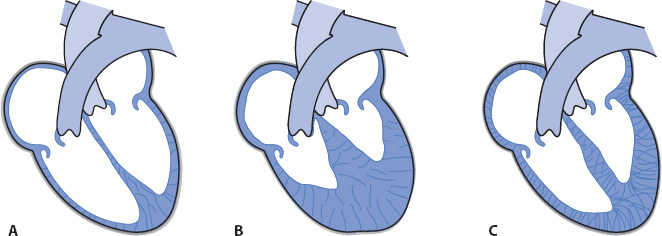
Figure 19-1. Types of cardiomyopathies. (A) Dilated (cardiac dilatation and impaired contractility). (B) Hypertrophic (decreased size of ventricular chambers and increased ventricular muscle mass). (C) Restrictive (decreased ventricular compliance).
KNOWLEDGE COMPETENCIES
1. Describe the etiology, pathophysiology, clinical presentation, patient needs, and principles of management of:
• Cardiomyopathy
• Valvular disease
• Pericarditis
• Aortic aneurysm
• Cardiac transplantation
2. Compare and contrast the pathophysiology, clinical presentation, patient needs, and management approaches of:
• Cardiomyopathy
• Valvular disease
• Pericarditis
• Aortic aneurysm
• Cardiac transplantation
3. Identify indications for, complications of, and nursing management of patients receiving intra-aortic balloon pump and ventricular assist device therapy.
Cardiomyopathy is a disease involving destruction of the cardiac muscle fibers, causing impairment of myocardial function and decreased cardiac output (CO). The body responds to this with initiation of several neuroendocrine responses including activation of the sympathetic nervous system and renin-angiotensin-aldosterone chain. The prevailing result is marked vasoconstriction, retention of sodium and water, and further myocyte injury. This process contributes to remodeling of ventricular myocytes and the downward spiral of cardiomyopathy. The cause of cardiomyopathy is often unknown. Cardiomyopathies are commonly classified into three types: dilated, hypertrophic, and restrictive (Figure 19-1).

Figure 19-1. Types of cardiomyopathies. (A) Dilated (cardiac dilatation and impaired contractility). (B) Hypertrophic (decreased size of ventricular chambers and increased ventricular muscle mass). (C) Restrictive (decreased ventricular compliance).
Dilated cardiomyopathy, the most common type of cardiomyopathy, is commonly caused by coronary artery disease and is associated with impaired myocardial contractility and increased ventricular filling pressures. Coronary artery disease contributes to ventricular remodeling thereby reducing ejection fraction. The two case studies presented later in this chapter involve patients with dilated cardiomyopathy. Hypertrophic cardiomyopathy may occur in both the young and the elderly. Hypertrophic cardiomyopathy is often categorized as obstructive or nonobstructive. Ventricular hypertrophy occurs in both types. The diagnosis of obstructive hypertrophic cardiomyopathy is made if hypertrophy of the intraventricular septum is also present. This is the congenital form and is often referred to as hypertrophic obstructive cardiomyopathy (HOCM). In the past other terms used to describe this type of cardiomyopathy were idiopathic hypertrophic subaortic stenosis (IHSS), and asymetric septal hypertrophy (ASH). The hypertrophied septum obstructs left ventricular outflow tract just below the aortic valve, thereby limiting ejection. Blood volume is “trapped” within the left ventricular chamber.
Restrictive cardiomyopathy is the least common of the three types. A classic finding for this type of cardiomyopathy is ventricular fibrosis caused by infiltration of the cardiac myocytes with abnormal cells such as sarcoid or amyloid disease. The fibrotic muscle tissue becomes very rigid with decreased compliance, thus limiting distention during diastole.
A variety of conditions may cause or contribute to the development of cardiomyopathy (Table 19-1). As noted previously, coronary artery disease is the most common cause of dilated cardiomyopathy in the United States.
TABLE 19-1. ETIOLOGY OF CARDIOMYOPATHY

Dilated cardiomyopathy begins with gradual destruction of the myocardial fibers impairing myocardial contraction. As the disease progresses, left ventricular dilatation occurs with increased blood volume in the left ventricle at the end of diastole. Additionally, ventricular compliance is reduced, which contributes to an increase in filling pressures (ie, left ventricular end diastolic pressure) and a decrease in CO. Left atrial volume and pressure eventually increase as the atrium struggles to overcome the higher LVEDP and eject blood into the left ventricle. The increased left atrial pressure often leads to an increased pulmonary capillary pressure as the higher filling pressures are reflected back into the pulmonary vascular bed. Right ventricular failure will eventually occur as the right ventricle has a limited capacity to increase its force of contraction against the higher pressures in the pulmonary vascular bed. Eventually, the right ventricle will dilate along with the left ventricle. In addition the atrioventricular valves (mitral and tricuspid) may develop insufficiency due to the dilated chambers stretching the papillary muscle and interfering with the closure of the valves.
Patients with hypertrophic cardiomyopathy have a greatly thickened ventricular wall (see Figure 19-1). It is not uncommon for the ventricular chamber size to be dramatically reduced due to hypertrophy. In obstructive cardiomyopathy, the intraventricular septum is also involved in the hypertrophic process, whereas in nonobstructive cardiomyopathy the septum is relatively normal. Common causes of the nonobstructive form include aortic stenosis and hypertension. The hypertrophied ventricle becomes rigid, causing a reduced ventricular compliance and distensibility. Myocardial contractility becomes impaired, resulting in a decreased stroke volume and CO.
If HOCM is present, left ventricular systolic ejection will be further compromised by obstruction of the outflow tract as the anterior leaflet of the mitral valve presses against an enlarged intraventricular septum.
Stress is placed on the left atrium as it attempts to propel blood forward into the stiff left ventricle. It is not uncommon for left atrial enlargement to develop as the left atrium is forced to contract against high left ventricular resistance.
The ventricles of patients with restrictive cardiomyopathy become rigid as fibrotic tissue infiltrates the myocardium. The stiffness of the ventricles decreases the compliance, or distensibility, of the ventricles, thus limiting ventricular filling and increasing end-diastolic pressures. Myocardial contractility is impaired, leading to decreases in CO. As with the other types of cardiomyopathy, atrial workload is increased as the atria attempt to propel blood forward into stiff ventricles. It is common for the atrioventricular valves to become insufficient and for the pressures in the pulmonary vascular bed and peripheral venous bed to increase, leading to the development of edema.
Patients may be asymptomatic for lengthy periods of time (months to years) prior to being diagnosed with a cardiomyopathy. By the time patients develop symptoms myocardial contractility may be significantly impaired. The heart rate increases initially as the heart attempts to maintain an adequate CO. As the disease progresses and/or during physical exertion, the impaired myocardium is no longer able to maintain an adequate CO to meet the metabolic demands of the tissues in spite of the increased heart rate.
1. Inability to maintain adequate CO:
• Fatigue
• Weakness
• Sinus tachycardia
• Pulses alternans
• Narrowed pulse pressure
• Decreased CO
2. Increased left ventricular filling pressures (LVEDP)
• Dyspnea
• Orthopnea
• Paroxysmal nocturnal dyspnea
• Crackles
• S3/S4
• Arrhythmias (atrial fibrillation, ventricular tachycardia or fibrillation)
• Systolic murmur associated with mitral valve insufficiency
• Abnormal hemodynamic profile:
• Increased pulmonary artery systolic (PAS) and pulmonary artery diastolic (PAD) pressures
• Elevated pulmonary artery occlusion pressure (PAOP)
• Increased systemic vascular resistance (SVR)
• Elevated V wave on PAOP waveform with mitral valve insufficiency
3. Increased right ventricular filling pressures:
• Peripheral edema
• Jugular vein distention (JVD)
• Hepatomegaly
• Elevated V wave on the right atrial (RA) waveform and systolic murmur associated with tricuspid valve insufficiency
4. Increased atrial pressures:
• Palpitations
• S4 may develop as the atria attempt to eject blood into stiff ventricles.
• Atrial arrhythmias may occur, such as premature atrial complexes (PACs) or atrial fibrillation (AF), due to increased atrial pressure
• Elevated A wave on PAOP waveform
• Elevated RA pressures
• Elevated A wave on the RA waveform
1. Inability to maintain adequate CO:
• Angina
• Syncope
• Fatigue
• Sinus tachycardia
• Ventricular fibrillation
• CO is initially normal, then decreases
2. Increased ventricular filling pressures:
• Dyspnea
• Orthopnea
• Arrhythmias, such as premature ventricular contractions or ventricular tachycardia
• Abnormal hemodynamic profile:
– Elevated PAS and PAD pressures
– Elevated PAOP pressure
– Increased SVR
3. Increased atrial pressure:
• S4 may develop as the atria attempt to eject blood into rigid ventricles.
• Atrial arrhythmias may occur (eg, PAC, AF) due to the increased atrial pressure.
• Palpitations.
• Elevated A wave on PAOP waveform.
• Elevated RA pressure.
4. Left outflow tract obstruction:
• Systolic murmur as blood flows through a narrowed outflow tract due to septal hypertrophy; heard at apex
Signs and symptoms of restrictive cardiomyopathy and pericarditis are similar. Diagnosis can usually be made after an echocardiogram.
1. Inability to maintain adequate CO:
• Activity intolerance
• Weakness
• Sinus tachycardia
• Arrhythmias
• Decreased CO/cardiac index (CI)
2. Increased left ventricular filling pressures:
• Dyspnea
• JVD
• S3
• Narrowed pulse pressure
• Systolic murmur with mitral valve insufficiency
• Abnormal hemodynamic profile:
• Elevated PAS, PAD, and PAOP pressures
• Elevated SVR
• Elevated V wave on PAOP waveform with mitral valve insufficiency
3. Increased right ventricular pressures:
• Peripheral edema
• Hepatomegaly
• Jaundice
• JVD
• Systolic murmur with tricuspid valve insufficiency
• Kussmaul sign (increased neck vein distention with inspiration)
• Elevated V wave on the RA waveform if tricuspid valve insufficient
4. Increased atrial pressures:
• Palpitations.
• S4 may develop as the atria attempt to eject blood into rigid ventricles.
• Atrial arrhythmias may occur (eg, PACs, AF) due to the increase in atrial pressure.
• Elevated A wave on PAOP waveform.
• Elevated RA pressure.
• Elevated A wave on the RA waveform.
• Chest x-ray: Left ventricular dilation with potential enlargement and dilatation of all four cardiac chambers
• 12-lead ECG: ST-segment and T-wave changes; left axis deviation; left ventricular hypertrophy and bundle branch block (left bundle branch block most common)
• Echocardiography: Dilated left ventricle with an increase in chamber size (other chambers may be enlarged also); diminished ventricular contractility; decreased septal wall movement; elevated ventricular volumes and decreased ejection fraction
• Chest x-ray: Normal or left atrial and ventricular hypertrophy
• 12-lead ECG: ST-segment and T-wave changes; septal Q waves due to septal hypertrophy; left ventricular hypertrophy
• Echocardiography: Thickened ventricular walls with a decrease in chamber size; left ventricular outflow obstruction created by thickened ventricular septum and motion of mitral valve leaflet
• Chest x-ray: Normal or slight enlargement of left atria and ventricle
• 12-lead ECG: ST-segment and T-wave changes; low QRS amplitude
• Echocardiography: Thickened ventricular walls; enlarged atria; diminished ventricular contractility; decreased ventricular volumes; elevated ventricular end-diastolic pressures
The primary objectives in the management of cardiomyopathy are to treat the underlying cause (if known); maximize cardiac function; assist the patient and family members to cope with a debilitating, chronic disease; and prevent complications associated with cardiomyopathy.
Dilated Cardiomyopathy
1. Improve myocardial oxygenation: As ventricular dilatation occurs, ventricular wall tension increases, increasing the myocardial workload and oxygen consumption. Oxygen therapy should be initiated as necessary to increase oxygenation delivery. Pulse oximetry, mixed venous oxygenation saturation (SVO2), and arterial blood gases are helpful in guiding sufficient oxygen therapy. If the patient has an SVO2 catheter or PA catheter, monitoring the SVO2 is also a very accurate means of assessing oxygenation status.
2. Increase myocardial contractility: Inotropic agents including β1 receptor stimulating agents (eg, dobutamine) and phosphodiesterase inhibitors (eg, milrinone) produce a positive inotropic effect (eg, strengthen myocardial contractility) and cause mild vasodilation, thereby reducing the workload of the failing ventricle.
3. Decrease preload and afterload: Diuretics decrease excess fluid and ventricular end-diastolic volumes; fluid and sodium restrictions also may be necessary. Vasodilators (eg, isosorbide dinitrate, hydralazine) dilate arterial and venous vessels, decreasing venous return and resistance to ventricular systolic ejection (afterload).
4. Administer beta blockers (eg, metoprolol, carvedilol) to reduce risk/prevent sudden cardiac death (VF, VT), as well as prevent further deterioration of the myocytes.
5. Administer ACE inhibitors or ARBs to block the negative effects of angiotensin II on the cardiac cells, as well as reduce ventricular afterload.
6. Mechanical cardiac assist devices (eg, intra-aortic balloon therapy, ventricular assist device [VAD] therapy), and in some critical situations extracorporeal membrane oxygenation (ECMO), may be instituted to assist with improving CO/CI and oxygen delivery to the tissues.
7. Dual-chamber biventricular pacemaker/implantable cardioverter defibrillator: Refer to Chapter 9, Cardiovascular System (section Improvement of Left Ventricular Function).
8. Ventricular reconstruction procedure: This is a surgical procedure focusing on removal of a ventricular aneurysm and scar tissue on the left ventricle, usually a result of a myocardial infarction (MI). The left ventricle is returned to its normal shape and is able to contract more efficiently.
9. Cardiac transplantation may be necessary if medical therapy does not relieve patient symptoms.
Hypertrophic Cardiomyopathy
The management of the patient with hypertrophic cardiomyopathy focuses on promoting myocardial relaxation and decreasing left ventricular obstruction.
1. Decrease myocardial contractility: Use beta-blockers to decrease heart rate, contractility, and myocardial oxygen consumption.
2. The following medications are usually contraindicated in patients with hypertrophic cardiomyopathy:
• Diuretics, because a decrease in fluid volume decreases ventricle filling pressures and CO.
• Inotropes (eg, dobutamine, milrinone), because an increase in contractility contributes to an increase in the left ventricular outflow obstruction.
• Vasodilators (eg, nitroglycerin, nitroprusside), because they decrease end-diastolic volume, leading to an increase in left ventricular outflow obstruction.
3. Reduce physical and psychological stress: Patients with hypertrophic cardiomyopathy are at an increased risk for sudden cardiac death, which may occur during stressful periods. It is important that strenuous physical activity be limited. In addition, sudden changes in position should be avoided, because the heart cannot respond to fluid shifts created by sudden position changes. Valsalva maneuver should also be avoided. Psychological stress should also be decreased. Teach patients strategies to enhance self-relaxation. Relaxation therapy may include rhythmic breathing, biofeedback, and imagery.
4. Cardiac surgery: Myectomy may be indicated for individuals who do not respond to medical management and have severe left ventricular outflow obstruction. Myectomy involves removal of a portion of the enlarged intraventricular septum in an attempt to decrease left ventricular outflow obstruction and improve myocardial functioning.
5. Ethanol ablation: In recent years, a new therapy for HOCM has emerged. Absolute alcohol (98% ethanol) is instilled into selected septal perforator branches of the left anterior descending coronary artery, resulting in a therapeutic MI. The resultant outcome is reduction of left ventricular outflow obstruction and improved CO. The procedure is performed in the cardiac catheterization laboratory by the interventional cardiologists. It has been found to be associated with less risk than myectomy because it is less invasive. Long-term outcomes of this procedure have yet to be determined.
Restrictive Cardiomyopathy
Decrease preload: Diuretics, sodium and fluid restrictions, and vasodilators decrease ventricular end-diastolic volumes. The rigid ventricle is very sensitive to small fluid changes, significantly increasing ventricular end-diastolic pressure.
For most patients, cardiomyopathy is a chronic, potentially life-threatening disease. Patients and their families often face an uncertain long-term prognosis. Emotions may vacillate as the family struggles to cope with the implications of the disease and its effect on lifestyle. Emphasis is placed on assisting the patient to remain active and to cope with a progressive disease. Involvement of the family unit in symptom management is also important. Relaxation therapy can benefit not only the patient, but also the family. It is important to discuss end-of-life issues as well as discuss options for palliative and/or hospice care for symptom management when it is apparent the patient is declining and all other medical options have either been tried or deemed not appropriate.
1. Arrhythmias: Continuous electrocardiogram (ECG) monitoring; observe for potential side effects of cardiac medications; encourage family to learn cardiopulmonary resuscitation (CPR).
2. Hemodynamic instability may require that the patient be managed in an ICU for invasive monitoring with insertion of a pulmonary artery catheter. The patient will be managed based on trends in hemodynamic parameters (ie, RA, PAS, PAD, and PAOP pressures; CO; CI; SVR; and PVR).
3. Thromboembolic event: Anticoagulation is necessary for patients with severely compromised left ventricular function and for patients experiencing AF. In both circumstances, thrombi may develop due to increased fluid volume and stasis.
4. Endocarditis: Antibiotic prophylaxis is recommended for patients with valve involvement. Prophylaxis should be given prior to dental work, surgery, or other invasive procedures.
Heart valvular disorders result from both congenital and acquired causes. Valves on the left side of the heart are more commonly affected because they are constantly exposed to higher pressures. Normally, when a valve opens, there are no pressure gradients between the chambers or vessels above and below the valve. As the heart valve disease progresses, pressure gradients between the two structures develop.
Heart valvular disorders are commonly classified as valve stenosis or valve insufficiency. A stenotic valve has a narrowed opening, ie, it does not open fully thereby reducing the amount of blood flowing through it. An insufficient valve does not close properly, thus permitting some blood to flow backward instead of forward. Heart valve insufficiency is also referred to as valvular regurgitation. Valvular dysfunction may affect one or more valves.
The development of valvular heart disease is usually a gradual process. As the case study below illustrates, the patient’s valvular problems began with a bacterial endocarditis 15 years prior to the onset of her symptoms of mitral valve insufficiency.
Heart valve disorders are caused by either congenital or acquired diseases (Table 19-2). Congenital valve disorders may affect any of the four valves causing stenosis or insufficiency. An example of a congenital valve disorder is an aortic valve with only two, instead of three, cusps. The bicuspid valve is associated with an increase in turbulence as blood flows through the narrowed orifice. The individual may become asymptomatic later in life when fibrotic tissue and calcium deposits form on the abnormal valve, leading to stenosis. This is often referred to as “senile aortic stenosis.”
TABLE 19-2. ETIOLOGY OF VALVULAR DISORDERS
There are three types of acquired valve disorders: degenerative disease, rheumatic disease, or infective endocarditis. Degenerative disease may occur as the valve is damaged over time due to constant mechanical stress. This may occur with aging, or may be aggravated by conditions such as hypertension. Hypertension places significant pressure on the aortic valve, often causing insufficiency.
Individuals who develop rheumatic fever often experience valvular disease years later. Rheumatic disease contributes to gradual fibrotic changes of the valve, in addition to calcification of the valve cusps. Shortening of the chordae tendineae also may occur. Rheumatic fever commonly affects the mitral valve.
Infective endocarditis may occur as a primary or secondary infection. The valve tissue is destroyed by the infectious organism. Table 19-2 lists other conditions that cause valvular heart disease.
Several processes occur that together cause stenosis or narrowing of the mitral valve orifice (Figure 19-2). Gradual fusion of the commissures (the valve leaflet edges) and fibrosis of the valve leaflets are common. In addition, calcium deposits may invade the valve leaflets, further impeding their movement. As the mitral valve becomes increasingly stenotic, the left atrium has to generate significant amounts of pressure to propel blood forward through the mitral valve and into the left ventricle. Left atrial pressures are commonly increased, with left atrial dilatation occurring as the stenosis worsens. Increased left atrial pressures may lead to increased pulmonary vascular pressures contributing to the development of right-sided ventricular failure.
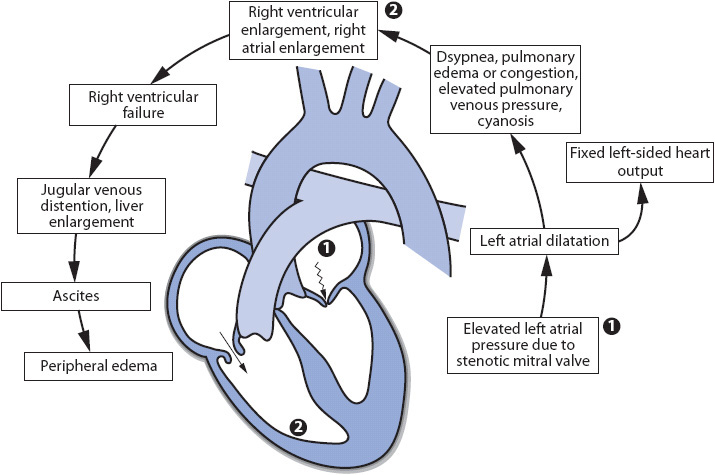
Figure 19-2. Cardiovascular effects of mitral stenosis.
Adequate closure of the mitral valve is important so that blood is ejected forward into the aorta, not backward into the left atrium, during ventricular systole. Damage to the mitral valve can affect the valve’s ability to close properly (Figure 19-3). During ventricular systole, as blood is ejected forward into the aorta, blood is also ejected backward through the insufficient mitral valve. This abnormal blood flow contributes to an increase in left atrial volume, pressure, and eventually dilatation. Increased left atrial pressures may lead to increased pulmonary vascular pressures and right-sided heart failure. The left ventricle usually dilates and hypertrophies over time as end-diastolic volumes increase and CO decreases.
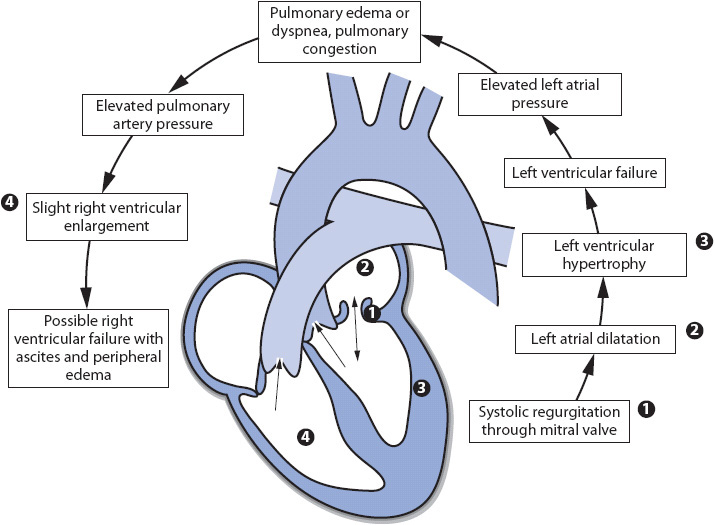
Figure 19-3. Cardiovascular effects of mitral insufficiency.
Mitral insufficiency is often associated with dilated cardiomyopathy. As the left ventricle dilates, the papillary muscles are stretched and no longer able to maintain closure of the mitral valve during ventricular systole. Acute mitral insufficiency may occur due to dysfunction or rupture of the papillary muscles. Papillary muscle contributes to preventing the valve leaflets from everting back into the left atrium during ventricular systole. Papillary muscles may rupture during an acute MI if blood supply to the tissue is diminished or absent during the infarct. Loss of a papillary muscle causes sudden, severe insufficiency of the mitral valve, resulting in rapid increase in both left ventricular and atrial volumes and pressures. The pulmonary vascular system is quickly affected by the high left-sided pressures, with pulmonary edema developing acutely. In acute mitral insufficiency, there is no time for the heart to compensate for the sudden increases in volume and pressure, as there is with longstanding mitral insufficiency.
A similar process occurs in aortic stenosis as in mitral stenosis (Figure 19-4). Fusion of the commissures, fibrosis of the valve leaflets, and calcium deposits may occur on the aortic valve leaflets, impeding their movement. When aortic stenosis presents, the left ventricle has to generate a significant amount of pressure to propel blood forward through the aortic valve into the aorta. Increased left ventricular pressure contributes to left ventricular dilatation and hypertrophy, as well as decreases in CO. Left atrial volume and pressure may increase as the left atrium must generate more pressure to eject blood into the left ventricle. Left atrial dilatation may eventually occur. The elevated left-sided pressures are reflected back into the pulmonary vascular system and to the right side of the heart, eventually causing right heart failure.
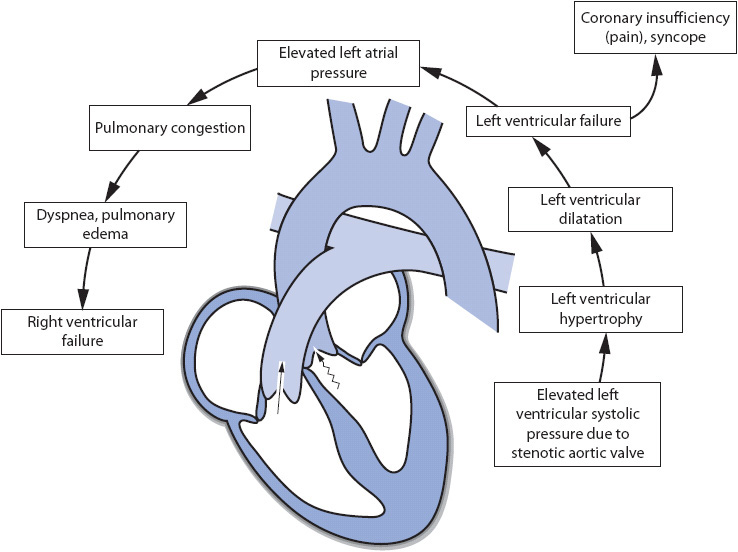
Figure 19-4. Cardiovascular effects of aortic stenosis.
A similar process also occurs in aortic insufficiency as in mitral insufficiency (Figure 19-5). Adequate closure of the aortic valve is even more important than adequate closure of the mitral valve. If the aortic valve does not close properly, blood flows backward from the aorta into the left ventricle during diastole. This can seriously affect forward blood flow into the aorta, and thus CO. This causes significant increases in the volume and pressure of the left ventricle contributing to the gradual development of left ventricular dilatation and hypertrophy. As with other left-sided valvular disease, pulmonary vascular pressures increase contributing to the development of right heart failure.
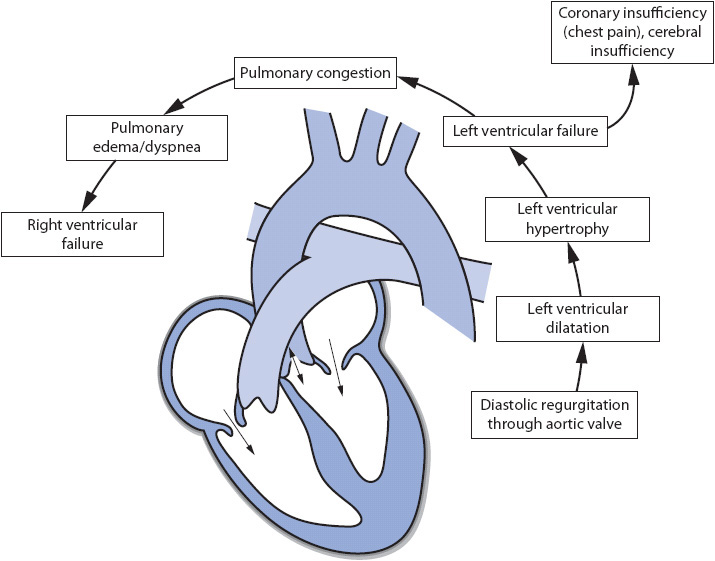
Figure 19-5. Cardiovascular effects of aortic insufficiency.
Fused commissures or fibrosis of the valve leaflets may also narrow the tricuspid valve orifice. RA pressures increase as the right atrium attempts to propel blood forward into the right ventricle. Eventually, RA dilatation occurs and the increased right atrial pressure is reflected back into the venous system.
Damage to the tricuspid valve that prevents complete closure during ventricular systole causes the abnormal ejection of blood through the tricuspid valve into the right atrium. Right atrial volumes and pressures increase, eventually leading to dilatation and possible decreases in CO. In recent years tricuspid insufficiency commonly occurs with dilated cardiomyopathy. As the right ventricle dilates, the papillary muscles are stretched and are unable to maintain closure of the valve during ventricular systole. This frequently accompanies mitral insufficiency.
Pulmonic stenosis develops as the pulmonic valve orifice becomes narrowed. Right ventricular pressures increase as the right ventricle attempts to eject blood forward into the pulmonary artery. Over time, right ventricular dilatation may occur, with decreases in right-sided CO. The increased pressure may back up into the right atrium, causing an increase in volume and pressure, and eventually leading to dilatation. This can lead to volume and pressure increases in the venous system.
Closure of the pulmonic valve prevents blood from backing up from the pulmonary artery into the right ventricle during diastole. An insufficient pulmonic valve permits blood to flow backward into the right ventricle during diastole. Right-sided CO decreases as blood flows backward instead of forward. An increase in right ventricular volume and pressure occurs, which may eventually lead to dilatation. The increased pressures may be reflected back into the right atrium and the venous system. Pulmonic stenosis and insufficiency are rarely seen in adults and are much more common in children. They are usually caused by a congenital defect.
The following signs and symptoms are found in all of the valvular disorders of the left side of the heart:
• Dyspnea
• Fatigue
• Increased pulmonary artery pressures (PAS, PAD, PAOP)
• Decreased CO
Mitral Stenosis
• Palpitations
• Hemoptysis
• Hoarseness
• Dysphagia
• JVD
• Orthopnea
• Cough
• Diastolic murmur
• Atrial arrhythmias (PACs, AF)
• Elevated A wave on PAOP pressure waveform
Mitral Insufficiency
• Paroxysmal nocturnal dyspnea
• Orthopnea
• Palpitations
• S3 and/or S4
• Crackles
• Systolic murmur
• Atrial arrhythmias
• Elevated V wave on PAOP pressure waveform
Aortic Stenosis
• Angina
• Syncope
• Decreased SVR
• S3 and/or S4
• Systolic murmur
• Narrowed pulse pressure
Aortic Insufficiency
• Angina
• S3
• Diastolic murmur
• Widened pulse pressure
• de Musset sign (nodding of the head)
The following signs and symptoms are found in all of the valvular disorders of the right side of the heart:
• Dyspnea
• Fatigue
• Increased RA pressures
• Peripheral edema
• Hepatomegaly
• JVD
Tricuspid Stenosis
• Atrial arrhythmias
• Diastolic murmur
• Decreased CO
• Elevated A wave on RA pressure waveform
Tricuspid Insufficiency
• Conduction delays
• Supraventricular tachycardia
• Systolic murmur
• Elevated V wave on RA pressure waveform
Pulmonic Stenosis
• Cyanosis
• Systolic murmur
• Elevated A wave on RA pressure waveform
Pulmonic Insufficiency
• Diastolic murmur
• Elevated A wave on RA pressure waveform
• Chest x-ray: Shows specific cardiac chamber enlargement, pulmonary congestion, presence of valve calcification
• 12-lead ECG: Useful in the diagnosis of right ventricular, left ventricular, and left atrial hypertrophy
• Echocardiogram: Demonstrates the size of the four cardiac chambers, presence of hypertrophy, specific valve dysfunction, ejection fraction, and amount of regurgitant flow, if present
• Radionuclide studies: Identify abnormal ejection fraction during inactivity and activity
• Cardiac catheterization: Determines cardiac chamber pressures, ejection fraction, regurgitation, and pressure gradients, if present
The primary objectives in the management of valvular disorders are to maximize cardiac function, reduce anxiety, and prevent complications.
Medical Management
1. Improve oxygenation delivery: As ventricular dilatation occurs, there is an increase in ventricular wall tension, myocardial workload, and oxygen consumption. Oxygen therapy should be initiated, as necessary, to increase oxygen saturation. Pulse oximetry, arterial blood gases, and mixed venous oxygenation saturation (SVO2) monitoring in the ICU are helpful in guiding sufficient oxygen therapy.
2. Decrease preload: Diuretics decrease excess fluid and ventricular end-diastolic volumes. Fluid and sodium restrictions also may be necessary. (Exception: Preload usually is not decreased in patients with aortic insufficiency, because decreased left ventricular end-diastolic volumes may accentuate decreases in CO.)
3. Decrease afterload: Afterload reduction may be indicated for patients with increased SVR and impaired left ventricular function (eg, aortic stenosis or mitral insufficiency).
4. Improve contractility: Inotropic agents (eg, dobutamine, milrinone) increase myocardial contractility and improve CO.
5. Modify activity: Activity limitation helps reduce myocardial oxygen consumption. Teach patients the importance of rest between activities.
6. Balloon valvuloplasty may be an option for stenotic mitral or aortic valves: A percutaneous catheter is inserted via the femoral artery under fluoroscopy and the balloon is inflated at the stenotic lesion in an effort to force open the fused commissures and improve valve leaflet mobility.
Cardiac surgery is indicated when medical management does not alleviate patient symptoms. Patients may have better surgical outcomes if surgery is done prior to left ventricular dysfunction.
1. Valve repair: An increasing trend today is to have dysfunctional valves repaired instead of replaced. The hemodynamic function of the inherent valve is superior to any prosthetic valve. In addition, the risks associated with valve replacement are avoided. An open commissurotomy may be performed to relieve stenosis of any of the four heart valves. During open commissurotomy, the fused commissures are incised, thus mobilizing the valve leaflets. Valve leaflet reconstruction also may be done to patch tears in valve leaflets using pericardial patches for the repair. Chordae tendineae reconstruction may be performed to elongate fibrotic tendineae or to shorten excessively stretched tendineae. An annuloplasty ring may also be inserted to correct dilatation of the valve annulus.
2. Prosthetic valve replacement: Replacement of the native valve with a prosthetic, or artificial, valve is done for severely damaged valves or when repair is not possible. The entire native valve is removed and replaced with a mechanical or biological (porcine, bovine, or allograft [homograft or autograft]) prosthetic valve.
3. Postoperative management after cardiac surgery is similar to coronary artery bypass surgery management (see Chapter 9, Cardiovascular System). Special considerations for patients having valve repair or replacement include the following:
• Maintain adequate preload: Patients with valvular heart disease are usually accustomed to increased end-diastolic volumes. Although the valve is repaired, the heart needs time to adjust to the hemodynamic changes. Most patients do better in the postoperative phase if fluids are adjusted based on presurgical RA and PAOP pressures.
• Monitor for conduction disturbances: The mitral, tricuspid, and aortic valves lie in close proximity to conduction pathways. Conduction disorders may be treated by temporary or permanent cardiac pacing.
• Initiate anticoagulation therapy: Anticoagulation therapy is usually initiated for patients having valve replacement after the epicardial pacing wires are removed. This may be as early as the first postoperative day.
4. If the patient had AF or flutter pre-operatively, the surgeon may perform a Maze procedure, ablating the area around the pulmonary veins in an effort to prevent return of the atrial dysrhythmia post-operatively.
5. Transcatheter Aortic Valve Replacement (TAVR): In recent years, technological advances have allowed for the development of a minimally-invasive approach to aortic valve replacement. This approach introduces a prosthetic tissue valve into place via a stent-like introducer catheter. The valve may be inserted through the femoral artery and placed across the native aortic valve. Another approach is trans-apical where a small incision is made in the anterior chest wall and the device is deployed through the left ventricular apex into the aortic valve position. Both approaches avoid the use of cardio-pulmonary bypass. Currently, this procedure is limited to patients who are older (eighth or ninth decade) and who are too debilitated to tolerate the traditional surgical approaches to aortic valve replacement. The patient often experiences near immediate relief of symptoms and is discharged home within a couple of days.
6. An evolving method is the use of a transaortic approach. In this approach a mini-sternotomy is performed and the valve is accessed via the aorta. The benefit of this approach may include a decreased risk of postoperative bleeding than the transapical approach.
Teach the patient relaxation techniques. Deep breathing or imagery may help alleviate anxiety especially when symptoms of valve dysfunction occur.
1. Arrhythmias: Continuous ECG monitoring; observe for side effects of specific cardiac medications.
2. Hemodynamic instability: Pulmonary artery pressure (PAP) monitoring, manage patient based on trends in hemodynamic monitoring.
3. Thromboembolic event: Anticoagulation is necessary for patients with severely compromised left ventricular function or AF, and after valve surgery. Lifelong anticoagulation therapy is indicated for patients after mechanical valve replacement. Short-term anticoagulation therapy is usually initiated for patients having a biological valve replacement.
4. Endocarditis: Antibiotic prophylaxis is recommended for patients with valve disorders and for patients with prosthetic valves. Prophylaxis should be given prior to dental work, surgery, or other invasive procedures. Prior to discharge, teach the patient and family the importance of prophylaxis.
5. Prosthetic valve dysfunction: Biological valve dysfunction usually develops slowly with gradual signs and symptoms (eg, presence of a new murmur, dyspnea, syncope). Mechanical valve dysfunction may occur slowly or suddenly. Rapid valve dysfunction requires an emergency intervention as the patient presents with signs and symptoms of acute cardiac failure (hypotension, tachycardia, low CO/CI, heart failure, cardiac arrest).
Pericarditis is a chronic or acute inflammation of the pericardial lining of the heart. Acute pericarditis usually occurs secondary to another disease process and usually resolves within 6 weeks. Chronic pericarditis, however, may last for months.
Pericarditis may lead to pericardial effusion or cardiac tamponade. Pericardial effusion occurs as fluid builds up within the pericardial sac. Cardiac tamponade can occur as the pericardial fluid compresses the heart, restricts ventricular end-diastolic filling, and compromises cardiac function.
The case study is an example of the importance of accurate diagnosis of patients with chest pain. The pain of pericarditis may be similar to anginal pain, but the treatment is very different.
A number of different conditions and situations can cause pericarditis (Table 19-3). Common causes include MI, infections, neoplasm, radiation therapy, and uremia.
TABLE 19-3. ETIOLOGY OF PERICARDITIS

Normally, the pericardial sac contains a small amount of clear serous fluid, typically less than 50 mL. This fluid lies between the visceral and parietal pleura and lubricates the surface of the heart as it expands and contracts. An inflammation of the pericardium causes friction between the visceral and parietal pleura.
Inflammation of the pericardium causes an increase in pericardial fluid production, with increases of up to 1 L or more. A gradual buildup of fluid may have little compromising effect on the heart as the pericardium expands and normal hemodynamics is not altered. A sudden increase in pericardial fluid, however, dramatically impairs the hemodynamic status.
Chronic pericarditis causes fibrotic changes within the pericardial lining. The visceral and parietal pleura eventually adhere to each other, restricting the filling of the heart. This condition may be referred to as constrictive pericarditis. The pressure created by the constricted pericardium affects the heart’s ability to distend properly, causing decreases in end-diastolic volume and CO. These changes may contribute to increases in ventricular end-diastolic atrial pressures, leading to increases in pulmonary vascular and venous system pressures.
• Sharp, stabbing, burning, dull, or aching pain in the substernal or precordial area, which increases with movement, inspiration, or coughing, or when the patient is in a recumbent position
• Pericardial friction rub
• Fever
• Sinus tachycardia
• Dyspnea, orthopnea
• Cough
• Fatigue
• Narrowed pulse pressure
• Hypotension
• Arrhythmias
• Elevated cardiac pressures (PA, PAOP, RA)
• Decreased CO
• Peripheral edema
• JVD
• Dyspnea
• Anorexia
• Fatigue
• Abdominal discomfort
• Weight gain
• Activity intolerance
• JVD
• Peripheral edema
• Hepatomegaly
• Kussmaul sign (increase in RA pressure during inspiration)
• Chest x-ray: Normal or enlarged heart; chronic pericarditis may reveal a decrease in heart size.
• ECG: ST-segment elevation in precordial leads (V leads) and leads I, II, or III; T-wave inversion after ST segment returns to isoelectric line; decrease in QRS voltage.
• Echocardiogram: Presence of increased fluid in pericardial sac; chronic, constrictive pericarditis may demonstrate a thickened pericardium and diminished ventricular contractility.
• Laboratory: Elevated sedimentation rate and elevated WBC (white blood cell); causative organisms may be identified from blood cultures.
• CT/MRI scan: Detects a thickened pericardium for patients with chronic pericarditis.
The primary principles of management of pericarditis are to correct the underlying cause, relieve pain and promote comfort, relieve pericardial effusion, and prevent and manage complications associated with pericarditis.
Promoting Comfort and Relieving Pain
1. Decrease pain: Teach the patient that chest pain may be decreased or relieved by sitting up and/or leaning forward. Analgesics (eg, aspirin) and nonsteroidal anti-inflammatory agents administered around the clock assist in pain relief.
2. Promote relaxation: Teach the patient relaxation techniques such as progressive muscle relaxation and visualization. This may assist the patient to cope. Relaxation techniques that include deep breathing should be avoided because pericardial pain usually increases with deep inspiration.
3. Limit activity: This is especially important during the acute period of inflammation. Activity can be gradually increased as fever and chest pain decrease. Assist patients to find a position of comfort. Patients often are more comfortable sitting up and leaning slightly forward.
Correcting the Underlying Cause
1. Decrease pericardial inflammation: Nonsteroidal anti-inflammatory agents (eg, indomethacin, ibuprofen) assist to decrease inflammation of the pericardium and the associated pain. Chronic, recurrent pericarditis may require corticosteroid therapy.
2. Eliminate infection: If the cause of the pericarditis is an infectious process, appropriate medications, including antibiotic therapy, are necessary.
Relieving Pericardial Effusion
1. Pericardiocentesis: A needle or small catheter is introduced subxyphoid into the pericardial sac and fluid is withdrawn via the needle or is attached to a catheter and drained into a vacuum bottle. This procedure is performed to remove the excess fluid in the pericardium to improve myocardial function. Culture specimens of the drained fluid should be obtained and sent to the laboratory for analysis. The drain may be left in for several days until the volume of drainage is minimal.
2. Pericardiotomy/pericardial window: This is a surgical procedure in which a section of the pericardium is removed in an effort to decrease pericardial pressure on the heart and to allow pericardial fluid to drain more readily. Often a drain may be inserted into the pericardium and tunneled down across the diaphragm into the peritoneal cavity. This permits the excess fluid to drain continuously into the peritoneal space where it is eventually absorbed into the lymph system. It may be performed for recurrent pericardial effusions.
3. Pericardiectomy: This involves surgically removing the entire pericardium. This may be necessary for chronic pericarditis that is refractory to other interventions.
Preventing and Managing Complications
1. Monitor for signs and symptoms of acute heart failure: These include hypotension; tachycardia; increased respiratory rate; extreme dyspnea; decreased oxygen saturation; decreased peripheral pulses; and decreased urinary output. Oxygen therapy and inotropic agents assist in improvement of myocardial contractility. Assessment of the need for surgical intervention for pericarditis may be indicated.
2. Cardiac tamponade: Monitor for signs and symptoms of cardiac tamponade. These include hypotension, tachycardia, tachypnea, dyspnea, pulsus paradoxus, narrowed pulse pressure, muffled heart sounds, and distended neck veins. Emergency pericardiocentesis is necessary to prevent further hemodynamic compromise.
An aortic aneurysm is an area of aortic wall dilatation. Aneurysms are most prevalent in men, commonly occurring during their early fifties to late sixties. Without treatment, mortality associated with aortic aneurysms is high.
Aneurysms frequently are classified by types (Figure 19-6). A fusiform aneurysm is characterized by distention of the entire circumference of the affected portion of the aorta. A saccular aneurysm is characterized by distention of one side of the aorta. The distention of a saccular aneurysm resembles a bulging sac. Aneurysms may also be classified according to their location (Figure 19-7):
• Ascending: Between the aortic valve and the innominate artery
• Transverse: Between the innominate artery and the left subclavian artery
• Descending: From the left subclavian artery to the diaphragm
• Thoracoabdominal: From above the diaphragm to the aortic bifurcation
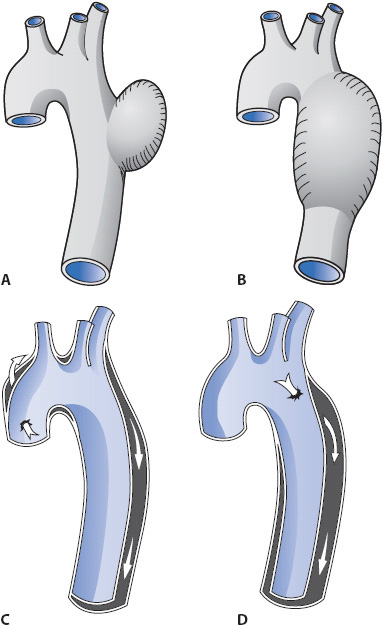
Figure 19-6. Diagram of different types of aortic aneurysms. (A) Fusiform aneurysm. (B) Saccular aneurysm. (C, D) Two aortic dissections. (From: Underhill SL, Woods SL, Sivarajan ES, Halpenny CJ. Cardiac Nursing. Philadelphia, PA. JB Lippincott; 1982: 680.)
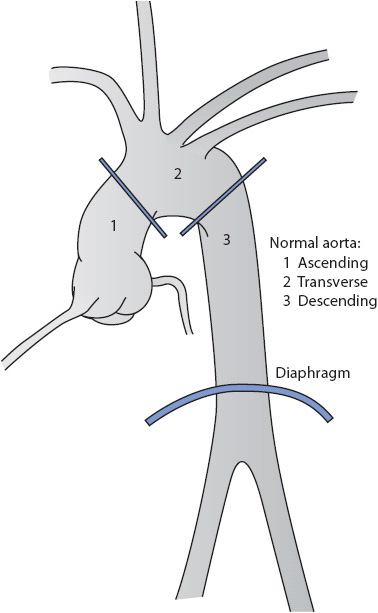
Figure 19-7. Classification of aortic aneurysms according to location. (From: Seifert PC. Cardiac Surgery. St Louis, MO: Mosby Yearbook; 1994:321.)
Aneurysms have the potential to dissect or rupture. Dissection occurs when the intimal aortic wall is disrupted and blood extends into the aortic vessel layers (Figure 19-6C and 19-6D). Rupture occurs when all three layers of the aorta are disrupted and massive hemorrhage occurs. Both dissection and rupture are life-threatening events. The case study demonstrates the sudden onset of signs and symptoms associated with aortic rupture and the emergent need for life-saving interventions.
Aortic aneurysms are caused by a variety of conditions, including atherosclerosis, cystic medial necrosis, genetic link, congenital abnormality, hypertension, Marfan’s syndrome, and trauma to the chest.
The aorta is composed of three layers: the intima, media, and tunica adventitia. Aneurysm development is initiated by degeneration of smooth muscle cells and elastic tissue in the medial layer of the aorta. This weakens the vessel wall, potentially leading to dilatation of all layers of the aorta. The aortic wall may be further weakened with age, as well as from hypertension.
As the aortic aneurysm gradually expands, there is an increase in the risk for aortic dissection. Dissection is caused by a tear in the intima. Blood leaves the central aorta via the intimal tear and flows through the medial layer of the aorta (Figure 19-6C and 19-6D). This creates a false lumen. As the amount of blood increases in the medial layer, the pressure in the false lumen increases, compressing the central aorta (Figure 19-6D). This compression may decrease or totally obstruct blood flow through the aorta and/or its arterial branches. Dissections are classified as acute if they have occurred less than 2 weeks since the onset of symptoms. They are classified as chronic if they occurred more than 2 weeks since the onset of symptoms.
Two additional classifications exist for identifying the location of aortic dissections (Figure 19-8). The first (Stanford classification) classifies the dissection as type A, involving the ascending aorta, or type B, involving the descending aorta (distal to the left subclavian artery). Type A requires immediate surgical intervention whereas type B is managed medically until surgery is deemed necessary. Another classification system for aortic dissection has three categories for the dissection: type I, the original intimal tear begins in the ascending aorta and the dissection extends to the descending aorta; type II, the original intimal tear begins and is contained in the ascending aorta; and type III, the original intimal tear begins and is contained in the descending aorta.
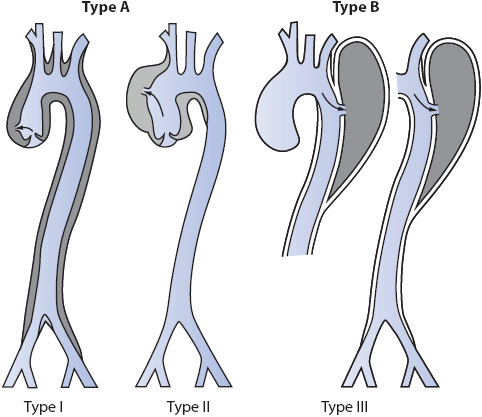
Figure 19-8. Classification for the location of aortic dissections. The Stanford system classifies aortic dissections based on involvement (type A) of the ascending aorta or noninvolvement (type B). The DeBakey system classifies dissections into types I, II, or III. (From: DeBakey ME. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:131; adapted from Seifert PC. Cardiac Surgery. St Louis, MO: Mosby Yearbook; 1994:321.)
Patients rarely demonstrate early signs of an aortic aneurysm. Diagnosis is commonly made during a routine physical examination or chest x-ray. Signs and symptoms of an aortic aneurysm occur as the aneurysm enlarges and compresses adjacent organs, structures, and/or nerve pathways.
Thoracic Aneurysm
• Ripping, tearing, or splitting pain, located at the anterior chest or posterior chest between the scapula, of an intense or excruciating nature
• Dysphagia
• Hoarseness, cough
• Dyspnea
• Different blood pressures when comparing right and left arms
• Different pulses when comparing right and left peripheral pulses
Abdominal Aneurysm
• Dull, constant abdominal or low back or lumbar pain
• Abdominal mass
• Pulsations in the abdomen
• Reduced lower extremity pulses
• Nausea and/or vomiting
Aortic Dissection
• Sudden intense pain in chest or back (or sudden increase in the intensity of pain)
• Dyspnea
• Syncope
• Abdominal discomfort or bloating
• Extremity weakness
• Oliguria or hematuria
• Hemiparesis, hemiplegia, or paraplegia
• Speech or visual disturbances
• Decreased hemoglobin and hematocrit
Aortic Rupture
• Sudden cessation of pain
• Recurrence of pain
• Signs and symptoms of shock, with the exception of blood pressure (high in rupture), including tachycardia, increased respiratory rate, pallor, moist skin, and restlessness
• Chest x-ray: Shows the dilated aorta, widening of the mediastinum, and mediastinal mass
• CT/MRI scan: Determines the size of the aorta, size of the aneurysm, extent of a dissection, involvement of additional arterial branches, lumen diameter, and wall thickness
• Echocardiogram: Can visualize the location and size of the aneurysm,
• Aortography: Determines the origin, size, and location of the aneurysm and involvement of additional arterial branches
The primary objectives in the management of aortic aneurysm are relieving pain and anxiety, lowering BP and thereby decreasing stress on the aneurysm, surgical repair if necessary, patient teaching, and prevention of complications.
Administer narcotics (eg, morphine) as necessary. Unrelieved pain is likely to increase anxiety, tachycardia, and hypertension, all of which may aggravate the condition. Relaxation therapy, with deep breathing exercises or imagery, may be extremely helpful.
1. Decrease afterload: Vasodilators (eg, nitroprusside, nicardipine, esmolol) may be prescribed to lower blood pressure and thus pressure on the aneurysm. Blood pressures should be maintained as low as possible (systolic blood pressure 90-120 mm Hg), without compromising perfusion to vital organs.
2. Decrease preload: Limit oral and IV fluids, decrease sodium intake, and administer diuretics as indicated. A decrease in preload decreases the circulating blood volume, thus decreasing pressure on the aneurysm.
3. Decrease myocardial contractility with beta-blockers (eg, esmolol, labetalol). A decrease in the strength of each cardiac contraction decreases the pulsatile pressure on the aneurysm.
1. Follow-up: If the patient is to be medically managed, follow up chest x-rays, CT scans, MRI scans, and/or ultrasounds will be needed at 6-month intervals to assess the status of the aneurysm. The importance of these studies should be stressed.
2. Diet modification: Teach the patient and family the importance of following a low-sodium diet. Consult a nutritionist for recipes and tips for food preparation.
3. Smoking cessation: Assist patient with programs available to assist with smoking cessation.
4. Physical/psychological stress modification: Teach the patient and family the hazardous effects of stress and the importance for modification. Discuss activity limitations and relaxation therapy.
5. Medications: Teach the patient and family the importance of compliance with the medication regimen. Stress that the medications are essential even though the patient may be asymptomatic.
Surgery is indicated for acute aneurysm rupture, aortic dissection in the ascending aorta, aortic dissection refractory to medical therapy, and asymptomatic patients with a fusiform aneurysm 6 cm or more in diameter (normal diameter is 2.5-3 cm).
1. During surgery the aortic aneurysm is resected and a prosthetic graft is sutured in place. The original aortic wall may be wrapped around the prosthetic graft for additional support.
2. If an acute dissection or rupture occurs and the patient is waiting for the operating room team to arrive:
• Administer narcotics for pain.
• Titrate vasodilators to maintain the patient’s blood pressure as low as possible (90-120 mm Hg if tolerated). This decreases the pressure on the aneurysm.
• Administer fluids to prevent hypovolemia.
• Administer blood replacement products to maintain adequate hemoglobin and hematocrit levels. If rupture occurs, the chest and abdomen will be opened emergently. The patient has a high risk of mortality or complications, including cerebral anoxia, severe hypovolemic shock, and multisystem organ dysfunctions (MODS).
3. Postoperative management:
• Same interventions as described to relieve pain and anxiety and decrease stress on the aorta wall. It is important to decrease pressure on the repaired aorta so that suture lines can heal and bleeding is kept to a minimum.
• Continuous ECG and hemodynamic monitoring.
• In the ICU, continuous spinal pressure monitoring (for surgical repair or insertion of an endograft stent for descending thoracic aortic dissection) will be done for the purpose of draining spinal fluid as necessary to maintain pressure at 10 mm Hg or less. Spinal cord swelling may occur following surgery. Maintaining a low spinal pressure has been shown to reduce the incidence of lower extremity paralysis.
• Complete assessment (including a focused neurologic assessment) every 1 to 2 hours.
• Gradual rewarming of the patient is important. Prevent postoperative shivering, which increases blood pressure and places additional stress on suture lines.
• Ventilator management to maximize oxygenation.
• Activity may be progressed according to institution standards and surgeon preference.
• Monitor renal function (urine output, blood urea nitrogen [BUN]), and creatinine, especially if the aorta was cross-clamped above the renal arteries.
• Initiate anticoagulation. Anticoagulation therapy is initiated for patients receiving prosthetic valves.
Preventing and Managing Complications
1. Hemorrhage: Hourly assessment of vital signs and hemodynamic parameters. Daily hemoglobin and hematocrit.
2. Arrhythmias: Continuous ECG monitoring; daily 12-lead ECGs.
3. Hemodynamic instability: Arterial and PAP monitoring; manage hemodynamic parameters based on trends.
4. Altered perfusion: Arteries originating from the aorta may be compromised, leading to MI, cerebral insufficiency/cerebrovascular accident, bowel necrosis, renal failure, paraplegia, and limb ischemia. Assess and monitor the patient for these conditions.
5. Aortic insufficiency: Aortic insufficiency may develop if the aneurysm is located in the ascending aorta. Enlargement or dissection of the aneurysm may dilate or damage the aortic valve, causing signs of acute heart failure and pulmonary edema.
From the early work of Dr. Christian Barnard in 1967, cardiac transplantation has evolved over nearly four decades to a standard modality for the treatment of end-stage cardiac disease. When medical, surgical, or pharmacologic interventions have failed to improve quality of life and functional capacity, cardiac transplantation offers patients improved survival. The international survival rate is 80% to 90%, 75% at 1 year, and 56% at 10 years. The primary indications for cardiac transplantation include cardiomyopathies or ischemic heart disease. Other indications include heart valve disease, congenital heart disease, and myocarditis.
Patients usually have a less than 1-year survival without cardiac transplant and are in New York Heart Association (NYHA) functional class III or IV or American Heart Association (AHA) Stage D. Because of the shortage of available organs, the patient must pass an extensive screening process to ascertain that he or she is appropriate for the candidate list (Table 19-4). Patients must be emotionally stable and free of alcohol, drug addictions, and tobacco use. They must demonstrate a commitment to the rigors of being a candidate and eventual recipient through compliance with their medical regimens.
TABLE 19-4. GENERAL INDICATIONS FOR CARDIAC TRANSPLANTATION
The period of waiting for an available donor can be extremely stressful for the patients and their families. It is important to explore their perceptions of the transplant process, what outcomes they are anticipating, and what methods they have utilized to cope in the past. Support group participation or meetings with a psychiatric clinical nurse specialist or nurse practitioner may be beneficial. Fear of death and acute illness may heighten the patient’s anxiety. Family members may need proximity to the patient, and this may assist in alleviating anxiety. Incorporating their involvement in direct patient care may enhance their coping abilities.
The greatest delay for cardiac transplantation occurs because of the shortage of donors. When a brain-dead donor is identified, he or she must be carefully managed to maintain cardiovascular stability and avoid electrolyte and renal complications. The United Network for Organ Sharing (UNOS) coordinates the allocation of organs based on a nationwide waiting list. The donor must be of a compatible ABO blood type to the recipient and of similar body size and weight. The recipient is tested for relative immunologic compatibility with the donor to avoid hyperacute rejection. Panel-reactive antibody screening is performed using the recipient’s serum with a random pool of lymphocytes. If no lymphocyte destruction occurs, the cross-match is negative and the transplant may proceed. The donor’s cardiac function must be normal as assessed by an echocardiogram, nuclear studies, or cardiac catheterization. The donor should have stable hemodynamic profiles on minimal inotropic support.
This process may take several hours, and it is imperative that the patient and family be frequently updated and made aware of the clinical plan of care. Pretransplant teaching should be reviewed to clarify misconceptions and correct knowledge deficits. If CO is compromised, decreased cerebral perfusion may compromise the attention span. During this time, the recipient needs close monitoring to maintain cardiovascular stability. The recipient may require antiarrhythmic therapy, inotropes, diuretics, or after-load reduction agents to achieve major organ perfusion adequate for cellular function. Anticoagulation therapy may be instituted to decrease risk of embolization secondary to AF, reduced left ventricular function, or peripheral venous stasis.
The most unstable patient may be maintained on a cardiac assist device such as the intra-aortic balloon pump (IABP), VAD, or ECMO to promote stabilization or to “bridge” him or her to transplantation.
In the past, there were two surgical options for cardiac transplantation. Today, almost all are orthotopic transplants in which the recipient’s heart is removed and replaced by the donor’s heart in the normal anatomic position (Figure 19-9). The surgical approach is a median sternotomy; the recipient’s heart is incised at the superior and inferior vena cavae, pulmonary artery, and aorta. The donor’s and recipient’s vena cavae, aortas, and pulmonary arteries are aligned and anastomosed. This technique is called the bicaval technique. Another technique which is rarely done today is the biatrial technique. This method involves removing the native heart but leaving the superior/posterior aspects of both atria. This will leave the native SA node intact and may result in double P waves on the ECG tracing (Figure 19-9). The fact that the donor’s heart is denervated results in no sympathetic or parasympathetic influence, so the donor’s heart must rely on noncardiac mediators to increase CO.
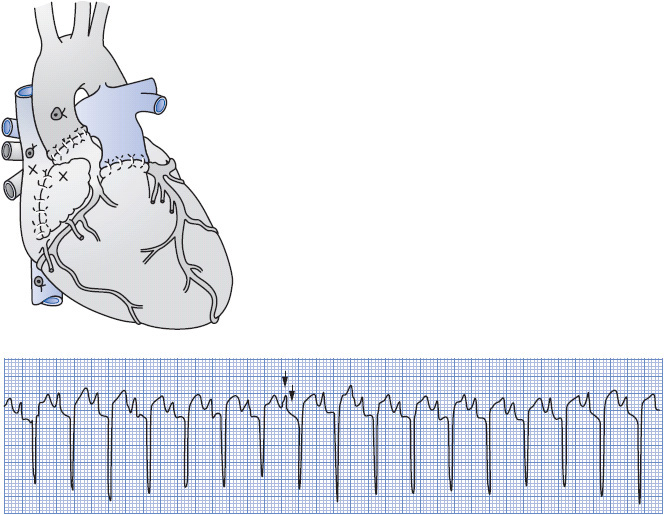
Figure 19-9. Orthotopic method of transplantation using a biatrial approach. Both the donor and the recipient SA nodes are intact (X). This results in an ECG tracing as shown. Note the double P wave at independent rates. (Reproduced with permission from Morton PG, Fontaine DK. Critical care nursing: A holistic approach, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2009.)
The other surgical option was a heterotopic approach, which is interesting from a historical perspective. It was used in about 5% of cardiac transplants at one point and was also known as a piggyback approach. The donor’s heart was placed to the right side of the pleural cavity and performed as an auxiliary pump for the native heart (Figure 19-10). This was used as an option in a size mismatch between donor and recipient or for severe pulmonary hypertension. This approach is rarely performed any more.
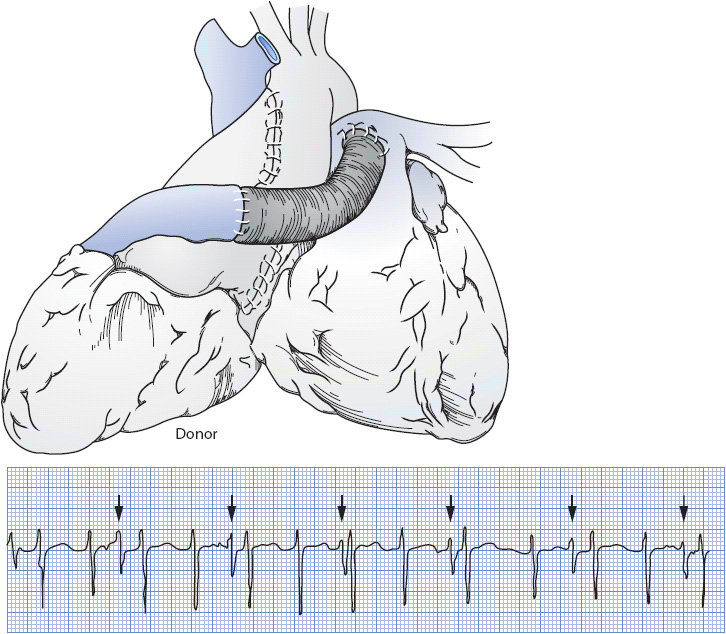
Figure 19-10. Heterotopic method of transplantation. The donor heart is anastomosed with a Dacron graft to the recipient’s heart. This results in an ECG tracing as shown. Note the “extra” QRS at an independent rate. (Reproduced with permission from Smeltzer SC, Bare BG. Brunner & Suddarth’s textbook of medical-surgical nursing, 10th ed. Philadelphia: Lippincott Williams & Wilkins, 2004.)
The postsurgical care is similar to care following conventional open heart surgery (see Chapter 9, Cardiovascular System). The primary objectives in the early postoperative period include stabilizing cardiovascular function, monitoring altered immune response and graft protection, and providing posttransplant psychological adjustment.
Stabilizing Cardiovascular Function
1. Cardiac denervation: Postoperatively there is loss of vagal influence, and the patient usually has a higher resting heart rate than normal.
• The posttransplant patient requires more stabilization prior to exercise or position changes to avoid orthostasis due to these effects from denervation. With loss of vagal tone, should the sinus rate decrease, there is a stronger potential for junctional rhythms to result.
• Surgical manipulation and postoperative edema may decrease donor SA node automaticity, and therefore the patient may require temporary pacing or isoproterenol (Isuprel) to increase the heart rate.
• Should arrhythmias such as SVT (supraventricular tachycardia) occur beta-blockers or calcium channel blockers are used to decrease heart rate in these circumstances. It is important to assess the patient for response to isoproterenol, because the drug can increase myocardial oxygen consumption.
• Denervation creates a more long-term concern in these patients because the patient no longer experiences angina if the myocardium becomes ischemic. Pain impulses are not transmitted to the brain, so patients must be taught to report other signs of declining cardiac function (ie, decreased exercise tolerance). This is seen in chronic rejection where even with diffuse coronary artery disease, the patient does not experience angina. The patient transplanted for ischemic cardiac disease may find this difficult to comprehend.
2. Ventricular failure: Any element of pulmonary hypertension can result in right ventricular dysfunction and eventually compromise left ventricular function also. Inotropic and vasodilating agents may be required to enhance cardiac function. It is essential to rule out any cardiac injury during harvesting and implantation that may have an impact on cardiac function. In reviewing the operative procedure, rule out reperfusion injuries or postbypass problems.
3. Bleeding: Risk factors include cardiopulmonary bypass (CPB), altered coagulation factors if right ventricular failure compromised hepatic function, and preoperative anticoagulation therapy. The recipient’s pericardium may be enlarged from pretransplant cardiomegaly. With a smaller donor heart there is more room for blood accumulation without early detection. If there is greater than 100 to 200 mL/h of bleeding for 2 hours, the patient may need to be reexplored. All medications should be reviewed for potential effect on platelet function and coagulation factors.
After cardiac transplantation, the patient is pharmacologically managed with immunosuppressive treatment for graft protection, titrating for the best graft function with the least adverse effects. By virtue of these agents, patient survival has been tremendously enhanced, with a decrease in the need for retransplantation.
1. Immunosuppression. Most patients are maintained on triple-therapy immunosuppression: cyclosporine, mycophenolate mofetil (CellCept), and corticosteroids.
• Cyclosporine creates a “selective immunosuppression” by selectively inhibiting T cells. T cells dependent on humoral immunity continue intact and no bone marrow suppression occurs. T-cell lymphocytes become unresponsive to interleukin (IL)-1, ultimately preventing maturation of helper and cytotoxic T cells. Adverse effects include hypertension, nephrotoxicity, hepatotoxicity, hirsutism, tremors, and gum hyperplasia. When the first intravenous (IV) dose is administered, it is important to assess the patient closely for potential histamine-type reactions with cardiovascular collapse. This is related to the IV solution preparation and is not seen with the oral preparation. A daily trough level is measured to assess therapeutic dosage and avoid toxicity.
• Basiliximab (Simulect) is an immunosuppressive agent that is an IL-2 antagonist. It is indicated for patients with renal insufficiency related to their chronic low CO because it is renal sparing. This drug is given preoperatively and then 2 to 4 days postoperatively.
• Mycophenolate mofetil has potent cytotoxic effects on lymphocytes. It inhibits the proliferative responses of T and B lymphocytes to both mitogenic and allospecific stimulation. It also suppresses antibody formation against B lymphocytes. It is given in 1.5-g dose twice a day. The side effects include gastrointestinal tract ulceration, nausea, vomiting, and diarrhea. It has severe neutropenic effects and can cause anemia, leukopenia, and thrombocytopenia.
• Corticosteroids are administered to both prevent and treat rejection. They are able to decrease antibody production and inhibit antigen-antibody production, as well as interfere with production of mediators IL-1 and IL-2. Both their anti-inflammatory and immunosuppressive properties offer the patient benefits. Immediately postoperatively, they are administered in high doses, and then tapered over the next 6 months. However, if the patient experiences two or more episodes of acute rejection, he/she remains on a maintenance dose. In situations of acute or chronic rejection, the patient may be “pulsed” with steroids. These doses are 500 to 1000 mg IV every day for 3 days, during which other steroids are discontinued. The patient then resumes another tapering wean to maintenance dose steroids. Complications from steroid treatment are numerous and include infection, hyperlipidemia, diabetes, hypertension, osteoporosis, sodium and water retention, metabolic alkalosis, peptic ulceration, pancreatitis, increased appetite, adrenopituitary suppression, lymphocytopenia, opportunistic infections, and aseptic necrosis of femoral and humoral heads. The patient often receives ulcer prophylaxis with a histamine blocker or antacids. Strict fluid and electrolyte balance must be maintained, and close assessment must be maintained for glucose intolerance. The anti-inflammatory response may mask an infection; therefore, identification of malaise, anorexia, myalgias, change in wound appearance, cough, or sore throat must be reported. With all these immunosuppressive agents, the patient has an intrinsic risk for malignancies and needs comprehensive teaching regarding this and all preventive therapies to follow.
• Newer therapies offer further improvement in transplant outcomes. Muromonab-CD3 (Orthoclone OKT3), a monoclonal antibody, may be given to reverse acute rejection, although it is rarely used. Antibodies that react with T3 cells’ surface antigens are produced, interfering with T-cell antigen recognition and making it more difficult for active T cells to recognize the target organ. Muromonab-CD3 is administered for a 10- to 14-day course of therapy as a daily bolus dose of 5 to 10 mg IV. There is a danger of flash pulmonary edema; therefore, the patient is premedicated with steroids, acetaminophen, and diphenhydramine. Vital signs are monitored every 15 minutes for 1 hour after the dose is given with emergency intubation and resuscitative equipment available. While receiving the treatment of Muromonab-CD3 cyclosporine is usually held and then titrated back up during the last 3 days of treatment. CD3 levels are monitored in the laboratory on the fourth and tenth days of therapy to assess effectiveness. Some centers utilize a monoclonal or polyclonal antibody for induction therapy in the immediate postoperative period. Others reserve medications such as Muromonab-CD3 for rescue therapy.
2. Infection risk. The immunosuppressive drugs decrease the normal immune response, increasing the risk for nosocomial or suprainfections (Table 19-5). In the immediate posttransplant period, when steroid doses are highest, the patient is more vulnerable to these infections. Infections are a major cause of morbidity and mortality, and prevention and early detection are crucial.
• The most challenging aspect of determining an infection is the clinical presentation, which is often masked by immunosuppressive therapy. The patient’s temperature may not elevate as high as in nonimmunosuppressed patients and the WBC may not elevate as rapidly. It is imperative to assess the individual trend in each patient and have a strong suspicion if patients appear more fatigued, complain of sore throats, develop a new cough, or run low-grade temperatures. Bacterial, fungal, viral, and protozoal infections may compromise the posttransplant recipient.
• Aggressive skin care to decrease dermal injuries, adequate nutrition and hydration, removing all invasive devices as soon as possible, and limiting unnecessary procedures may assist in reducing risks for sepsis. Patients and families should receive thorough education regarding transmission of infections. Antimicrobial therapy is instituted postoperatively while invasive devices are in place but should be utilized appropriately to avoid growth of antibiotic-resistant organisms. Thorough skin and oral assessments should be incorporated into daily assessment to rule out viral or fungal infections.
3. Assessing for rejection. Routinely, the patient undergoes a posttransplant endomyocardial biopsy to rule out rejection (Figure 19-11). Under fluoroscopy, utilizing a cardiac bioptome via the right internal jugular vein into the right ventricle, multiple (three to five) samples are taken of the myocardium to rule out rejection. The patient is then treated with the appropriate protocol (pulsed steroids or monoclonal antibodies). These biopsies are performed serially posttransplant during clinic visits to monitor for rejection. Other diagnostic procedures such as transesophageal echocardiogram and chest x-ray every 6 months may be performed. Cyclosporine levels are measured monthly. These data provide further guidance for earlier detection of rejection.
TABLE 19-5. COMMON INFECTIONS IN CARDIAC RECIPIENTS

Figure 19-11. Endomyocardial biopsy technique. (From: Macdonald SN. Heart transplantation. In: Smith SL, ed. Tissue and Organ Transplantation: Implications for Professional Nursing Practice. St Louis, MO: Mosby-Year Book, 1990, used with permission from AACN.)
Many emotions impact on the posttransplant patient. Often the patient and family have altered their roles and responsibilities during the illness. The posttransplant goal is to encourage role readjustment and resumption of preillness activities of daily living. The return to independence may frighten them after the security of the hospital environment.
1. They must be supported and assisted toward their return to home and with the long-term plan of care.
2. Involvement in a transplant support group may benefit the patient and family, reduce anxieties, and clarify misconceptions. Meeting other recipients may validate their feelings and enhance the patient’s adjustments.
3. Some recipients experience body image concerns related to hirsutism and increased weight. Reviewing cosmetic methods for dealing with these changes may decrease their concerns.
4. Weight loss may be enhanced through dietary counseling and participation in cardiac rehabilitation activities.
5. Quality-of-life issues should be explored with patients to heighten the positive side of transplantation and the future that awaits them.
6. Steroids may cause periods of mood swings from episodes of depression to euphoria. Counseling with the patient and family may reduce confusion over the cause of personality changes. During pulsed steroid therapy it is very important to assess for steroid psychosis. Closer monitoring and reassurance during this therapy may assist in diminishing this side effect.
Patients with cardiogenic shock following an MI, coming off CPB, or with cardiomyopathies may require additional assistance when CO remains low despite maximal medical therapy. IABP support offers 8% to 12% augmentation to the patient’s CO, but this may be inadequate, requiring placement of a VAD. Greater support for the failing ventricle(s) can be provided with a VAD. The goals of utilizing a VAD are to reduce myocardial ischemia and workload, limit permanent cardiac damage, and restore adequate organ perfusion.
Appropriate candidates for VAD include those patients with end-stage cardiac disease, cardiomyopathies, post-CPB cardiogenic shock, and acute MI with cardiogenic shock. Another indication for insertion is to “bridge” the patient prior to cardiac transplantation until a suitable donor is located. Post-MI, a patient may be bridged in the hope of myocardial recovery and eventual weaning from the device. VADs (left ventricular devices) have been approved for “destination therapy”; for example, once the VAD is inserted and the patient recovers, the patient is discharged home. The patient is also taken off the transplant list or moved to a lower priority of need. Recently, there have been reports of myocardial recovery after VAD placement. This has allowed for surgical removal of the VAD. The VAD is removed when the myocardium has recovered to the point of consistently ejecting an adequate CO. After VAD removal the patient is monitored closely for reoccurrence of heart failure symptoms.
The appropriate selection of a candidate for these devices is based on hemodynamic criteria. If preload has been maximized, afterload reduced, and drug therapy instituted to maximal levels, and yet the patient continues to have cardiovascular compromise, a VAD may be critical to achieve survival. Appropriate parameters to consider for VAD placement are:
• CI < 2 L/min/m2
• SVR > 2100 dyne/s/cm5
• Mean arterial pressure > 60 mm Hg
• Left or right atrial pressure > 20 mm Hg
• Urine output > 30 mL/h
• PAOP > 15-20 mm Hg
The exclusion criteria for use of a VAD include the following:
• Acute cerebral vascular damage
• Cancer with metastasis
• Renal failure (unrelated to cardiac failure)
• Severe hepatic disease
• Coagulopathy
• Severe systemic sepsis, resistant to therapy
• Severe pulmonary disease
• Severe peripheral vascular disease
• Psychological instability
• Alcohol, drug addiction, or tobacco use
The VAD unloads the native ventricle or ventricles by way of artificial ventricles or a blood pump. CO is enhanced by blood circulating at a physiologic rate and by augmenting systemic and coronary circulation.
Ventricular assist device support is predominately utilized for the left ventricle. However, if the right ventricle is compromised, support can be provided to both ventricles. This would necessitate separate VADs, yet the systems would function in tandem.
Ventricular assist devices can be used for postcardiotomy support as a bridge to recovery, a bridge to transplant, or as destination therapy. VADs can be nonpulsatile pumps (roller, centrifugal, or axial flow) or pulsatile pumps (pneumatically or electromagnetically driven). Previously, most VADs were inserted in the operating room but recently percutaneous VADs have been approved as a bridge to recovery, placement of another VAD, or to transplant. The percutaneous VADs are often inserted in the cardiac catheterization laboratory. There are several approaches for cannula insertion depending on the type of device being used and also, if the VAD is being used as a biventricular device or for one side of the heart alone.
Examples of VADs that are used as a bridge to recovery following cardiac surgery include the Biomedicus system and the Abiomed 5000 BVS system. The Biomedicus is a continuous-flow centrifugal pump and usually requires a perfusionist at the bedside for monitoring and troubleshooting. The Abiomed 5000 BVS system is easily managed by the nurse caring for the patient. There are smaller VADs that are emerging and that can be inserted in the cardiac catheterization laboratory using a percutaneous approach via the femoral vein and/or femoral artery. These include the Tandem Heart® and the Abiomed Impella® devices. The Tandem Heart involves inserting a cannula into the right atrium via the femoral vein. The cannula is introduced across the fossa ovalis into the left atrium. Arterialized blood is removed from the left atrium, circulated through an axial flow device, and reintroduced into the arterial circulation via a cannula that has been inserted into the aorta via the femoral artery. The Abiomed Impella is inserted percutaneously through the femoral artery, up the aorta, and introduced across the aortic valve. The device is designed to augment the patient’s CO. Blood is removed from the left ventricle and delivered into the ascending aorta.
Ventricular assist devices commonly used as bridge to transplant include the Heart Mate II (Figure 19-12), Heart Ware VAD, and Thoratec paracorporeal VAD. The Heart Mate II and Heart Ware VAD are axial flow devices while the Thoratec PVAD is a pulsatile flow device. The pulsatile flow devices produce a palpable pulse while the axial flow devices are usually not associated with a palpable pulse initially. Often, as the heart regains contractility over a period of several weeks, the pulse will return.
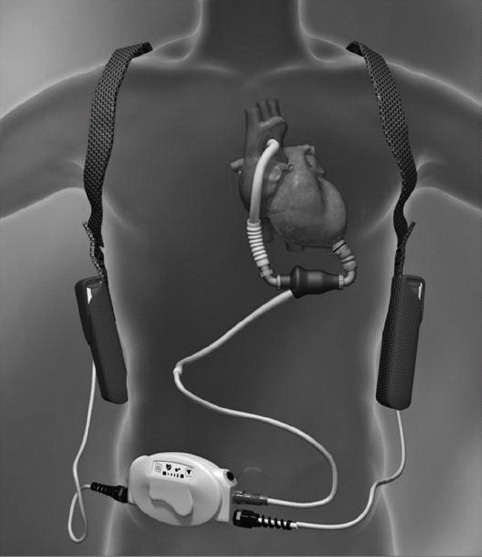
Figure 19-12. HeartMate II left ventricular assist system. (Used with permission from Thoratec Corporation Pleasanton, CA.)
The HeartMate II is the only device currently approved for destination therapy. Destination therapy implies that the patient is not a candidate for transplant and will not be placed on the waiting list for a transplant.
The devices used as a bridge to transplant or for destination therapy are all left ventricular assist devices (LVADs) with the exception of the Thoratec device (PVAD), which can be used as a right VAD as well. They all require an incision from the sternal notch to the umbilicus. A large cannula is inserted into the apex of the left ventricle and connected to the inflow port of LVAD with an outflow cannula inserted into the aorta. The LVAD is implanted outside the peritoneum just below the diaphragm. The drive line (power cord) is brought through the skin and connected to a power source. The drive line exit site represents a major source for infection to occur.
The LVAD has a monitor that provides the flow rate (similar to CO) and other information pertinent to the device. These devices can achieve flow rates that support adequate oxygen delivery to the tissues while reducing cardiac workload.
The plan for weaning should revolve around hemodynamic stability and the patient’s other physiologic systems’ response. Neurologic, pulmonary, renal, and hematologic systems must be recovered from multiorgan insults. Assessment of CO, CI, SVR, PAOP, mean arterial pressure, and SVO2 guides decisions for initiating weaning. Pharmacologic support should be at a stable level with good major organ perfusion.
The arterial line waveform is assessed for the dicrotic notch appearance, indicating that there is adequate left ventricular pressure for aortic opening. The VAD is turned down at small increments to assess tolerance throughout the weaning process. Heparin must be initiated before weaning and the device never set at less than 2 L/min flow to avoid clot formation. At completion of weaning, the patient returns to the operating room for surgical removal.
The primary objectives in managing the patient with a VAD are to optimize CO, maximize coping, and prevent complications.
Optimizing Cardiac Output
1. Initially, in the ICU, the risk of biventricular failure still is paramount after the device’s insertion and the patient must be closely evaluated. Cardiovascular profiles are measured every 2 to 4 hours and changes in CO and CI or the device flow rates are reported to the physician. Pharmacologic support should be titrated to achieve the most stable mean arterial pressure, adequate flow rates and adequate SVO2.
2. The VAD should be assessed for proper function to achieve an improved cardiovascular profile. As myocardial recovery occurs, more support occurs from the heart and less from the VAD. The patient can then support CO without as much mechanical support.
The patient and family may be overwhelmed by the suddenness of the disease, the progressive care unit environment, the equipment related to the VAD, and the threat of loss of life. Transplantation, if discussed, may significantly increase their stress. They may require intense information sharing and clarification of misconceptions.
1. Promote emotional and psychological adaptation and assess for nonverbal clues of fear or anxiety. Frequent updates regarding goals for the day and present plan of care need to be provided in an interdisciplinary manner. The advanced practice nurse and the patient’s primary nurse may coordinate this process.
2. Realistic information related to prognosis needs to be addressed with the patient and family. Often, 20% to 40% of patients on VAD die awaiting a donor heart, and families need support to cope with this possibility. Early involvement with social work and chaplains also may assist patients and families. Closely assess for other situational stressors and review prior coping strategies the patient or family found helpful.
3. If the device has been implanted for the purpose of destination therapy, the patient and family need to be taught the following:
• How to perform dressing changes
• How to change from the battery-powered unit to the base power unit and the reverse
• How to hand pump the device should there be a total loss of power if appropriate for the type of device
• What equipment the patient should always have when leaving home or the base power unit
The patient and family education process should begin in the ICU as soon as the patient is awake and alert. The nursing staff on the progressive care unit should continue the education process and assist with skills check off as the patient and family become competent when performing the skills mentioned above. Prior to discharge home, the emergency medical services (EMS) system in the patient’s home community should be notified about the patient returning to his community.
Preventing Complications
1. Thromboembolism: Anticoagulation therapy may include heparin, dextran, or aspirin to reduce the risk for thromboembolism. Peripheral vascular impairment may occur secondary to vascular catheters. Frequent neurovascular checks should be performed and any change reported immediately. Assess for the five P’s of vascular complications:
• Pallor
• Pain
• Paresthesia
• Paralysis
• Pulselessness
2. Bleeding: Monitor hemoglobin, hematocrit, and coagulation factors frequently. Assess all catheter sites and wounds for oozing. The patient needs to be evaluated for spontaneous oozing or occult bleeding. The patient needs close monitoring of therapy so that he or she is safely anticoagulated but not in an abnormal range should a sudden match for a transplantation heart occur. Ideally the partial thromboplastin time (PTT) should be 1.5 times normal or activated clotting levels should be appropriate for the device. The anticoagulation therapy may increase the propensity for cardiac tamponade to occur. This is a surgical emergency and may require reoperation for stabilization. Clues to this complication include the following:
• Elevated atrial pressures/neck vein distention
• Reduced CO as pump cannot fill properly
• Elevated pulmonary pressures
• Diastolic equalization
• Reduced mean arterial pressure
• Declining MVO2
3. Right ventricular failure: Observe for development of elevated central venous pressure/neck vein distention combined with low to normal PAOP.
4. Arrhythmias: Possible treatment with medications or electrical cardioversion may be required. Biventricular support may maintain nearly normal hemodynamics during arrhythmias. Assess the effect of arrhythmias on CO and augment the VAD accordingly. Treat all electrolyte abnormalities aggressively to enhance contractility. Validate with physicians whether CPR may be performed for asystole, depending on the specific VAD.
5. Decreased renal function: Possible etiologies in the VAD patient for reduced renal function include hypoperfusion before VAD insertion, prolonged CPB time, massive transfusions, and hemolysis with release of hemoglobin. Assess daily BUN and creatinine values for further decline in renal function. It is imperative that all medications be assessed for nephrotoxicity and doses be based on creatinine clearance. Adequate vasopressor therapy in the dopaminergic range is beneficial to enhance renal perfusion. Maintain adequate fluid balance so preload is within normal limits. Monitor urinalysis for potential abnormalities, and avoid any period of hypotension, which could further insult the kidneys.
6. Infection: The large cannulas exiting the skin create great portals of entry for pathologic organisms. Patients on VAD support are so metabolically stressed that they are more prone to infections, and strict precautions need to be followed. It is imperative they not become colonized, especially if they are pretransplant, because sepsis could preclude their receiving a heart. The best plan of action is prevention and includes:
• Strict hand washing before and after all patient care activities.
• Strict aseptic technique.
• Pan-culture for temperature > 101°F (38.33°C).
• Monitor wounds for erythema, exudate, or edema.
• Assess for shift to the left on differential count.
7. Immobility: Dermal injury may arise from the degree of immobility during the patient’s acute phase of illness. Meticulous skin care and frequent position changes assist in reducing problems. If the patient is hemodynamically stable, early mobilization is important as soon as possible. Having the patient up in chair and ambulating early will maintain muscle strength. Aggressive nutritional support assists in decreasing the degree of catabolism. Immobility may result in significant muscle mass loss and negative nitrogen balance. Bedside physical therapy is crucial until the patient is more stable and can begin ambulating. Foot splints may be applied to diminish the risk of foot drop.
8. Poor device performance: Dangers related to VAD mechanical problems include thrombus formation, inflow obstructions, or device failures. Frequent device evaluation is needed, particularly with any change in the patient’s clinical status. Device failure may result in inadequate or no systemic perfusion, so emergency measures must be implemented rapidly (Table 19-6).
TABLE 19-6. EMERGENCY MEASURES FOR VAD FAILURE OR CARDIAC ARREST
Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders Elsevier; 2012.
Carlson KK, ed. Advanced Critical Care Nursing. St Louis, MO: Saunders Elsevier; 2008.
Fuster V, Walsh RA, Harrington RA, eds. Hurst’s The Heart. 13th ed. New York, NY: McGraw Hill Companies, 2011.
Hardin S, Kaplow R. Cardiac Surgery Essentials for Critical Care Nursing. Sudbury, MA: Jones & Bartlett Publishing; 2010.
Moser DK, Riegel B. Cardiac Nursing: A Companion to Braunwald’s Heart Disease. Canada: Saunders; 2008.
Woods SL, Froelicher ESS, Motzer SA, Bridges EJ. Cardiac Nursing. 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005.
Albert NM. Fluid management strategies in heart failure. Crit Care Nurs. 2012;32(2):20-31.
Gheorghiade M, Filippatos GS, Felker GM. Diagnosis and management of acute heart failure syndromes. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease. 9th ed. Philadelphia, PA: Saunders Elsevier; 2011:517-542.
Joyce D, Mallidi HR, Hunt SA. Surgical treatment for heart failure. In: Fuster V, Walsh RA, Harrington RA, eds. Hurst’s The Heart, 13th ed. New York, NY: McGraw Hill Companies, 2011.
Lee CS, Tkacs NC. Current concepts of neurohormonal activation in heart failure. AACN Adv Crit Care. 2008;19(4):364-385.
Leeper B, Legge D. Resynchronization therapy for management of heart failure. Crit Care Nurs Clin N Am. 2003;15(4):467-476.
Lewis PS, Boyd CM, Hubert NE, Steele MC. Ethanol-induced therapeutic myocardial infarction to treat hypertrophic obstructive cardiomyopathy. Crit Care Nurs. 2001;21(2):20-34.
Mann DL. Pathophysiology of heart failure. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease. 9th ed. Philadelphia, PA: Saunders Elsevier; 2011:487-504.
Mann DL, ed. Heart Failure: A Companion to Braunwald’s Heat Disease. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
Paul S. Diastolic dysfunction. Crit Care Nurs Clin N Am. 2003;15(4): 495-500.
Paul S. Ventricular remodeling. Crit Care Nurs Clin N Am. 2003;15(4): 407-412.
Piano MR, Prasun M. Neurohormone activation. Crit Care Nurs Clin N Am. 2003;15(4):413-422.
Quinn B. Pharmacologic treatment of heart failure. Crit Care Nurs Q. 2007;30(4):299-306.
Collins EG, White-Williams C, Jalowiec A. Spouse quality of life before and 1 year after heart transplantation. Crit Care Nurs Clin N Am. 2000;12(1):103-110.
DiNella JV, Bowman J. Heat transplantation. Crit Care Nurs Clin N Am. 2011;23(3):471-479.
Guido GW. Heart transplantation from an ethical perspective. Crit Care Nurs Clin N Am. 2000;12(1):111-121.
Klein DG. Current trends in cardiac transplantation. Crit Care Nurs Clin N Am. 2007;19(4):445-460.
McCaffery D. A review of transplant immunology. Crit Care Nurs Clin N Am. 2011;23(3):393-404.
McCalmont V, Ohler L. Cardiac transplantation: candidate identification, evaluation and management. Crit Care Nurs Q. 2008;31(3):216-229.
Pham MX, Berry GJ, Hunt SA. Cardiac transplantation. In: Fuster V, Walsh RA, Harrington RA, eds. Hurst’s The Heart, 13th ed. New York, NY: McGraw Hill Companies, 2011.
Schonder KS. Pharmacology of immunosuppressive medications in solid organ transplantation. Crit Care Nurs Clin N Am. 2011;23(3):405-423.
Blaisdell MW, Good L, Gentzler RD. Percutaneous transluminal valvuloplasty. Crit Care Nurs. 1989;9(3):62-68.
Hill KM. Surgical repair of cardiac valves. Crit Care Nurs Clin N Am. 2007;19(4):353-360.
Holloway S, Feldman T. An alternative to valvular surgery in the treatment of mitral stenosis: balloon mitral valvotomy. Crit Care Nurs. 1997;17(3):27-36.
Leeper B. Valvular disease and surgery. In: Carlson KK, ed. Advanced Critical Care Nursing. St Louis, MO: Saunders Elsevier; 2008:322-346.
Nauer KA, Schouchoff B, Demitras K. Minimally invasive aortic valve surgery. Crit Care Q. 2000;23(1):66-71.
Otto CM, Bonow RO. eds. Valvular Heart Disease. A Companion to Braunwald’s Heart Disease. 3rd ed. Philadelphia, PA: Saunders Elsevier, 2009.
Piaschyk M, Cyr AM, Wetzel A, et al. A journey through heart valve surgery. Crit Care Nurs Clin NA. 2011;23(4):587-605.
Dziadulewicz L, Shannon-Stone M. Postpericardiotomy syndrome: a complication of cardiac surgery. AACN Clin Issues in Crit Care Nurs. 1998;9(2):464-470.
Hamel W. Care of patients with an indwelling pericardial catheter. Crit Care Nurse. 1998;18(5):40-45.
Anderson LA. Abdominal aortic aneurysm. J Cardiovasc Nurs. 2001;15(4):1-14.
Cronewett JL, Johnson KW, Rutherford RB, eds. Vascular Surgery. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010.
Dolinger C, Strider DV. Endovascular interventions for descending thoracic aortic aneurysms: the pivotal role of the clinical nurse in post-operative care. Vasc Nurs. 2010;28:147-153.
Iacono LA. Naloxone infusion and drainage of cerebrospinal fluid as adjuncts to postoperative care after repair of thoracoabdominal aneurysms. Crit Care Nurs. 1999;19(5):37-47.
Lam, CH, Vatakencherry G. Spinal cord protection with a cerebrospinal fluid drain in a patient undergoing thoracic endovascular aortic repair. J Vasc Radio. 2010;21:1343-1346.
Leeper B, Lovasik D. Cerebrospinal drainage systems: external ventricular and lumbar drains. In: Littlejohns LR, Bader MK, eds. AACN-AANN Protocols for Practice: Monitoring Technologies in Critically ill Neuroscience Patients. Sudbury, MA: Jones & Bartlett Publishers; 2009:71-102.
Makkad B, Pilling S. Management of thoracic aneurysm. Semin Cardiothorac and Vasc Anesth. 2005;9(3):227-240.
Bond AE, Bolton B, Nelson K. Nursing education and implications for left ventricular assist device destination therapy. Prog Cardiovasc Nurs. 2004;29(3):95-101.
Bond AE, Nelson K, Germany CL, et al. The left ventricular assist device. Am J Nurs. 2003;103(1):32-41.
Camp D. The left ventricular assist device (LVAD): a bridge to heart transplantation. Crit Care Nurs Clin N Am. 2000;12(1): 61-68.
Christensen DM. Physiology of continuous-flow pumps. AACN Adv Crit Care. 2012;22(1):46-54.
Cianci P, Lonergan-Thomas H, Slaughter M, Silver MA. Current and potential applications of left ventricular assist devices. J Cardiovasc Nurs. 2003;18(1):17-22.
Delgado RM, Frazier OH, Razeghi P, Taegtmeyer H. Mechanical circulatory support in patients with heart failure. In: Mann DL, ed. Heart Failure: A Companion to Braunwald’s Heat Disease. Philadelphia, PA: Elsevier, Inc.; 2004.
Fleck D, Puhlan M. Ventricular assist devices. In: Weigand D, ed. AACN Procedure Manual. 6th ed. Philadelphia, PA: Saunders Elsevier; 2011:464-489.
Hagan K, Casanova-Ghosh E. Postcardiotomy cardiogenic shock: the role of ventricular assist devices. Crit Care Nurs Clin N Am. 2007;19(4):427-444.
Kurien S, Hughes KA. Anticoagulation and bleeding in patients with ventricular assist devices. AACN Adv Crit Care. 2012;23(1): 91-98.
Litton KA. Demystifying ventricular assist devices. Crit Care Nurs Q. 2011;34(3):200-207.
Myers TJ. Temporary ventricular assist devices in the intensive care unit as a bridge to decision. AACN Adv Crit Care. 2012;23(1): 55-68.
O’Shea G. Ventricular assist devices: what intensive care unit nurses need to know about post-operative management. AACN Adv Crit Care. 2012;23(1):69-83.
Puhlman M. Continuous-flow left ventricular assist device and the right ventricle. AACN Adv Crit Care. 2012;23(1):86-90.
Richards NM, Stahl MA. Ventricular assist devices in the adult. Crit Care Nurs Q. 2007;30(2):104-118.
Rose EA, Moskowitz AJ, Packer M, et al. The REMATCH trial: rationale, design and end points. Ann Thorac Surg. 1999;67:723-730.
Savage L. Quality of life among patients with a left ventricular assist device: what is new? AACN Clin Issues. 2003;14:64-72.
Stahovich M, Chillcott S, Dembitsky WP. The nest treatment option: using ventricular assist devices for heart failure. Crit Care Nurs Q. 2007;30(4):337-346.
Castellucci D. Intraaortic balloon pump management. In: Weigand D. ed. AACN Procedure Manual, 6th ed. Philadelphia, PA: Saunders Elsevier; 2011:443-463.
Quall SJ. Comprehensive Intraaortic Balloon Pumping. St Louis, MO: CV Mosby; 1984.
Bonow RO, Carabello BA, Chaterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease. Circulation. 2008;118:e523-e661.
Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HSFA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):475-539.
Nashimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 Guideline Update on Valvular Heart Disease. Focused Update on Infective Endocarditis: a report from the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:887-896.
Peura JL, Colvin-Adams M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection. A scientific statement from the American Heart Association. Circulation. 2012;126:2648-2667.