
NUCLEAR CHEMISTRY
NUCLEAR CHEMISTRY
GLOSSARY
alpha particle A type of particle given off in one type of radioactive decay. An alpha particle contains 2 protons and 2 neutrons and is symbolized as 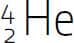 .
.
amu A unit of mass used for subatomic particles. 1 amu = 1.66 x 10-27 kg.
beta particle A type of particle given off in one type of radioactive decay. A beta particle is an electron and is symbolized as 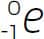 .
.
chemical reaction A process in which the atoms in one or more substances (the reactants) rearrange to form different substances (the products).
critical mass In nuclear fission, the minimum mass of fissile material needed to maintain a self-sustaining nuclear reaction.
Einstein’s equation E=mc2 An equation that relates mass to energy, which means that the two are interconvertible.
electron A subatomic particle with a negative charge and a mass of 0.00055 amu.
gamma ray A high energy photon given off in one type of radioactive decay and often in conjunction with other types. A gamma ray is symbolized as 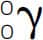 .
.
gene A strand of DNA that codes for a single protein.
isotope An atom that has the same number of protons as another atom, but a different number of neutrons.
metabolism The process by which living organisms convert certain compounds into the energy needed to survive and reproduce.
neutron A subatomic particle with no charge and a mass of 1 amu.
nuclear fission A nuclear reaction in which a large nucleus splits into smaller fragments and releases energy.
plutonium A synthetic chemical element with atomic number 94 used in nuclear chemistry, especially nuclear power and atomic bombs.
proton A subatomic particle with a positive charge and a mass of 1 amu.
radioactivity The emission of small energetic particles from the nuclei of certain unstable isotopes.
radiocarbon dating A method of determining the age of previously living material by measuring the C-14 content in the material.
radiopharmaceutical Pharmaceutical agents that are radioactive and used in the diagnosis and treatment of disease.
tracers A chemical compound where one or more atoms have been replaced with a radioactive isotope that allows a scientist to trace where the atom goes in a particular process.
uranium A radioactive chemical element with atomic number 92 used in nuclear chemistry, especially nuclear power and atomic bombs.
X-rays A form of electromagnetic radiation with wavelengths slightly longer than gamma rays and used to image bones and organs.
RADIOACTIVITY
the 30-second chemistry
As a scientific poet once wrote ‘Atoms … fly to bits with utmost facility’. Counterintuitively, some atoms spontaneously fall apart to produce rays and particles that can penetrate through various materials, including metals and our own bodies. Antoine Becquerel and Pierre and Marie Curie, working in Paris in the last decade of the nineteenth century, were the first to recognize this phenomenon in uranium minerals. Madame Curie called this ‘radioactivity’ (from the Latin radius, meaning ‘ray’) and soon found two mysterious, previously unknown elements (‘radium’ and ‘polonium’) that emitted more intense ‘radiation’ than uranium itself. Initially radioactivity was thought to exist in two types, designated logically enough by Ernest Rutherford as alpha and beta. (Gamma rays were discovered several years later.) Positively charged alpha particles, soon found to be energetic helium nuclei, He2+, had less ability to penetrate various substances. Negatively charged beta particles, soon found to be energetic electrons, were much lighter and able to penetrate a variety of materials well. Gamma rays, the most penetrating of all, were high-energy electromagnetic radiation. Most remarkably, it is now clear that certain types of atoms spontaneously ‘decay’ and spit out tiny charged particles and highly intense radiation.
3-SECOND NUCLEUS
Some atoms spontaneously ‘decay’, producing alpha and beta particles and gamma rays that can penetrate through various materials. This is called radioactivity.
3-MINUTE VALENCE
Radioactive elements can be used as ‘tracers’ to follow the pathway of a chemical reaction or monitor concentrations of elements in research, environmental, agricultural and medical settings. Radioactive elements are also used to establish the ages of various objects including once-living systems (carbon-14), early humanoids (potassium-40) and the Moon, Earth and various rocks and minerals (uranium and thorium isotopes).
RELATED TOPICS
See also
3-SECOND BIOGRAPHY
WILLIAM RAMSAY
1852–1916
Scottish chemist who wrote the 1902 poem ‘The Death Knell of an Atom’, which contains the following stanza ‘So the atoms, in turn, we now clearly discern,/Fly to bits with the utmost facility;/ They wend on their way, and in splitting, display/An absolute lack of stability.’
30-SECOND TEXT
Glen E. Rodgers
When atoms emit radiation they change their identity.
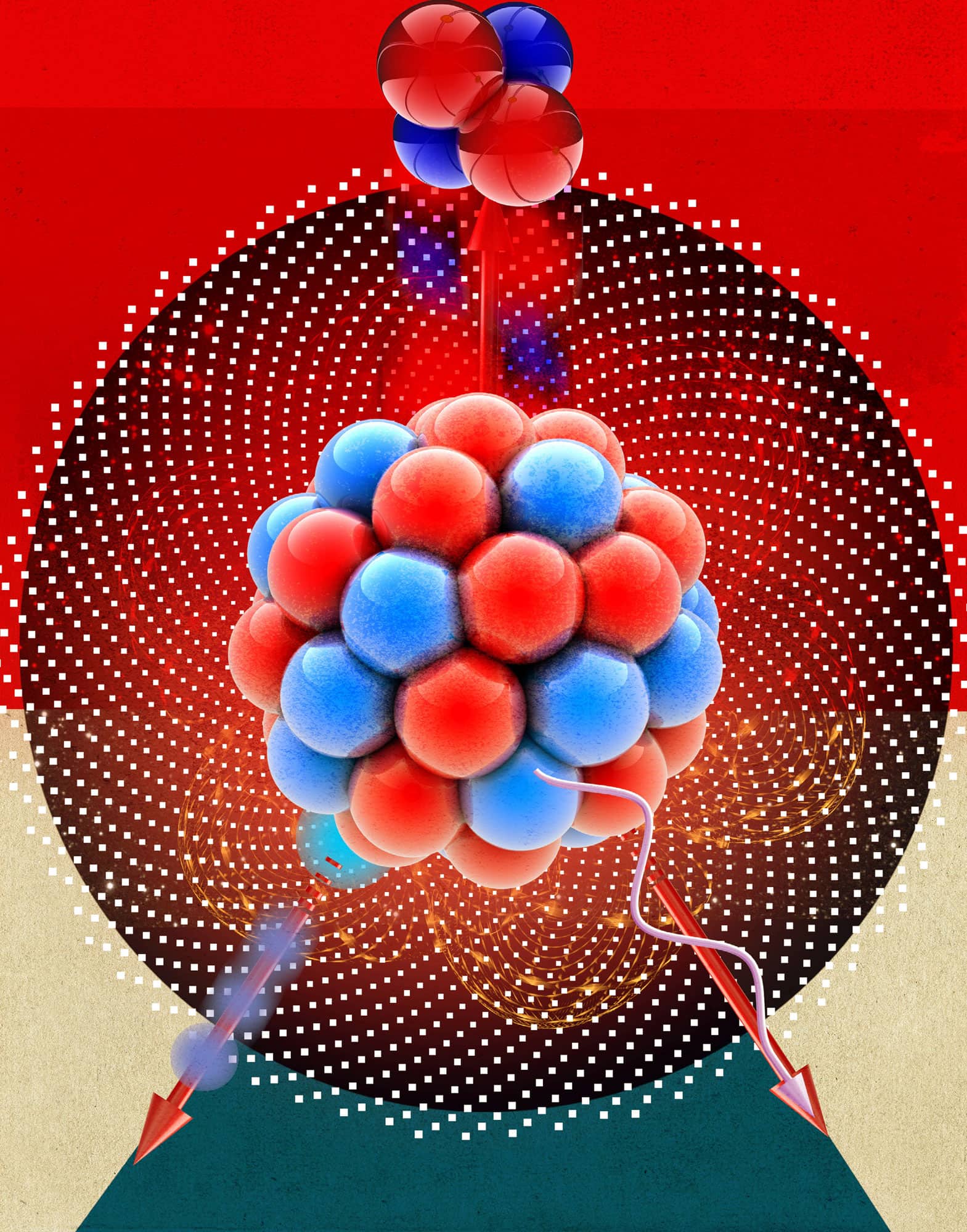
SPLITTING THE ATOM
the 30-second chemistry
In 1938 Otto Hahn shot neutrons at uranium atoms and was amazed to discover that they appeared to split roughly in half. He had discovered nuclear fission, whereby a large nucleus splits to form two smaller nuclei and several more neutrons. If a ‘critical mass’ of a sufficiently pure isotope of uranium or plutonium is present, these additional neutrons can go on to hit other fissionable nuclei and cause a chain reaction which, as calculated using Einstein’s equation E=mc2, releases inordinate amounts of energy, far in excess of that obtained from conventional chemical reactions. This energy can be harnessed to create electricity (nuclear energy) or to create explosions (nuclear bombs). The uranium-based bomb dropped on Hiroshima, Japan, on August 6, 1945, was called ‘Little Boy’. In this ‘gun-type’ assembly, the critical mass was obtained by firing a uranium ‘bullet’ into a hollow cylinder of uranium. The plutonium-based bomb dropped on Nagasaki, Japan, on August 9, 1945, was called ‘Fat Man’. In this implosion-type assembly, the critical mass was obtained using a lens assembly that fired small bits of plutonium all towards the centre of the bomb.
3-SECOND NUCLEUS
Firing neutrons into critical masses of fissionable materials splits the atoms apart and produces additional neutrons; the resulting chain reaction releases large amounts of energy.
3-MINUTE VALENCE
Why don’t atomic nuclei, particularly those containing dozens of positively charged and therefore mutually repulsive protons, just burst apart? It turns out that some do. Large nuclei of certain types of atoms of uranium or plutonium atoms act like wobbly drops of liquid tenuously held together by a nuclear surface tension that can be easily disrupted.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
MARIE CURIE
1867–1934
Polish-born French chemist who developed the theory of radioactivity
OTTO HAHN
1879–1968
German winner of the 1944 Nobel Prize in Chemistry, who discovered nuclear fission
30-SECOND TEXT
Glen E. Rodgers
In nuclear fission, a neutron causes an unstable nucleus to split, releasing large amounts of energy.

NUCLEAR WEIGHT LOSS
the 30-second chemistry
When an atom splits, either in a nuclear power plant or during the detonation of a nuclear weapon, a tremendous amount of energy is released. Where does all of this energy come from? The answer lies in Einstein’s remarkably simple equation that relates energy and matter: E=mc2 – energy equals mass times the speed of light squared. This equation states that energy and matter are really two different forms of the same thing. In other words, if energy is being released (created), then matter must disappear. That’s exactly what happens during nuclear fission. A large nucleus splits into two smaller ones, a little bit of the total mass is destroyed – and lots of energy is produced. Does this mean one or two protons or neutrons get vaporized? No, the fascinating part of this is that all of the protons and neutrons lose a little bit of their mass. However remarkable it may seem, this means that protons and neutrons do not always have the same mass. A proton in a uranium nucleus weighs more than a proton in an iron nucleus. After splitting, the same total number of protons and neutrons exist; they all weigh just a bit less. This is not a recommended weight loss programme for humans.
3-SECOND NUCLEUS
Protons and neutrons each lose a tiny bit of mass when nuclear fission occurs. This mass is converted into energy.
3-MINUTE VALENCE
If matter and energy are two sides of the same coin, then is it appropriate to imagine protons and neutrons as hard spheres? Is it better to think of them as little balls of energy? Because matter and energy are interconvertible, perhaps it doesn’t really matter. This is another indication that the subatomic universe is a very strange place.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
LISE MEITNER
1878–1968
Austrian physicist who performed the first mass/energy calculations on nuclear fission
ALBERT EINSTEIN
1879–1955
German-born physicist who provided mathematical equivalence to mass and energy
30-SECOND TEXT
Jeff C. Bryan
When an atom splits through nuclear fission, some of its mass is converted into energy.

THE EFFECT OF RADIATION ON LIFE
the 30-second chemistry
The types of radiation discussed in this chapter are rather unusual in that they have the power to knock electrons loose from atoms and molecules. As the radiation travels through matter, it transfers some of its energy to the molecules it passes by, much like a bullet fired into a pile of pea gravel. Since electrons bind atoms together in molecules, this subatomic violence can lead to broken chemical bonds. If enough radiation damage occurs in a single cell, a large number of molecules get broken and the cell could die. If it’s less damaged, it can repair itself. However, if the cell’s DNA is damaged, the cell could change (or mutate) in ways that cause it to grow abnormally (because DNA directs how cells grow). These mutations and abnormal cell growth can lead to cancer. This sounds bad, especially since humans, like all living things and the planet, are naturally radioactive. Fortunately, we’ve evolved with rather efficient cell repair mechanisms. It appears that below a certain threshold, radiation produces no negative health effects. Some set this threshold at 100 mSv (millisieverts, a unit of dose). For reference, the global average annual dose for natural and anthropogenic radiation is 2.8 mSv.
3-SECOND NUCLEUS
Radiation can break chemical bonds in living things, possibly leading to cell death or cancer.
3-MINUTE VALENCE
Radiation damage to DNA typically takes place indirectly. The most common chemical in living things is water, making it the odds-on favourite to have one of its electrons knocked loose. This forms H2O+, which falls apart into H+ and HO·. HO· is known as hydroxyl radical, and is very reactive. If it bumps into a DNA molecule, it will likely disrupt a chemical bond in the DNA.
RELATED TOPICS
See also
THE BIOLOGICAL BLUEPRINT: NUCLEIC ACIDS
3-SECOND BIOGRAPHIES
HERMANN J. MÜLLER
1890–1967
American biologist and winner of the 1946 Nobel Prize in Medicine who first observed changes in genes after exposure to X-rays
L. HAROLD GRAY
1905–65
English physicist and radiobiologist who pioneered studies of radiation effects on living things
30-SECOND TEXT
Jeff C. Bryan
DNA can be damaged by ionizing radiation.

NUCLEAR MEDICINE
the 30-second chemistry
Nuclear medicine involves the injection of a radioactive material (a radiopharmaceutical) into a patient to diagnose or treat a disease. An example is 18F-fluorodeoxyglucose (FDG), a radioactive sugar molecule. In our bodies, sugars tend to go to sites of metabolism. They also collect in cancerous tumours, because cancer is a sugar hog. Once the radiopharmaceutical is allowed to localize, radiation detectors can be positioned around the patient to generate three-dimensional images of the organs where the radiopharmaceutical has accumulated. The data collected from a nuclear medicine scan typically tells us more about how well the organ is functioning (physiology) than what it looks like (anatomy). By changing the chemical nature (size, shape, charge) of the radiopharmaceutical, we can obtain images from any organ in the body and determine how well it’s working. Nuclear medicine scans make patients more radioactive. But remember that we are all naturally radioactive; nuclear medicine simply adds more that concentrates in part of the body. The radiation dose is typically low enough not to have any measurable adverse effects, and the benefits of diagnosing or treating a disease you already have outweighs any nearly negligible risk that the radiation might pose.
3-SECOND NUCLEUS
Nuclear medicine uses radiopharmaceuticals to examine physiology and to treat diseases.
3-MINUTE VALENCE
As we gain a better understanding of human physiology, radiopharmaceuticals are becoming so sophisticated they can generate images and perform therapy at the cellular and molecular level. Imagine being able to kill cancer when it is so small that it can’t even be located by conventional means. We may soon be able to detect and treat cancer before any outward symptoms are apparent.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
GEORGE DE HEVESY
1885–1966
Hungarian-born winner of the 1943 Nobel Prize in Chemistry who first recognized that radioactive isotopes could be used to study complex chemical processes such as metabolism
HAL ANGER
1920–2005
American electrical engineer and biophysicist who invented the cameras that are still widely used in nuclear medicine
30-SECOND TEXT
Jeff C. Bryan
Radioactive substances can be used to image internal organs.
