Functions of the Cortex
More than a century ago, surgeons found damaged cortical areas during autopsies of people who had been partially paralyzed or speechless. This rather crude evidence did not prove that specific parts of the cortex control complex functions like movement or speech. A laptop with a broken power cord might go dead, but we would be fooling ourselves if we thought we had “localized” the Internet in the cord.
Motor Functions
Scientists had better luck in localizing simpler brain functions. For example, in 1870, German physicians Gustav Fritsch and Eduard Hitzig made an important discovery: Mild electrical stimulation to parts of an animal’s cortex made parts of its body move. The effects were selective: Stimulation caused movement only when applied to an arch-shaped region at the back of the frontal lobe, running roughly ear-to-ear across the top of the brain. Moreover, stimulating parts of this region in the left or right hemisphere caused movements of specific body parts on the opposite side of the body. Fritsch and Hitzig had discovered what is now called the motor cortex.
Mapping the Motor Cortex
Lucky for brain surgeons and their patients, the brain has no sensory receptors. Knowing this, in the 1930s, Otfrid Foerster and Wilder Penfield were able to map the motor cortex in hundreds of wide-awake patients by stimulating different cortical areas and observing responses. They discovered that body areas requiring precise control, such as the fingers and mouth, occupy the greatest amount of cortical space (Figure 12.2). In one of his many demonstrations of motor behavior mechanics, Spanish neuroscientist José Delgado stimulated a spot on a patient’s left motor cortex, triggering the right hand to make a fist. Asked to keep the fingers open during the next stimulation, the patient, whose fingers closed despite his best efforts, remarked, “I guess, Doctor, that your electricity is stronger than my will” (Delgado, 1969, p. 114).
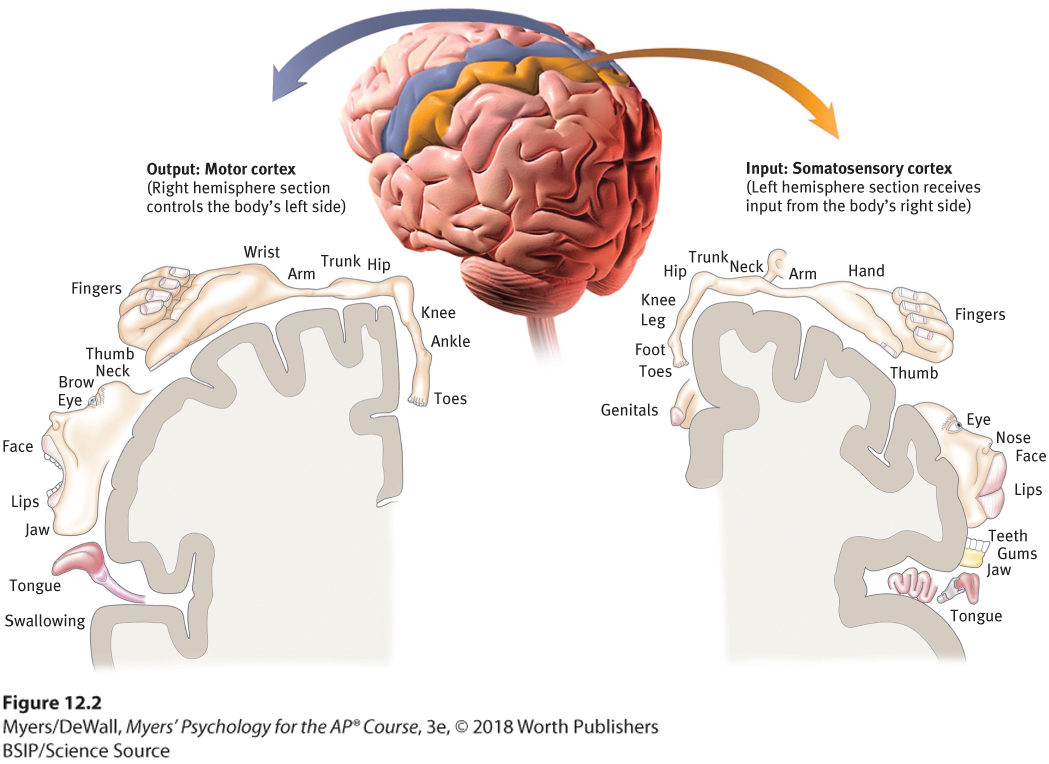
Figure 12.2 Motor cortex and somatosensory cortex tissue devoted to each body part
As you can see from this classic though inexact representation, the amount of cortex devoted to a body part in the motor cortex (in the frontal lobes) or in the somatosensory cortex (in the parietal lobes) is not proportional to that body part’s size. Rather, the brain devotes more tissue to sensitive areas and to areas requiring precise control. Thus, the fingers have a greater representation in the cortex than does the upper arm.
More recently, scientists were able to predict a monkey’s arm motion a tenth of a second before it moved—by repeatedly measuring motor cortex activity preceding specific arm movements (Gibbs, 1996). Such findings have opened the door to research on brain-controlled computer technology.
Brain-Computer Interfaces
By eavesdropping on the brain, could we enable a paralyzed person to move a robotic limb? Could a brain-computer interface command a cursor to write an email or do an online search? To find out, brain researchers implanted 100 tiny recording electrodes in the motor cortexes of three monkeys (Nicolelis, 2011; Serruya et al., 2002). As the monkeys gained rewards by using a joystick to follow a moving red target, the researchers matched the brain signals with the arm movements. Then they programmed a computer to monitor the signals and operate the joystick. When a monkey merely thought about a move, the mind-reading computer moved the cursor with nearly the same proficiency as had the reward-seeking monkey. In follow-up experiments, both monkeys and humans have learned to control a robot arm or wheelchair that could help them receive food (Collinger et al., 2013; Hochberg et al., 2012; Rajangam et al., 2016; Velliste et al., 2008; see Figure 12.3).
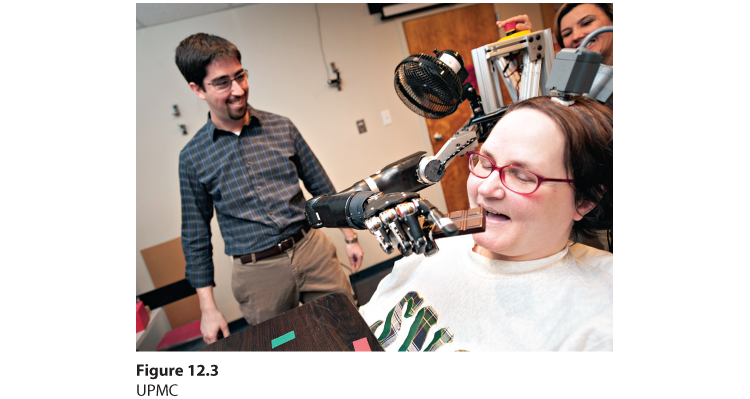
Figure 12.3 Mind over matter
A neurodegenerative disease caused Jan’s complete paralysis. Yet, thanks to a tiny, 96-electrode implant in her motor cortex, she and others have learned to direct a robotic arm with their thoughts (Collinger et al., 2013; Hochberg et al., 2012).
Research has also recorded messages not from the arm-controlling motor neurons but from a brain area involved in planning and intention (Leuthardt et al., 2009; Musallam et al., 2004). In one study, a monkey seeking a juice reward awaited a cue telling it to reach toward a spot flashed on a screen in one of up to eight locations. A computer program captured the monkey’s thinking by recording the associated activity. By matching this neural activity to the monkey’s subsequent pointing, the mind-reading researchers could program a cursor to move in response to the monkey’s thoughts. Monkey think, computer do.
If this technique works, why not use it to capture the words a person can think but cannot say (for example, after a stroke)? Cal Tech neuroscientist Richard Andersen (2005) and colleagues (2004) have speculated that researchers could implant electrodes in speech areas, then “ask a patient to think of different words and observe how the cells fire in different ways. So you build up your database, and then when the patient thinks of the word, you compare the signals with your database, and you can predict the words they’re thinking. Then you take this output and connect it to a speech synthesizer. This would be identical to what we’re doing for motor control.” With this goal in mind, the U.S. Army has invested millions of dollars in neuroscientists’ efforts to build a helmet that might read and transmit soldiers’ thoughts (Piore, 2011).
Clinical trials of such cognitive neural prosthetics are now under way with people who have severe paralysis or have lost a limb (Andersen et al., 2010; Nurmikko et al., 2010). The first patient, a 25-year-old man with paralysis, was able to mentally control a TV, draw shapes on a computer screen, and play video games—all thanks to an aspirin-sized chip with 100 microelectrodes recording activity in his motor cortex (Hochberg et al., 2006). Since then, others with paralysis who have received implants have learned to direct robotic arms with their thoughts (Collinger et al., 2013; Hochberg et al., 2012). And then there is Ian Burkhart, who lost the use of his arms and legs several years ago at the age of 19. Ohio State University brain researchers implanted recording electrodes in his motor cortex (Bouton et al., 2016). Using a technique called machine learning, they instructed Ian to stare at a computer screen that showed a moving hand. Next, Ian imagined moving his own hand. Brain signals from his motor cortex began feeding into the computer, which got the message that Ian wanted to move his arm and thus stimulate those muscles. The result? Ian, with his very own paralyzed arm, grasped a bottle, dumped out its contents, and picked up a stick. “The things I do in the lab,” Ian said, “I would love to [do] in everyday life” (Marcus, 2016). If everything psychological is also biological—if, for example, every thought is also a neural event—microelectrodes could someday detect complex thoughts well enough to enable people to control their environment with ever-greater precision (see Figure 12.4).
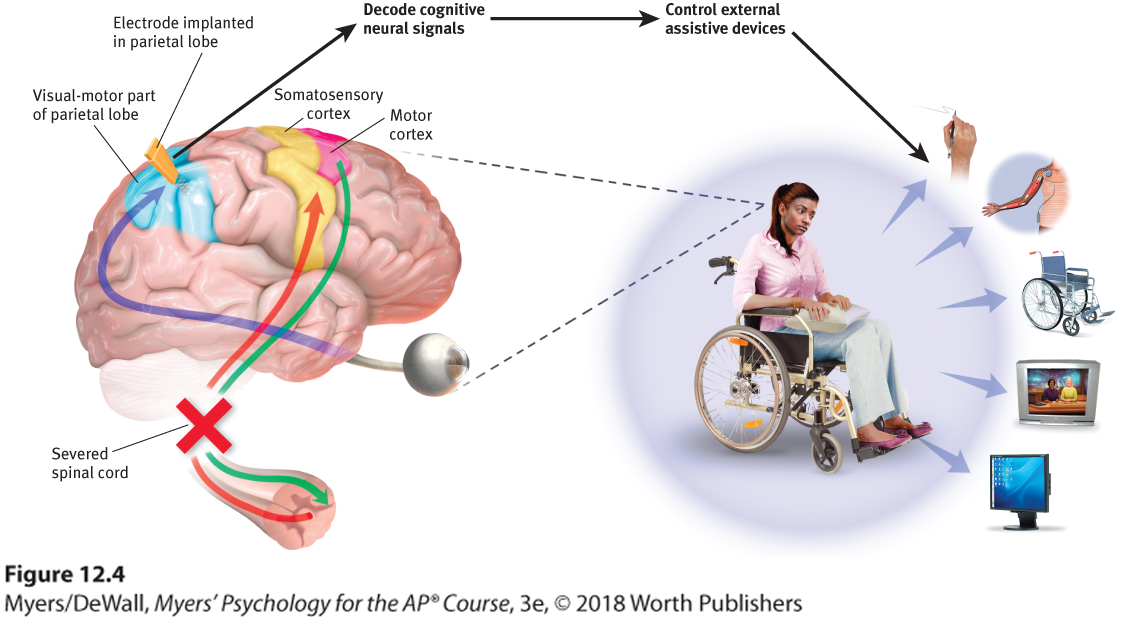
Figure 12.4 Brain-computer interaction
Researchers planted electrodes in a parietal lobe region involved with planning to reach out an arm. This enabled the person’s thoughts to move a robotic limb, stimulate muscles that activate a paralyzed limb, navigate a wheelchair, control a TV, and use the Internet (Andersen et al., 2010).
Sensory Functions
If the motor cortex sends messages out to the body, where does the cortex receive incoming messages? Wilder Penfield identified a cortical area—at the front of the parietal lobes, parallel to and just behind the motor cortex—that specializes in receiving information from the skin senses, such as touch and temperature, and from the movement of body parts. We now call this area the somatosensory cortex (Figure 12.2). Stimulate a point on the top of this band of tissue and a person may report being touched on the shoulder; stimulate some point on the side and the person may feel something on the face.
The more sensitive the body region, the larger the somatosensory cortex area devoted to it (Figure 12.2). Your supersensitive lips project to a larger brain area than do your toes, which is one reason we kiss rather than touch toes. Rats have a large area of the brain devoted to their whisker sensations, and owls to their hearing sensations.
Scientists have identified additional areas where the cortex receives input from senses other than touch. Any visual information you are receiving now is going to the visual cortex in your occipital lobes, at the back of your brain (Figures 12.5 and 12.6). If you have normal vision, you might see flashes of light or dashes of color if stimulated in your occipital lobes. (In a sense, we do have eyes in the back of our head!) Having lost much of his right occipital lobe to a tumor removal, a friend of mine [DM’s] was blind to the left half of his field of vision. Visual information travels from the occipital lobes to other areas that specialize in tasks such as identifying words, detecting emotions, and recognizing faces.
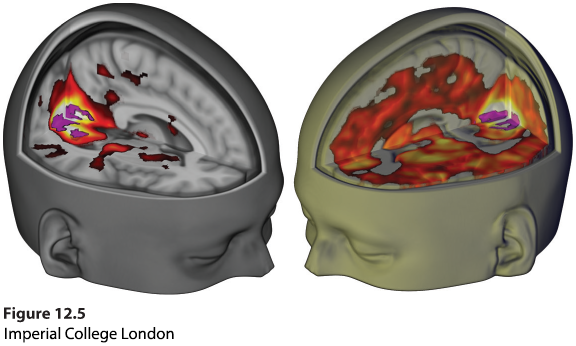
Figure 12.5 Seeing without eyes
The psychoactive drug LSD often produces vivid hallucinations. Why? It dramatically increases communication between the visual cortex in the occipital lobe and other brain regions. The fMRI (functional MRI) scan above right shows, a research participant with closed eyes who is under the influence of LSD (color represents increased bloodflow). The fMRI above left shows the same person when given a placebo (Carhart-Harris et al., 2016; Cormier, 2016).
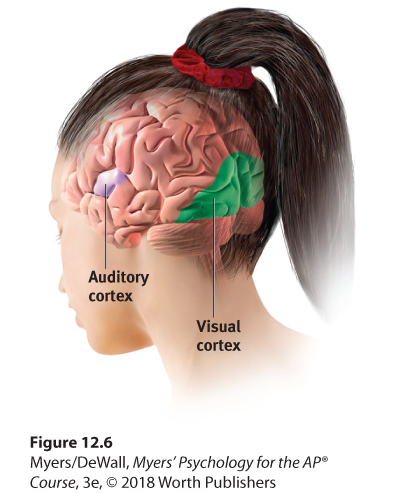
Figure 12.6 The visual cortex and auditory cortex
The visual cortex in the occipital lobes at the rear of your brain receives input from your eyes. The auditory cortex in your temporal lobes—above your ears—receives information from your ears.
Any sound you now hear is processed by your auditory cortex in your temporal lobes (just above your ears; see Figure 12.6). Most of this auditory information travels a circuitous route from one ear to the auditory receiving area above your opposite ear. If stimulated in your auditory cortex, you might hear a sound. MRI scans of people with schizophrenia have revealed active auditory areas in the temporal lobes during the false sensory experience of auditory hallucinations (Lennox et al., 1999). Even the phantom ringing sound experienced by people with hearing loss is—if heard in one ear—associated with activity in the temporal lobe on the brain’s opposite side (Muhlnickel, 1998).
Association Areas
So far, we have pointed out small cortical areas that either receive sensory input or direct muscular output. Together, these occupy about one-fourth of the human brain’s thin, wrinkled cover. What, then, goes on in the remaining vast regions of the cortex? In these association areas (identified in the Thinking Critically About box below), neurons are busy with higher mental functions—many of the tasks that make us human. Electrically probing an association area won’t trigger any observable response. So, unlike the somatosensory and motor areas, association area functions cannot be neatly mapped. Does this mean we don’t use them? See Thinking Critically About: Using More Than 10 Percent of Our Brain.
Association areas are found in all four lobes. The prefrontal cortex in the forward part of the frontal lobes enables judgment, planning, and processing of new memories (de la Vega et al., 2016). People with damaged frontal lobes may have high intelligence test scores and great cake-baking skills. Yet they would not be able to plan ahead to begin baking a cake for a birthday party (Huey et al., 2006). And if they did begin to bake, they might forget the recipe (MacPherson et al., 2016).
Frontal lobe damage also can alter personality and remove a person’s inhibitions. Consider the classic case of railroad worker Phineas Gage. One afternoon in 1848, Gage, then 25 years old, was using a tamping iron to pack gunpowder into a rock. A spark ignited the gunpowder, shooting the rod up through his left cheek and out the top of his skull, leaving his frontal lobes damaged (Figure 12.7). To everyone’s amazement, Gage was immediately able to sit up and speak, and after the wound healed, he returned to work. But having lost some of the neural tracts that enabled his frontal lobes to control his emotions, the affable, soft-spoken man was now irritable, profane, and dishonest (Van Horn et al., 2012). This person, said his friends, was “no longer Gage.” His mental abilities and memories were unharmed, but his personality was not. Gage later lost his railroad job, but over time, he adapted to his injury and found work as a stagecoach driver (Macmillan & Lena, 2010).
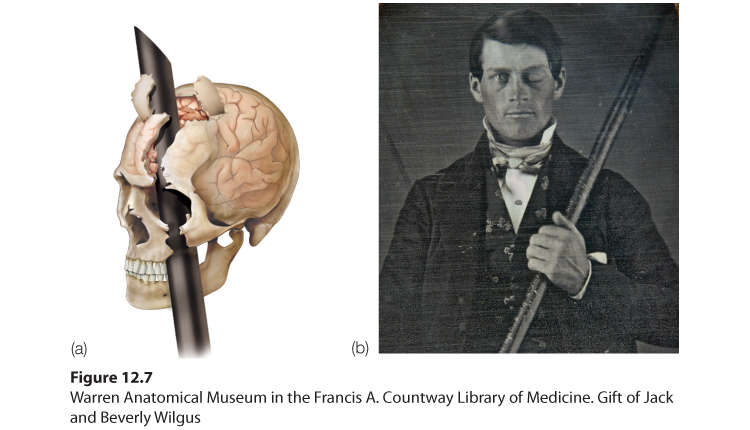
Figure 12.7 A blast from the past
(a) Phineas Gage’s skull was kept as a medical record. Using measurements and modern neuroimaging techniques, researchers have reconstructed the probable path of the rod through Gage’s brain (Van Horn et al., 2012). (b) This photo shows Gage after his accident. (The image has been reversed to show the features correctly. Early photos, including this one, were actually mirror images.)

Studies of others with damaged frontal lobes have revealed similar impairments. Not only may they become less inhibited (without the frontal lobe brakes on their impulses), but their moral judgments may seem unrestrained. Cecil Clayton lost 20 percent of his left frontal lobe in a 1972 sawmill accident. Thereafter, his intelligence test score dropped to an elementary school level and he displayed increased impulsivity. In 1996, he fatally shot a deputy sheriff. In 2015, when he was 74, the State of Missouri executed him (Williams, 2015).
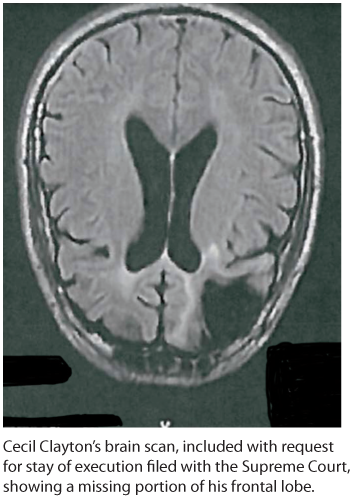
Missing frontal lobe brakes With part of his left frontal lobe (in this downward-facing brain scan) lost to injury, Cecil Clayton became more impulsive and killed a deputy sheriff. Nineteen years later, his state executed him for this crime.
Would you advocate pushing one person in front of a runaway trolley to save five others? Most people would not, but those with damage to the prefrontal cortex are often untroubled by such ethical dilemmas (Koenigs et al., 2007). The frontal lobes help steer us away from violent actions (Molenberghs et al., 2015; Yang & Raine, 2009). With their frontal lobes ruptured, people’s moral compass seems to disconnect from their behavior.
Association areas also perform other mental functions. The parietal lobes, parts of which were large and unusually shaped in Einstein’s normal-weight brain, enable mathematical and spatial reasoning (Burrell, 2015; Ibos & Freedman, 2014). Stimulation of one parietal lobe area in brain-surgery patients produced a feeling of wanting to move an upper limb, the lips, or the tongue without any actual movement. With increased stimulation, patients falsely believed they had moved. Curiously, when surgeons stimulated a different association area near the motor cortex in the frontal lobes, the patients did move but had no awareness of doing so (Desmurget et al., 2009). These head-scratching findings suggest that our perception of moving flows not from the movement itself but rather from our intention and the results we expected.
On the underside of the right temporal lobe, another association area enables us to recognize faces. If a stroke or head injury destroyed this area of your brain, you would still be able to describe facial features and to recognize someone’s gender and approximate age, yet be strangely unable to identify the person as, say, Taylor Swift, or even your grandmother.
Nevertheless, to reemphasize, we should be wary of using pictures of brain “hot spots” to create a new phrenology that locates complex functions in precise brain areas (Beck, 2010; Shimamura, 2010; Uttal, 2001). During a complex task, a brain scan shows many islands of brain activity working together—some running automatically in the background, and others under conscious control (Chein & Schneider, 2012).
Similarly, the acquisition, development, and use of language depends on both specialized neural networks and their integration. Nineteenth-century research by French physician Paul Broca and German investigator Carl Wernicke led to the discovery of specialized language brain areas. Damage to Broca’s area disrupts speaking, while damage to Wernicke’s area disrupts understanding. Today’s neuroscience, however, has shown that language functions are distributed across other brain areas as well.
Your memory, language, and attention result from the synchronized activity among distinct brain areas and neural networks (Knight, 2007). Ditto for religious experience. More than 40 distinct brain regions become active in different religious states, such as prayer and meditation, indicating that there is no simple “God spot” (Fingelkurts & Fingelkurts, 2009). The point to remember: Our mental experiences arise from coordinated brain activity.