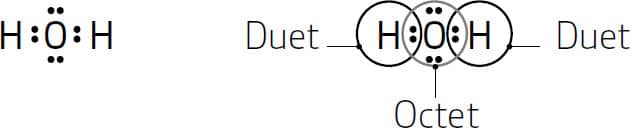
The shared electrons (those between two elements) count towards the octet (or duet) of both atoms. So in this Lewis structure, each hydrogen atom has a duet and oxygen an octet.
3-SECOND NUCLEUS
In the Lewis model for chemical bonding, atoms bond to obtain octets – eight electrons in their valence shell.
3-MINUTE VALENCE
The most powerful pieces of scientific knowledge are theories (or models). Theories explain not only what happens in nature but also why it happens. The Lewis model for chemical bonding explains why, for example, water is H2O and not some other combination of atoms. The Lewis model is simple, however, and other more sophisticated models are even more powerful at predicting and explaining chemical bonding.
RELATED TOPICS
See also
WHERE ELECTRONS ARE WITHIN AN ATOM
VALENCE BOND & MOLECULAR ORBITAL THEORIES
3-SECOND BIOGRAPHY
GILBERT N. LEWIS
1875–1946
American chemist and University of California, Berkeley chemistry professor who constructed the Lewis model for chemical bonding
30-SECOND TEXT
Nivaldo Tro
The Lewis model shows how atoms share electrons to obtain octets.
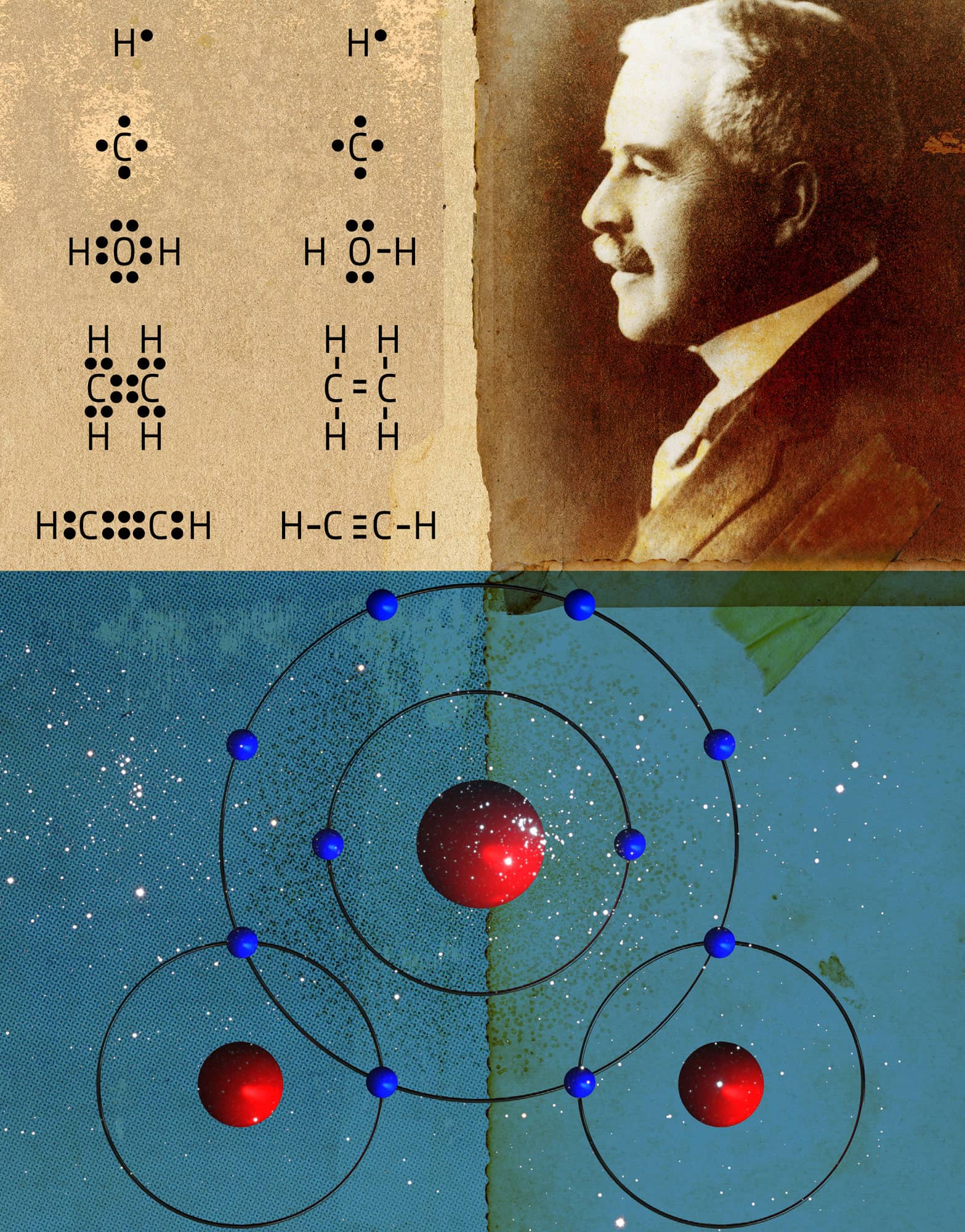
VALENCE BOND & MOLECULAR ORBITAL THEORIES
the 30-second chemistry
Chemists use three different models to explain chemical bonding: the Lewis model, valence bond theory and molecular orbital theory, each increasingly complex but also increasingly powerful. The Lewis model requires nothing more than paper and pencil to enable chemists to predict and explain a great deal of chemical behaviour. Valence bond and molecular orbital theory, by contrast, both require more complex calculations, usually on a computer. In valence bond theory, a chemical bond is modelled as the overlap between half-filled atomic orbitals. As the orbitals overlap, the energy of the electrons in those orbitals decreases, stabilizing the molecule relative to its constituent atoms. A molecular orbital is to a molecule what an atomic orbital is to an atom. Each molecule has its own unique set of molecular orbitals that depend on the constituent atoms and their arrangement in space. If the overall energy of the electrons in the molecular orbitals is lower than in the constituent atoms’ atomic orbitals, the resulting molecule is stable. Both valence bond theory and molecular orbital theory can accurately predict details about molecular structure including molecular geometries, bond lengths and bond strengths.
3-SECOND NUCLEUS
In the valence bond model, a chemical bond is the overlap between half-filled atomic orbitals. In molecular orbital theory, atomic orbitals are completely replaced by molecular orbitals.
3-MINUTE VALENCE
The Lewis model for chemical bonding is practical and useful; however, it also has limits. We know, for example, that electrons are not stationary dots that sit between atoms. Two more powerful bonding models – valence bond theory and molecular orbital theory – take into account the quantum mechanical nature of electrons and provide even more powerful predictions and explanations of chemical bonding.
RELATED TOPICS
See also
WHERE ELECTRONS ARE WITHIN AN ATOM
THE LEWIS MODEL FOR CHEMICAL BONDING
3-SECOND BIOGRAPHIES
JOHN EDWARD JONES
1894–1954
English mathematician, physicist and pioneer of computational chemistry
LINUS PAULING
1901–94
American chemist who made significant contributions to valence bond theory
30-SECOND TEXT
Nivaldo Tro
Molecular orbital theory predicts that oxygen should be a magnetic liquid, which it is. The simpler bonding theories fail to predict this property.

OPPOSITES ATTRACT
the 30-second chemistry
We know from previous entries that atoms can bond together by sharing electrons. But if the bonding atoms are different (two different elements), then the sharing is often not equal – one of the two atoms hogs the electron more than the other. The result is a polar bond, one that has a positive end on one side and a negative end on the other. In a molecule, polar bonds may add together to result in a polar molecule. Polar molecules interact strongly with one another because the positive end of one molecule is attracted to the negative end of its neighbour – just as the north pole of a magnet is attracted to the south pole of another magnet. These attractions affect the properties of the substances that the molecules compose. For example, polar substances tend to have higher melting and boiling points than their nonpolar counterparts because the attraction between neighbouring molecules makes the molecules more difficult to separate. Polar substances also tend not to mix well with nonpolar substances. For example, water and oil do not mix because water is very polar and oil is nonpolar. Water and ethyl alcohol (ethanol), by contrast, mix in all proportions because they are both polar.
3-SECOND NUCLEUS
The often uneven distribution of electrons that can result when two different atoms bond together results in a polar bond, which greatly affects a substance’s properties.
3-MINUTE VALENCE
The existence of liquid water on Earth’s surface can be attributed to polar bonds. Most small molecules are gases at room temperature, but water is among the very few that is a liquid. Why? Because water has highly polar bonds with hydrogen at one end and oxygen on the other. The small size of hydrogen allows the molecules to get very close together and interact strongly. This strong interaction makes it difficult to separate water molecules from one another.
RELATED TOPICS
See also
THE FORCES THAT HOLD MATTER TOGETHER
3-SECOND BIOGRAPHIES
JOHANNES DIDERIK VAN DER WAALS
1837–1923
Dutch physicist who was among the first to postulate forces between molecules
LINUS PAULING
1901–94
American chemist who quantified the polarity of chemical bonds
30-SECOND TEXT
Nivaldo Tro
A polar molecule has an asymmetrical charge distribution that causes an attraction to other polar molecules.
