Other Mechanisms That Maintain Self-Tolerance
As we have seen, negative selection of thymocytes can rid a developing T-cell repertoire of cells that express a high affinity for both ubiquitous self antigens and, thanks to the activity of AIRE, tissue-specific antigens. However, negative selection in the thymus (central tolerance) is not perfect. Autoreactive T cells do escape, either because they have too low an affinity for self to induce clonal deletion, or because they happen not to have browsed the “right” tissue-specific antigen/MHC combination. The body has evolved several other mechanisms to avoid autoimmunity, including what has become a major focus of interest for immunologists: the development in both the thymus and the periphery of a fascinating group of cells known as regulatory T cells.
TREG Cells Negatively Regulate Immune Responses
Regulatory T cells (TREG cells) inhibit the proliferation of other T-cell populations both directly and indirectly, effectively suppressing autoreactive immune responses. They express surface CD4 as well as CD25, the α chain of the IL-2 receptor. However, TREG cells (TREGs) are more definitively identified by their expression of a master transcriptional regulator, FoxP3, the expression of which is necessary and sufficient to induce differentiation to the TREG lineage.
Many TREGs develop in the thymus and appear to represent an alternative fate for autoreactive T cells. As we have seen, most thymocytes that express receptors with high affinity for self antigen die via negative selection. However, a small fraction commits to the regulatory T-cell lineage and leaves the thymus to patrol the body and thwart autoimmune reactions.
What determines whether a self-reactive thymocyte dies or differentiates into a TREG cell is still not fully understood. Thymocytes that experience strong, but not too strong, TCR signals appear more likely to commit to the TREG lineage than undergo apoptosis (see Figure 8-7). However, other factors, such as subtle differences in maturation state or stochastic differences in chromatin status, may also have an influence. Although all types of antigen-presenting cells, including mTECs and dendritic cells, can mediate TREG cell development, some studies suggest that TREGs encounter these cells in a unique microenvironmental niche. Such a niche may provide TREG precursors with the cytokines they need (IL-2 and IL-15) to survive and complete maturation.
TREG cells that develop in the thymus are referred to as thymic or tTREG cells. Peripheral or pTREG cells develop from conventional mature T cells exposed to antigen in the context of TGF-β and IL-10 cytokines (see Chapters 10 and 13). Although tTREG and pTREG cells both quell immune responses, they may also adopt distinct inhibitory functions in various tissues.
Animal studies show that members of the FoxP3+ TREG population inhibit development of autoimmune diseases such as experimentally induced inflammatory bowel disease, experimental allergic encephalitis, and autoimmune diabetes. Suppression by these regulatory cells is antigen specific because it depends on activation through the T-cell receptor. Exactly how TREGs quell responses is still debated, although they probably do so via a variety of means: directly inhibiting an antigen-presenting cell’s ability to activate T cells, directly killing T cells, indirectly inhibiting T-cell activity by secreting inhibitory cytokines IL-10 and TGF-β, and/or depleting the local environment of stimulatory cytokines such as IL-2 (Figure 8-11). See Chapter 14 for a description of one experiment that directly demonstrated that TREGs use more than one approach to inhibit autoreactive responses.
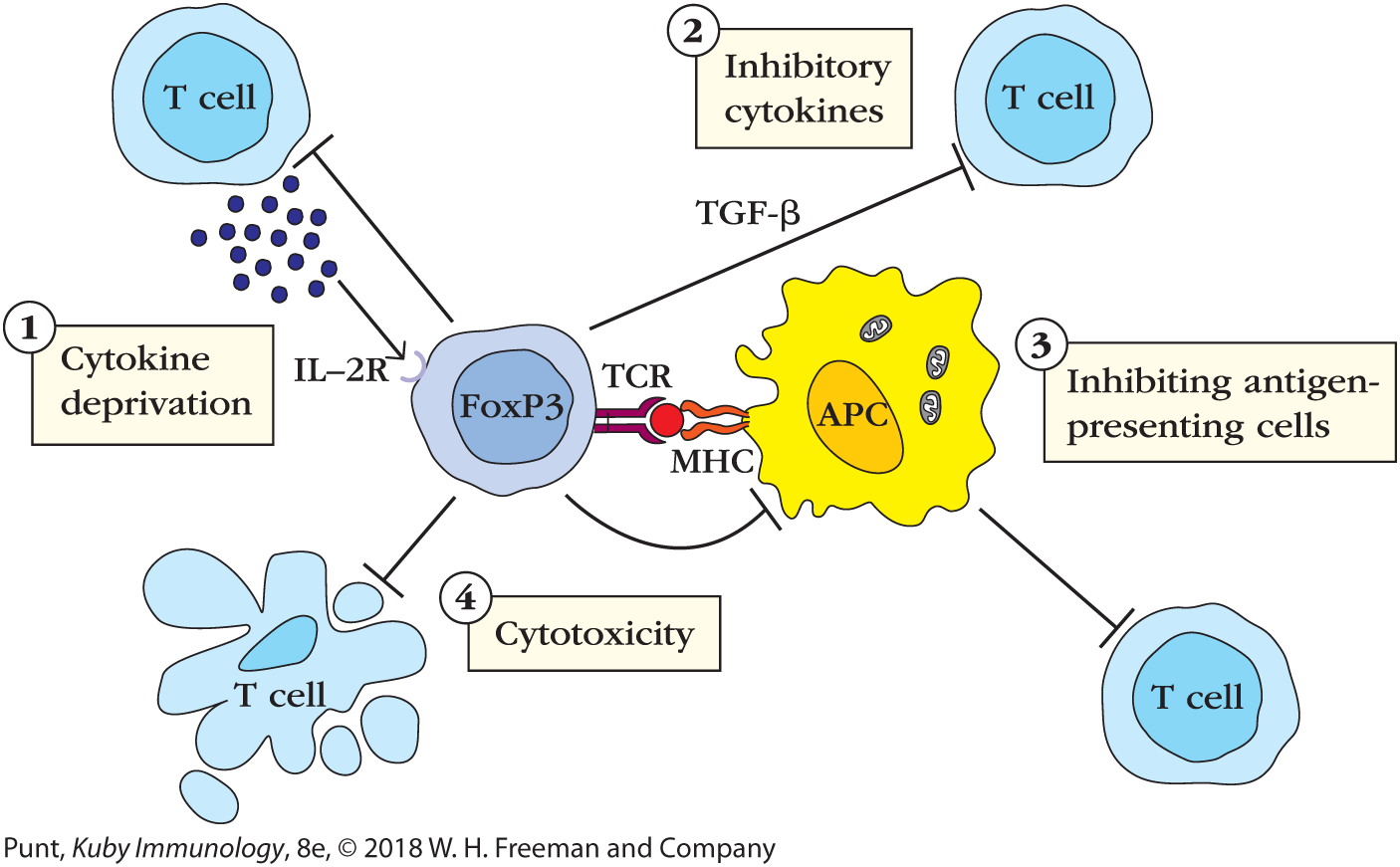
FIGURE 8-11 How regulatory T cells (TREGs) inactivate traditional T cells. Some possible mechanisms of TREG activity are illustrated in this schematic. These may all contribute to quelling immune responses in vivo. (1) Cytokine deprivation: TREGs express relatively high levels of high-affinity IL-2 receptors and can compete for the cytokines that activated T cells need to survive and proliferate. (2) Cytokine inhibition: TREGs secrete several cytokines, including IL-10 and TGF-β, which bind receptors on activated T cells and reduce signaling activity. (3) Inhibition of antigen-presenting cells: TREGs can interact directly with MHC class II–expressing antigen-presenting cells and inhibit their maturation, leaving them less able to activate T cells. (4) Cytotoxicity: TREGs can also display cytotoxic function and kill cells by secreting perforin and granzyme.
The existence of regulatory T cells that specifically suppress immune responses has clinical implications. Depletion or inhibition of TREG cells before immunization may enhance immune responses to conventional vaccines. Elimination of T cells that suppress responses to tumor antigens may also facilitate the development of antitumor immunity. Conversely, increasing the suppressive activity of regulatory T-cell populations could be useful in the treatment of allergic or autoimmune diseases. The ability to increase the activity of regulatory T-cell populations might also be useful in suppressing organ and tissue rejection. Regulatory T-cell immunotherapy has recently become a reality in clinical trials. Investigators are currently testing TREG effectiveness in preventing graft-versus-host disease (GvHD) after bone marrow transplantation, and clinicians hope to use them soon to quell the immune response that causes type 1 diabetes.
Peripheral Mechanisms of Tolerance Also Protect against Autoreactive Thymocytes
Although the thymus screens the developing T-cell repertoire remarkably efficiently, it is not perfect. Autoreactive T cells do escape. The body has evolved several other mechanisms to manage the autoreactive escapee in the periphery. Briefly, many antigens are “hidden” from autoreactive T cells because only a subset of cells (professional APCs) express the right costimulatory molecules needed to initiate the immune response. Autoreactive naïve T cells that can see an MHC/self-peptide combination on a nonprofessional antigen-presenting cell will not receive the correct costimulatory signals and, therefore, will not divide or differentiate. For example, if a thymocyte specific for a peptide made by a kidney cell escaped from the thymus, it would not be activated unless that peptide were first presented on a professional antigen-presenting cell. Kidney cells do not express the costimulatory ligands required for activating a CD4+ or a CD8+ T cell. High-affinity interactions with MHC/peptide combinations on cells that do not express costimulatory ligands can also lead to T-cell anergy—another peripheral tolerance mechanism that is described in more detail in Chapter 10. The clinical consequences of failures of central and peripheral tolerance are discussed in Chapter 16.