The Exogenous Pathway of Antigen Processing and Presentation
Extracellular material can gain access to vesicular compartments inside an APC through several means. Professional APCs can internalize particulate material by simple phagocytosis (also called “cell eating”), where material is engulfed by pseudopods of the cell membrane; or by receptor-mediated endocytosis, where the material first binds to specific surface receptors, followed by clathrin-mediated internalization. Macrophages and dendritic cells internalize antigen by both processes. Most other APCs, whether professional or not, demonstrate little or no phagocytic activity and therefore typically internalize exogenous antigen only by endocytosis (either receptor-mediated endocytosis or by pinocytosis, i.e., nonspecific “cell drinking”). B cells, for example, internalize antigen very effectively by receptor-mediated endocytosis, using their antigen-specific membrane immunoglobulin as the receptor. The one thing that all these pathways have in common is that the internalized components gain access to the cell but remain bound by a phospholipid bilayer (membrane) structure.
Peptides Are Generated from Internalized Antigens in Endocytic Vesicles
Once an antigen is internalized in this fashion it is typically degraded into peptides within compartments that make up the endocytic processing pathway. As the experiment shown in Figure 7-11 demonstrated, internalized antigen takes a mere 1 to 3 hours to travel through the endocytic pathway and ultimately appear at the cell surface in the form of MHC class II–peptide complexes. This endocytic antigen-processing and presentation pathway appears to involve several increasingly acidic compartments, including early endosomes (pH 6.0–6.5); late endosomes, or endolysosomes (pH 4.5–5.0); and lysosomes (pH 4.5). Internalized antigen progresses through these membrane-enclosed compartments, encountering hydrolytic enzymes and a lower pH in each successive compartment (Figure 7-16). Antigen-presenting cells have a unique form of late endosome, the MHC class II–containing compartment (MIIC), in which final protein degradation and peptide loading into MHC class II proteins occurs. Within the compartments of the endocytic pathway, antigen is degraded into short peptides of about 13 to 18 residues that ultimately meet up with and bind to preformed MHC class II molecules in late endosomes. Because the hydrolytic enzymes are optimally active under acidic conditions (low pH), antigen processing can be inhibited by chemical agents that increase the pH of the compartments (e.g., chloroquine) as well as by protease inhibitors (e.g., leupeptin).
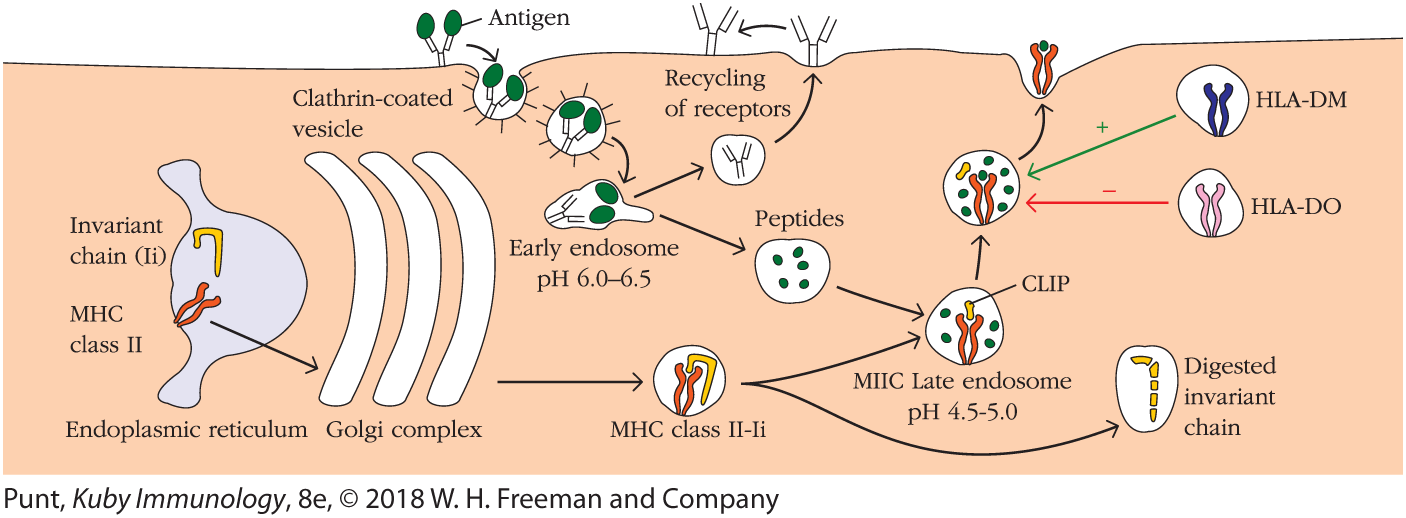
FIGURE 7-16 Generation of antigenic peptides and assembly of MHC class II molecules in the exogenous processing pathway. In the cell shown here, a B cell, exogenous antigen is internalized by receptor-mediated endocytosis (top left), with the membrane-bound antibody functioning as an antigen-specific receptor. Internalized antigen moves through several acidic compartments ending in specialized MIIC late endosomes, where it is degraded into peptide fragments. Within the rough endoplasmic reticulum (bottom left), a newly synthesized MHC class II molecule binds an invariant chain. The bound invariant chain prevents premature binding of peptides to the class II molecule and helps to direct the complex to endocytic compartments containing processed peptides derived from exogenous antigens. Digestion of the invariant chain leaves CLIP, a small fragment remaining in the binding groove of the MHC class II molecule. HLA-DM, a nonclassical MHC class II molecule present within the MIIC compartment, mediates exchange of antigenic peptides for CLIP. The nonclassical class II molecule HLA-DO may act as a negative regulator of class II antigen processing by binding to HLA-DM and inhibiting its role in the dissociation of CLIP from class II molecules.
The mechanism by which internalized antigen moves from one endocytic compartment to the next has not been conclusively demonstrated. However, it has been suggested that early endosomes from the periphery move inward to become late endosomes and eventually lysosomes. Alternatively, small transport vesicles may carry antigens from one compartment to the next. Eventually the endocytic compartments, or portions of them, return to the cell periphery, where they fuse with the plasma membrane. In this way, the surface receptors are recycled.
The Invariant Chain Guides Transport of MHC Class II Molecules to Endocytic Vesicles
Since APCs express both MHC class I and II molecules, newly formed MHC molecules of both classes reside simultaneously within the membrane of the RER. Therefore, some mechanism must exist to prevent MHC class II molecules from binding to the antigenic peptides destined for MHC class I molecules. When MHC class II molecules are synthesized within the RER they associate with a protein called the invariant chain (Ii, or CD74). This conserved, non–MHC-encoded protein interacts with the peptide-binding groove formed by the combination of the α and β chains of MHC class II, in essence blocking any endogenously derived peptides from binding while the class II molecule is still in the RER (see Figure 7-16). The invariant chain also appears to be involved in the folding of the class II α and β chains, their exit from the RER, and the subsequent routing of class II molecules to the endocytic processing pathway from the trans-Golgi network.
The role of the invariant chain in the routing of class II molecules has been demonstrated in transfection experiments with cells that lack genes encoding both the MHC class II molecules and the invariant chain. Immunofluorescence labeling of these cells transfected only with MHC class II genes revealed that, in the absence of invariant chain, class II molecules remain primarily in the ER and do not transit past the cis-Golgi, or their final compartment before breaking off into vesicles. However, in cells transfected with both the MHC class II genes and the Ii-encoding gene, the class II molecules were localized in the cytoplasmic vesicular structures of the endocytic pathway. The invariant chain contains sorting signals in its cytoplasmic tail that direct the transport of the MHC class II complex from the trans-Golgi network to the endocytic compartments.
Peptides Assemble with MHC Class II Molecules by Displacing CLIP
Experimental observations indicate that most MHC class II–invariant chain complexes are transported from the RER, where they are formed, through the Golgi complex and trans-Golgi network. From there they proceed through the endocytic pathway, moving from early endosomes to the MIIC late endosomal compartment in some cases. As the proteolytic activity increases in each successive compartment, the invariant chain itself is gradually degraded. However, a short fragment of the invariant chain, termed CLIP (for class II–associated invariant chain peptide), remains bound to the class II molecule after the majority of the invariant chain has been cleaved within the endosomal compartment. Like antigenic peptide, CLIP physically occupies the peptide-binding groove of the MHC class II molecule, preventing any premature binding of antigen-derived peptide.
A nonclassical MHC class II molecule called HLA-DM is required to catalyze the exchange of CLIP with antigenic peptides (see Figure 7-16). The DMα and DMβ genes are located near the TAP and LMP genes in the MHC complex of humans, with similar genes in mice (see Figure 7-7). Like other MHC class II molecules, HLA-DM is a heterodimer of α and β chains. However, unlike other class II molecules it is relatively nonpolymorphic and is not normally expressed at the cell membrane but is found predominantly within the endosomal compartment. HLA-DM has been found to associate with the MHC class II β chain and to function in removing or “editing” peptides, including CLIP, that associate transiently with the binding groove of classical class II molecules. Peptides that make especially strong molecular interactions with MHC class II, creating long-lived complexes, are harder for HLA-DM to displace, and thus become the repertoire of peptides that ultimately make it to the cell surface as MHC-peptide complexes.
As with MHC class I molecules, peptide binding is required to maintain the structure and stability of class II molecules. Once a peptide has bound, the MHC class II–peptide complex is transported to the plasma membrane, where the neutral pH appears to enable the complex to assume a compact, stable form. Peptide is bound so strongly in this compact form that it is difficult to replace a class II–bound peptide on the membrane with another peptide under physiologic conditions.
One additional nonclassical member of the MHC class II family, HLA-DO, also is relatively nonpolymorphic and associates with classical class II molecules. However, it appears to act as a negative regulator of antigen binding, modulating the function of HLA-DM and changing the repertoire of peptides that preferentially bind to classical class II molecules. In cells that express both HLA-DO and HLA-DM, these two molecules strongly associate in the ER and maintain this interaction all the way to the endosomal compartments. Although this interaction has been recognized for many years, the function of this negative regulator and the impact of this changed peptide repertoire has been only more recently resolved.
Originally observed only in B cells and in the thymus, the cellular expression profile of HLA-DO has recently been expanded to dendritic cells (DCs), where it is thought to play a role in the maintenance of self-tolerance (discussed further in Chapter 16). This phenomenon was studied by L. K. Denzin and colleagues, using diabetes-prone mice engineered to express human HLA-DO. DCs are known to be essential in the establishment of self-tolerance, as well as in the presentation of autoantigens to self-reactive T cells. Self-reactive T cells develop in these nonobese diabetic animals, and these cells ultimately destroy pancreatic beta cells, causing type 1 diabetes. In this study, the development of diabetes was blocked by the presence of the HLA-DO transgene in DCs. In addition, using specific monoclonal antibodies, the authors showed that the repertoire of peptides being presented to the autoreactive T cells was significantly altered, resulting in reduced efficiency in presenting key self-peptides. This and other work suggests that normal HLA-DO expression may play an important role in modulating HLA-DM behavior to ensure the presentation of a self-peptide repertoire that encourages tolerance to self-antigens. Interestingly, in wild-type mice and humans, HLA-DO expression is down-regulated following DC activation by antigen, releasing HLA-DM to carry out its normal function of encouraging the presentation of a diverse array of peptides, many of which will presumably be derived from the foreign proteins that stimulated these APCs.
Overview Figure 7-17 illustrates the endogenous pathway (left side) and compares it with the separate exogenous pathway (right side). Whether an antigenic peptide associates with class I or with class II molecules is partially dictated by the mode of entry into the cell, either exogenous or endogenous; the site of processing; the cell type; and the microenvironment surrounding that cell. However, in the next section, we will see that these assignments are not absolute.
Key Concepts:
- The invariant chain is gradually processed into a smaller fragment, called CLIP, that blocks the peptide-binding groove of classical class II molecules in late endosomes, where HLA-DM (a nonclassical class II molecule) mediates exchange of CLIP for exogenous antigen fragments in the endosome.
- The presence of HLA-DO, another nonclassical class II molecule, in some pAPCs (specifically DCs) may regulate the activity of HLA-DM and encourage class II presentation of self-peptides in a manner that encourages self-tolerance.