Biological Constraints on Conditioning
Ever since Charles Darwin, scientists have assumed that all animals share a common evolutionary history and thus share commonalities in their makeup and functioning. Pavlov and Watson, for example, believed the basic laws of learning were essentially similar in all animals. So it should make little difference whether one studied pigeons or people. Moreover, it seemed that any natural response could be conditioned to any neutral stimulus.
Biological Limits on Classical Conditioning
 Flip It Video: Garcia’s Research
Flip It Video: Garcia’s Research
In 1956, learning researcher Gregory Kimble proclaimed, “Just about any activity of which the organism is capable can be conditioned and . . . these responses can be conditioned to any stimulus that the organism can perceive” (p. 195). Twenty-five years later, he humbly acknowledged that “half a thousand” scientific reports had proven him wrong (Kimble, 1981). More than the early behaviorists realized, an animal’s capacity for conditioning is limited by biological constraints. For example, each species’ predispositions prepare it to learn the associations that enhance its survival—a phenomenon called preparedness. Environments are not the whole story. Biology matters.
John Garcia was among those who challenged the prevailing idea that all associations can be learned equally well. While researching the effects of radiation on laboratory animals, Garcia and Robert Koelling (1966) noticed that rats began to avoid drinking water from the plastic bottles in radiation chambers. Could classical conditioning be the culprit? Might the rats have linked the plastic-tasting water (a CS) to the sickness (UR) triggered by the radiation (US)?

John Garcia As the laboring son of California farmworkers, Garcia attended school only in the off-season during his early childhood years. After entering junior college in his late twenties, and earning his Ph.D. in his late forties, he received the American Psychological Association’s Distinguished Scientific Contribution Award “for his highly original, pioneering research in conditioning and learning.” He was also elected to the National Academy of Sciences.
To test their hunch, Garcia and Koelling exposed the rats to a particular taste, sight, or sound (CS) and later also to radiation or drugs (US) that led to nausea and vomiting (UR). Two startling findings emerged: First, even if sickened as late as several hours after tasting a particular novel flavor, the rats thereafter avoided that flavor. This appeared to violate the notion that for conditioning to occur, the US must immediately follow the CS.
Second, the sickened rats developed aversions to tastes but not to sights or sounds. This contradicted the behaviorists’ idea that any perceivable stimulus could serve as a CS. But it made adaptive sense. For rats, the easiest way to identify tainted food is to taste it; if sickened after sampling a new food, they thereafter avoid it. This response, called taste aversion, makes it difficult to eradicate a population of “bait-shy” rats by poisoning.

Taste aversion If you became violently ill after eating oysters, you would probably have a hard time eating them again. Their smell and taste would have become a CS for nausea. This learning occurs readily because our biology prepares us to learn taste aversions to toxic foods.
Humans, too, seem biologically prepared to learn some associations rather than others. If you become violently ill four hours after eating contaminated oysters, you will probably develop an aversion to the taste of oysters more readily than to the sight of the associated restaurant, its plates, the people you were with, or the music you heard there. (In contrast, birds, which hunt by sight, appear biologically primed to develop aversions to the sight of tainted food [Nicolaus et al., 1983].)
Garcia’s early findings on taste aversion were met with an onslaught of criticism. As the German philosopher Arthur Schopenhauer (1788–1860) once said, important ideas are first ridiculed, then attacked, and finally taken for granted. Leading journals refused to publish Garcia’s work: The findings are impossible, said some critics. But, as sometimes happens in science, Garcia and Koelling’s oft-replicated taste-aversion research is now basic textbook material.
“ Once bitten, twice shy.”
G. F. Northall, Folk-Phrases, 1894
It is also a good example of experiments that begin with the discomfort of some laboratory animals and end by enhancing the welfare of many others. In one conditioned taste-aversion study, coyotes and wolves were tempted into eating sheep carcasses laced with a sickening poison. Thereafter, they developed an aversion to sheep meat; two wolves later penned with a live sheep seemed actually to fear it (Gustavson et al., 1974, 1976). These studies not only saved the sheep from their predators, but also saved the sheep-shunning coyotes and wolves from angry ranchers and farmers who had wanted to destroy them. Similar applications have prevented baboons from raiding African gardens, raccoons from attacking chickens, and ravens and crows from feeding on crane eggs. In all these cases, research helped preserve both the prey and their predators, all of which occupy an important ecological niche (Dingfelder, 2010; Garcia & Gustavson, 1997).
Such research supports Darwin’s principle that natural selection favors traits that aid survival. Our ancestors who readily learned taste aversions were unlikely to eat the same toxic food again and were more likely to survive and leave descendants. Nausea, like anxiety, pain, and other bad feelings, serves a good purpose. Like a low-oil warning on a car dashboard, each alerts the body to a threat (Davidson & Riley, 2015; Neese, 1991).
“ All animals are on a voyage through time, navigating toward futures that promote their survival and away from futures that threaten it. Pleasure and pain are the stars by which they steer.”
Psychologists Daniel T. Gilbert and Timothy D. Wilson, “Prospection: Experiencing the Future,” 2007
Our preparedness to associate a CS with a US that follows predictably and immediately is adaptive. Causes often do immediately precede effects. But as we saw in the taste-aversion findings, our predisposition to associate an effect with a preceding event can trick us. When chemotherapy triggers nausea and vomiting more than an hour following treatment, cancer patients may over time develop classically conditioned nausea (and sometimes anxiety) to the sights, sounds, and smells associated with the clinic (Figure 29.2) (Hall, 1997). Merely returning to the clinic’s waiting room or seeing the nurses can provoke these conditioned feelings (Burish & Carey, 1986; Davey, 1992). Under normal circumstances, such revulsion to sickening stimuli would be adaptive.
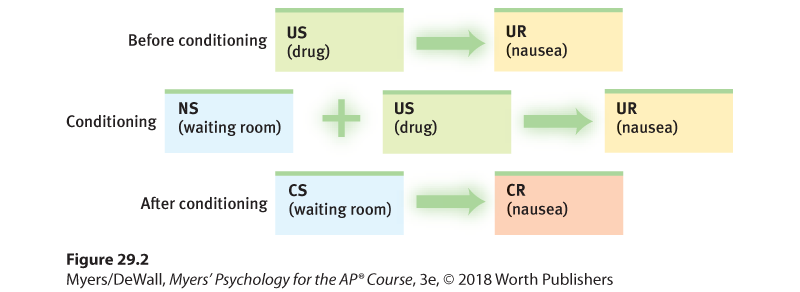
Figure 29.2 Nausea conditioning in cancer patients

Animal taste aversion As an alternative to killing wolves and coyotes that preyed on sheep, some ranchers have sickened the animals with lamb laced with a drug to create a taste aversion.
Biological Limits on Operant Conditioning
Nature also constrains each species’ capacity for operant conditioning. Science fiction writer Robert Heinlein (1907–1988) said it well: “Never try to teach a pig to sing; it wastes your time and annoys the pig.”
We most easily learn and retain behaviors that reflect our biological predispositions. Thus, using food as a reinforcer, you could easily condition a hamster to dig or to rear up, because these are among the animal’s natural food-searching behaviors. But you won’t be so successful if you use food as a reinforcer to shape face washing and other hamster behaviors that aren’t normally associated with food or hunger (Shettleworth, 1973). Similarly, you could easily teach pigeons to flap their wings to avoid being shocked, and to peck to obtain food: Fleeing with their wings and eating with their beaks are natural pigeon behaviors. However, pigeons would have a hard time learning to peck to avoid a shock, or to flap their wings to obtain food (Foree & LoLordo, 1973). The principle: Biological constraints predispose organisms to learn associations that are naturally adaptive.
In the early years of their work, animal trainers Marian Breland and Keller Breland presumed that operant principles would work on almost any response an animal could make. But along the way, they too learned about biological constraints. In one act, pigs trained to pick up large wooden “dollars” and deposit them in a piggy bank began to drift back to their natural ways. They dropped the coin, pushed it with their snouts as pigs are prone to do, picked it up again, and then repeated the sequence—delaying their food reinforcer. This instinctive drift occurred as the animals reverted to their biologically predisposed patterns.

Natural athletes Animals can most easily learn and retain behaviors that draw on their biological predispositions, such as horses’ inborn ability to move around obstacles with speed and agility.