
ORGANIC CHEMISTRY
ORGANIC CHEMISTRY
GLOSSARY
aldehydes A family of organic compounds containing a terminal C=O functional group.
aldosterone A hormone responsible for the regulation of sodium and potassium.
alkaloids Organic bases found in plants; often poisonous.
amines Organic compounds containing a nitrogen atom bonded to one or more carbon atoms.
aromatic ring A flat, ring-shaped chain of carbon atoms containing alternating single and double bonds.
carbonyl The C=O functional group.
carboxylic acid An organic compound containing the–COOH functional group.
covalent bond The joining of atoms by the sharing of one or more electrons.
cross-linked structure A common structure in polymers in which long chain-like molecules form bonds between the neighbouring chains.
cyclic structures Any structure containing one or more rings of atoms bonded together.
distillation A process by which a mixture of compounds with differing boiling points can be separated by heating the mixture and recondensing the gases that vaporize.
ester An organic compound containing the –COO –functional group.
functional group A characteristic atom or group of atoms present in a family of organic compounds that give the compounds certain characteristics.
homologous series A series of organic compounds that differ in length by one carbon atom.
hydrocarbon An organic compound containing only carbon and hydrogen.
hydroxyl group An –OH group characteristic of the organic family of alcohols.
isomers Two molecules with the same chemical formula but different structures.
ketone A family of organic compounds that contain the C=O functional group sandwiched between two other carbon atoms.
linear structure A chemical structure in which the atoms that compose the structure fall on a continuous line.
macromolecule A very large molecule, such as a polymer, typically containing thousands of atoms.
molar mass The mass of one mole of an element or compound.
monomer Repeating unit in a polymer.
organic compounds Compounds that contain carbon bonded to hydrogen, nitrogen, oxygen or sulfur.
paraffin wax A soft, flammable solid composed of a mix of long chain hydrocarbons.
progesterone A female sex hormone with the formula C21H30O2 that plays a role in the menstrual cycle and pregnancy.
testosterone A male sex hormone with the formula C19H28O2.
ORGANIC CHEMISTRY & VITALISM
the 30-second chemistry
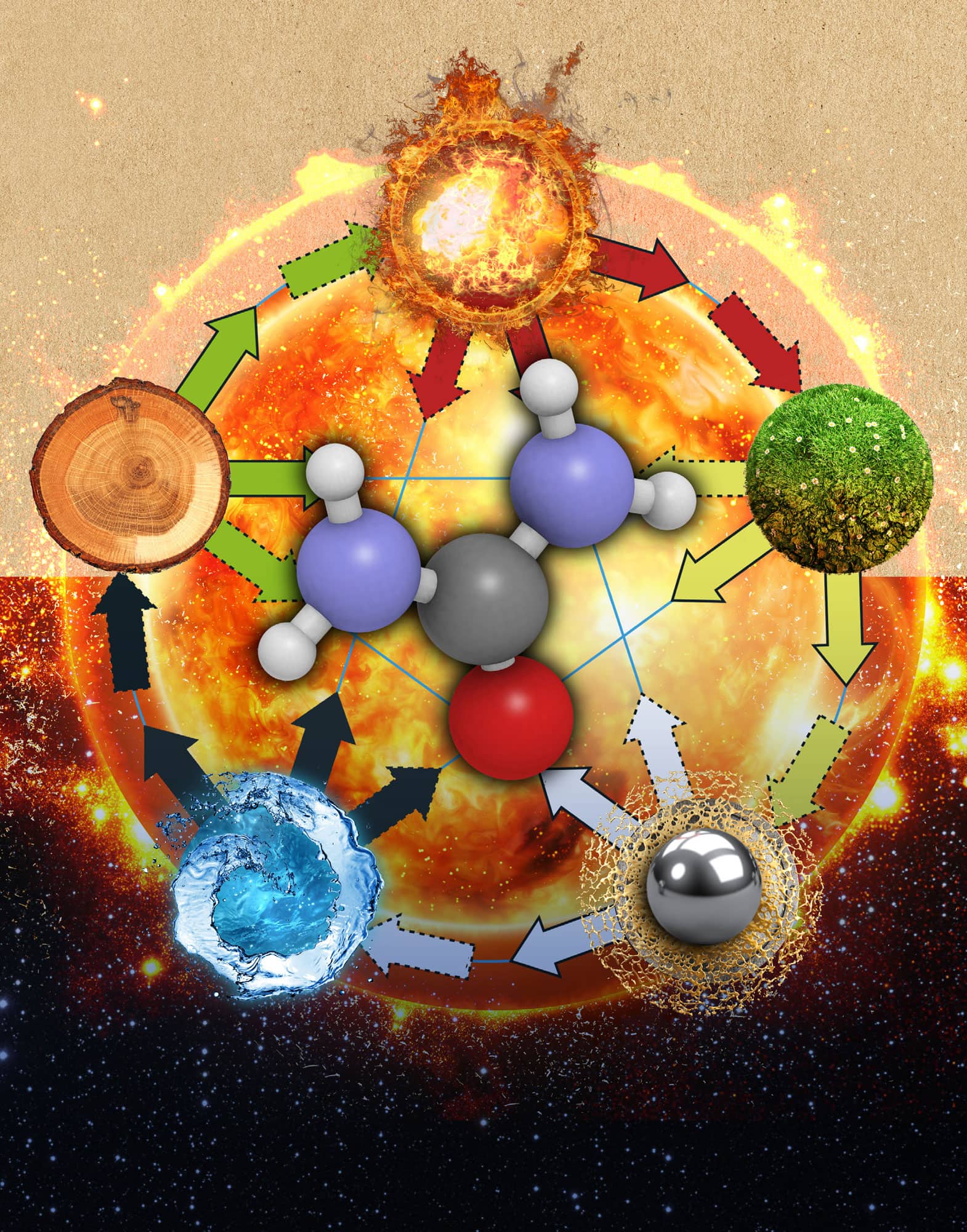
Early chemists divided compounds into two distinct types: organic and inorganic. Organic compounds were isolated from living organisms and tended to be quite fragile. For example, sugar is an organic compound (isolated from sugar cane or the sugar beet). If you heat sugar in a pan, it quickly decomposes. Inorganic compounds came from the Earth and tended to be more durable. For example, table salt is an inorganic compound (isolated from salt mines or from oceans). If you heat salt in a pan, the salt does not decompose – you simply get hot salt. Furthering the divide, early chemists were able to synthesize inorganic compounds in the laboratory, but not organic compounds. This divide fitted well with an eighteenth-century belief called vitalism, which suggested that all living organisms contained a vital force that separated them from non-living things. The vital force allowed living organisms to synthesize organic compounds, but a chemist could not synthesize an organic compound in a beaker because no vital force was present. Vitalism died a slow death in the middle of the nineteenth century because chemists began to synthesize organic compounds from inorganic ones. Today, hundreds of thousands of organic compounds have been synthesized, including many compounds central to life itself.
3-SECOND NUCLEUS
Vitalism, the belief that living things contained some unique force not contained in non-living things, was proved false and abandoned in the nineteenth century.
3-MINUTE VALENCE
The downfall of vitalism is significant because it meant that living things could be studied from a chemical point of view. The reactions that happen in living organisms are not qualitatively different from those that happen outside of living organisms. The revolution that has occurred in biology over the last half-century is largely due to understanding life from a molecular perspective.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
GEORG ERNST STAHL
1659–1734
German chemist and advocate of vitalism
FREDERICH WÖHLER
1800–82
German chemist who, in 1828, synthesized urea, an organic compound, from inorganic starting materials
30-SECOND TEXT
Nivaldo Tro
Urea (centre) was one of the first organic compounds synthesized from inorganic compounds.
HYDROCARBONS
the 30-second chemistry
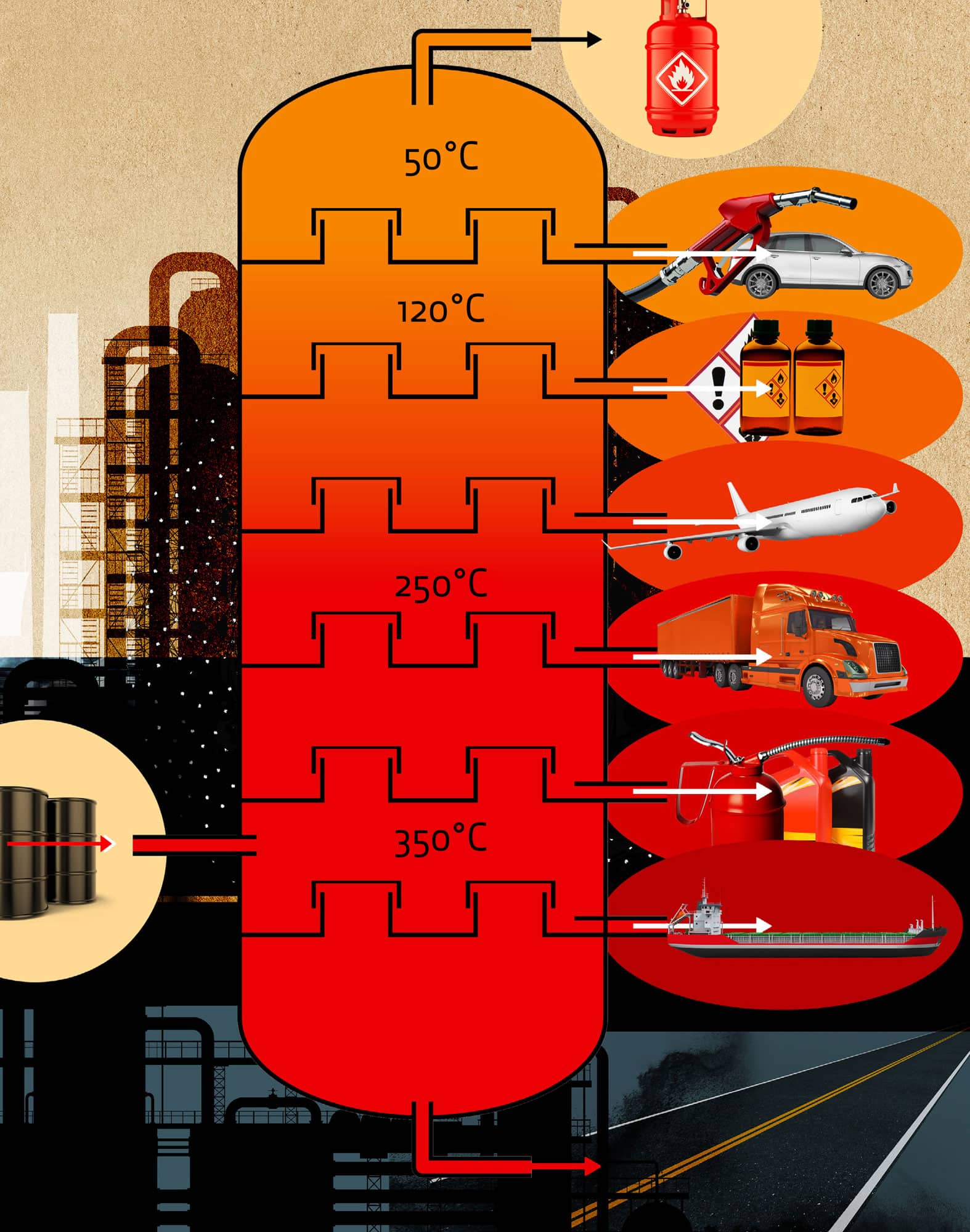
Hydrocarbons, the principle constituents of petroleum and natural gas, are organic compounds of hydrogen and carbon. Because carbon can bond, not only to other atoms like hydrogen, but also to itself (a process called ‘catenation’), there are many naturally occurring and synthetically produced hydrocarbon compounds. Hydrocarbons have had an unmatched influence on the economic, social and environmental development of modern society. They are a convenient source of energy and the starting materials to make many common products such as polymers, textiles and pharmaceuticals. Hydrocarbons in all physical forms find their way into our lives every day – in gas (natural gas), liquid (petrol) and solid (paraffin wax) forms. They are, in general, classified as ‘saturated’ (only single covalent bonds are present), ‘unsaturated’ (at least one double or triple covalent bond exists between carbon atoms) or ‘aromatic’ (at least one aromatic ring is present). Long chains of hydrocarbons can form linear, cross-linked or cyclic structures, with the number of carbon atoms ranging from one (methane) to thousands. Hydrocarbons have high potential energy, which can be released upon combustion – generating energy convenient for applications such as transportation and heating.
3-SECOND NUCLEUS
Petrol, a potpourri of many hydrocarbons, packs a large amount of energy in a small volume, which makes it a convenient source of fuel for transportation.
3-MINUTE VALENCE
Hydrocarbon-based ‘fossil fuels’ provide more than 80 per cent of the world’s energy – mainly as coal, petroleum and natural gas. They are the most convenient and economical sources of energy for most practical applications. However, hydrocarbons are non-renewable, with limited resources globally. The search for alternative and renewable sources of energy will reduce the global dependence on fossil fuels and help reduce their adverse impact on the environment.
RELATED TOPICS
See also
CARBON: IT’S NOT JUST FOR PENCILS
3-SECOND BIOGRAPHIES
JAMES YOUNG
1811–83
Scottish chemist known as the father of the petrochemical industry who first distilled paraffin, a saturated hydrocarbon, from petroleum
EDWIN L. DRAKE
1819–80
An American railroad conductor credited with drilling the first modern oil well, near Titusville, Pennsylvania
30-SECOND TEXT
Ali O. Sezer
Petroleum is separated into its hydrocarbon components due to the differences in the boiling points of those components.
ALCOHOLS
the 30-second chemistry
Isopropanol (C3H7OH), a popular antiseptic known as rubbing alcohol, and ethanol (C2H5OH), the intoxicating component of alcoholic drinks, belong to a very important class of organic compounds called alcohols. Alcohols contain the hydroxyl (–OH) functional group. The smaller alcohols are clear, volatile and flammable liquids with a biting odour. The alcohol family contains a homologous series of compounds from hydrocarbons in which a hydrogen atom is replaced by an –OH group. Methanol, ethanol and propanol are the first three members of this series. The –OH functional group is directly bonded to a carbon atom, which is also connected to one (primary alcohol), two (secondary alcohol) or three (tertiary alcohol) other carbon atoms. Ethanol and isopropanol are examples of primary and secondary alcohols, respectively. The –OH group makes alcohols highly polar, and the smaller ones are all miscible with water – meaning that they form homogenous solutions with water in all proportions. Water-solubility decreases noticeably as the number of carbon atoms (molecular mass) increases. Today alcohols find a wide range of uses, from perfume-making through food and pharmaceutical products to medical applications.
3-SECOND NUCLEUS
Beyond being the intoxicating component of alcoholic drinks, alcohols have an important place in organic chemistry – with many applications benefitting human life.
3-MINUTE VALENCE
Polyalcohols, also known as sugar alcohols, are alcohols with more than one –OH group in their molecular structure. These white, water-soluble solid compounds are industrially important – they are used as thickeners and sweeteners. Sorbitol, erythritol, xylitol and maltitol are popular polyalcohols commonly used in hard candy and artificial sweeteners. They do not get completely absorbed into the bloodstream so they are incapable of rapidly raising blood sugar.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
JOHANN TOBIAS LOWITZ
1757–1804
German-born Russian chemist who first obtained pure ethanol by filtering distilled ethanol through activated carbon
ARCHIBALD SCOTT COUPER
1831–92
Scottish chemist who first published the structural formula of ethanol, one of the first structural formulas determined
30-SECOND TEXT
Ali O. Sezer
Alcohols are a common family of organic compounds that includes substances such as ethanol (the alcohol in alcoholic beverages).

ALDEHYDES, KETONES & ESTERS
the 30-second chemistry
The pleasant odour and flavour of fresh almonds and peppermint are mainly due to benzaldehyde and menthone, two naturally occurring compounds whose molecule contains the carbonyl functional group (–C=O). Benzaldehyde is an example of a family of organic compounds called aldehydes. In an aldehyde, the –C=O group is at the end of a carbon chain. Menthone is an example of a family of organic compounds called ketones. In a ketone, the carbonyl group is in the middle of the carbon chain. Another common family of organic compounds is the ester family. In an ester, an oxygen atom interrupts the bonding of the carbonyl group to the carbon chain (–CO2C–). Benzyl acetate, a component in the smell of strawberries, pears and jasmine, is an ester. Small aldehydes and ketones (those with low molar masses), such as formaldehyde (an important industrial solvent) and acetone (nail-polish remover), have a strongly pungent odour. But aldehydes with increasing molar masses generally have more pleasant and fruity odours. Aldehydes, ketones and esters are commonly found naturally and manufactured industrially as odours and flavours in food and pharmaceutical products. Many solvents used in adhesives, paints, perfumes, plastics and fabrics also contain aldehydes, ketones and esters.
3-SECOND NUCLEUS
Aldehydes, ketones and esters are naturally occurring organic compounds responsible for the pleasant odours and flavours of many natural and synthetic products.
3-MINUTE VALENCE
Aldehydes, ketones and esters are found abundantly in living organisms. Humans and animals store energy as esters known as fats and oils. Testosterone and progesterone (the male and female sex hormones, respectively), aldosterone (which regulates blood sodium level) and pheromone (which is released by animals to trigger a social response) are all ketones. Retinal (retinaldehyde) is an aldehyde forming the basis of vision. Acetaldehyde causes the ‘hangover’ feeling after alcohol is metabolized in the liver.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
LEOPOLD GMELIN
1788–1853
German chemist who first introduced the terms ‘ester’ and ‘ketone’
JUSTUS VON LIEBIG
1803–73
German chemist considered the father of organic chemistry, who first used the term ‘aldehyde’
30-SECOND TEXT
Ali O. Sezer
Many of the odours you smell every day come from the naturally occurring families of aldehydes, ketones and esters.

CARBOXYLIC ACIDS & AMINES
the 30-second chemistry
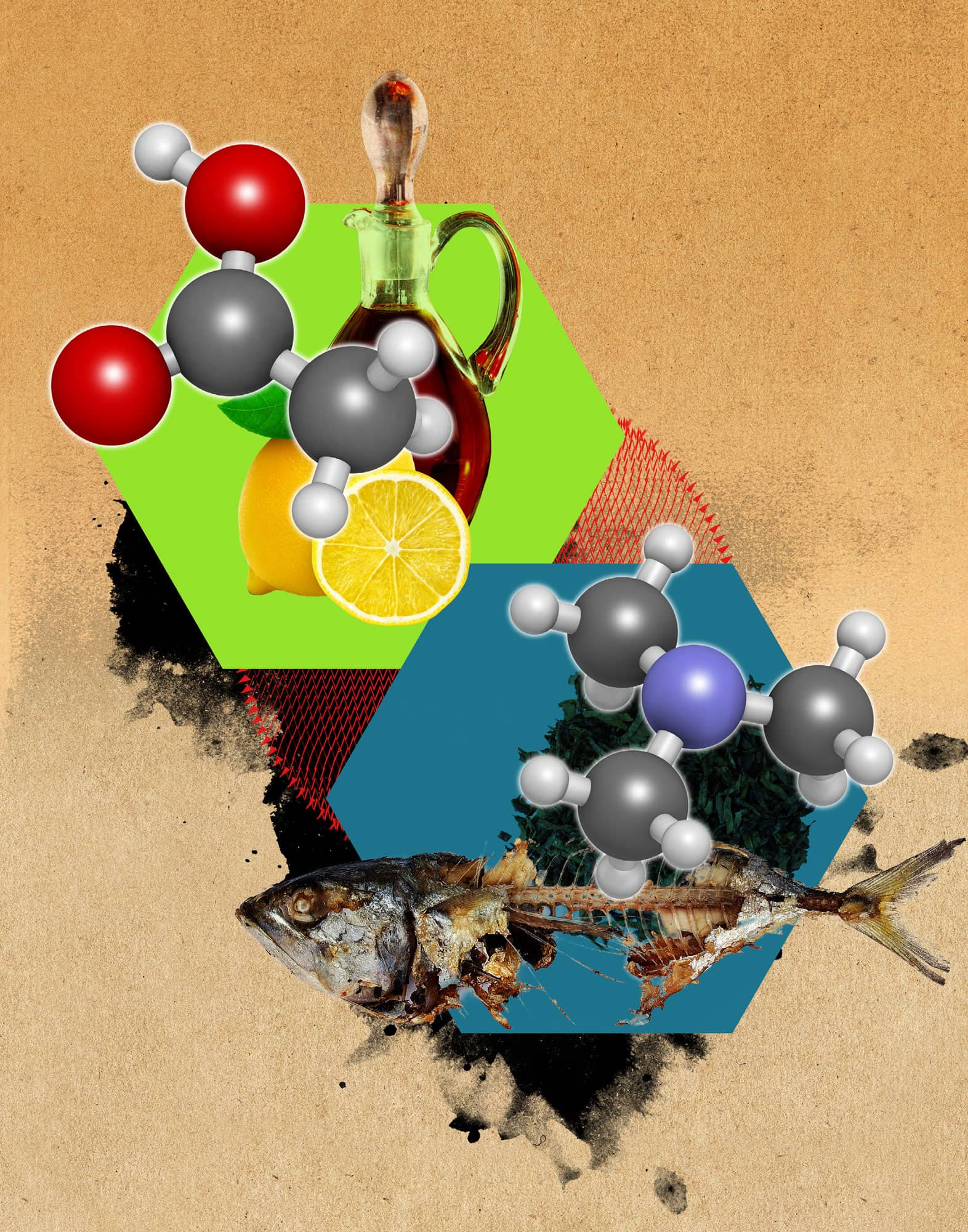
Carboxylic acids and amines are important and familiar families of organic compounds. Carboxylic acids are organic acids and can be identified by their –COOH functional group. Like all acids, carboxylic acids taste sour. Acetic acid, the active component of vinegar, is a carboxylic acid. The word vinegar originates from the French for wine (vin) and sour (aigre). When wine is left exposed to air, the ethanol oxidizes to form carboxylic acid, ruining the wine. Citric acid is another carboxylic acid; it is responsible for the sour taste of lemons and limes. Amines are organic bases and contain a nitrogen atom bonded to one or more carbon atoms. Many amines have an unpleasant odour. When living things die, their proteins are broken down into amines, which evaporate into the air. For example, the smell of rotting fish is due to trimethylamine, and that of decaying animal flesh to caderverine, both foul-smelling amines. Some plant-based amines, known as alkaloids, have the ability to alter sensory pathways. Caffeine, nicotine and cocaine are all alkaloids that stimulate the central nervous system, resulting in feelings of increased alertness and energy. Other alkaloids, such as morphine and codeine, have the opposite effect: they depress the central nervous system. Morphine is a powerful depressant used to treat extreme pain.
3-SECOND NUCLEUS
Carboxylic acids and amines are naturally occurring acids and bases, respectively.
3-MINUTE VALENCE
The reaction between a carboxylic acid and an amine is an acid-base reaction that links the two molecules together and forms water as a byproduct. This reaction is important in biochemistry because it is responsible for the linking of amino acids – which are molecules that have a carboxylic acid group on one side and an amine group on the other – to form proteins, the workhorse molecules in living organisms.
RELATED TOPICS
See also
3-SECOND BIOGRAPHY
HERMANN KOLBE
1818–84
German chemist who greatly contributed to the development of organic chemistry and was the first to synthesize acetic acid
30-SECOND TEXT
Nivaldo Tro
Acetic acid (top) is responsible for the smell of vinegar. Trimethylamine (bottom) is responsible for the foul smell of rotten fish.
CHEMISTRY COPYING NATURE
the 30-second chemistry
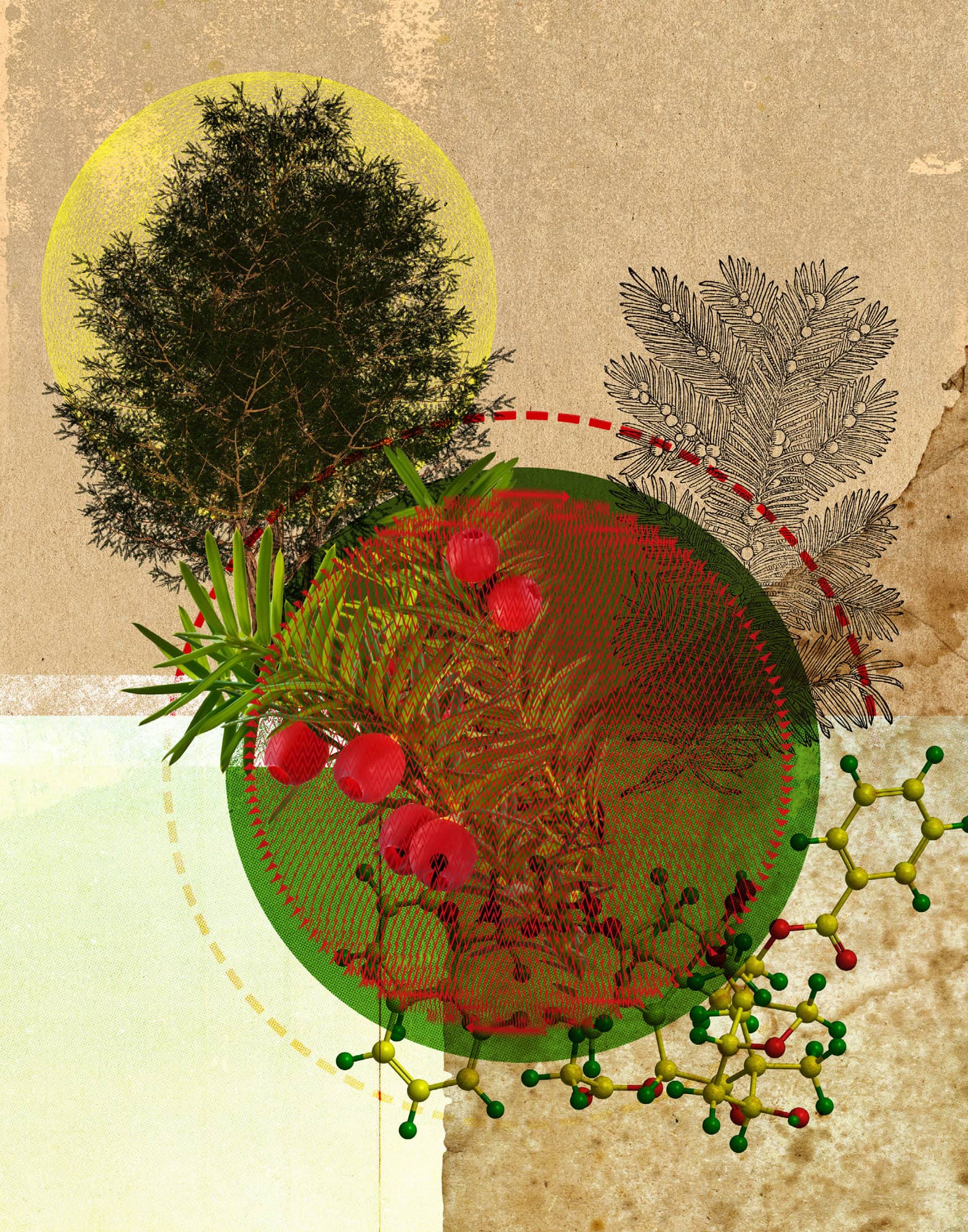
The Pacific yew tree is rather unremarkable. It grows in the Pacific Northwest to heights of about 10–15 m (30–50 ft) and has flat, green needles and red berries. However, its bark contains the miracle drug Taxol, now used to treat ovarian, lung, breast and colon cancer. The biological action of the yew tree has been known since Greek times, and Native Americans used the tree for medicinal purposes. Because of this known history, researchers in the 1960s included the tree in large-scale screening for cancer-fighting agents. The positive results eventually led researchers to isolate the active ingredient Taxol. But the amount of Taxol needed to treat a single cancer patient required the harvesting of several trees that were around 100 years old, creating environmental problems. As often happens with natural products, however, researchers came up with another way to obtain the compound: Taxol is now synthesized from a precursor found in the needles of the European yew tree. Because the trees don’t have to be cut down to harvest the needles, this route is sustainable. Today, millions of cancer patients have benefitted from this natural product. The story of Taxol is representative of the field of natural products research, an active area that produces many novel and useful compounds.
3-SECOND NUCLEUS
Plants, animals and microbes are sources of valuable and often healing chemical compounds.
3-MINUTE VALENCE
Natural products researchers scour living organisms for the useful chemicals they might contain. For example, penicillin (an antibiotic) was extracted from microbes, and the early form of aspirin was extracted from the bark of the willow tree. Often chemists later find ways to synthesize these compounds in the laboratory, but the myriad of compounds in nature often shows us the way.
30-SECOND TEXT
Nivaldo Tro
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
MONROE ELIOT WALL
1916–2002
American chemist credited with the co-discovery of Taxol
MANSUKH C. WANI
1925–
Indian American chemist credited with the co-discovery of Taxol
The yew tree is the source of the cancer-fighting agent Taxol.
POLYMERS
the 30-second chemistry
Polymers permeate most of human life. It is hard to imagine society without them, given the significant role natural and synthetic polymers play – from medicine to food packaging, and from clothing to housewares. German chemist Hermann Staudinger first demonstrated the existence of macromolecules (polymers) as large, chain-like molecules of smaller repeating units called monomers. Humans have known natural polymers, such as cotton and rubber, for thousands of years. But their chemical structure was long subject to debate until Staudinger proved the macromolecular structure of natural rubber – a Nobel Prize-winning discovery in 1953. The term polymer is derived from the Greek poly meros, meaning many parts. Many molecules can act as monomers, making it possible to create a wide range of polymeric materials with desired characteristics. Polyethylene, for example – one of the most common plastic materials found in packaging bags and bottles – is a chain-like molecule consisting of an ethylene monomer backbone. Polymers can have high molecular mass as monomers can link in a variety of ways to grow into very large molecules. Some are light, hard, strong and flexible; others exhibit unique chemical, thermal, electrical and optical characteristics.
3-SECOND NUCLEUS
Polymers, chain-like macromolecules consisting of chemically linked repeating units (monomers), play an unmatched role providing convenience in many aspects of human life.
3-MINUTE VALENCE
Polymers are in general known as electrical insulators. However, some organic polymers – consisting of monomer units linked by alternating single and double bonds in the carbon backbone – exhibit a low, inherent electrical conductivity, which can significantly be improved by chemically mixing in electron-donating and/or -receiving agents, a process called doping. Doped polymers, such as organic light emitting diodes (OLED), have revolutionized the electronics industry.
RELATED TOPICS
See also
THE LEWIS MODEL FOR CHEMICAL BONDING
3-SECOND BIOGRAPHIES
HERMANN STAUDINGER
1881–1965
German chemist who won the 1953 Nobel Prize in Chemistry for discovering the macromolecular structure of natural rubber
HIDEKI SHIRAKAWA
1936–
Japanese chemist who won the 2000 Nobel Prize in Chemistry for co-discovering the existence of conductive polymers
30-SECOND TEXT
Ali O. Sezer
Polymers are chain-like molecules that compose a range of substances such as plastics.
