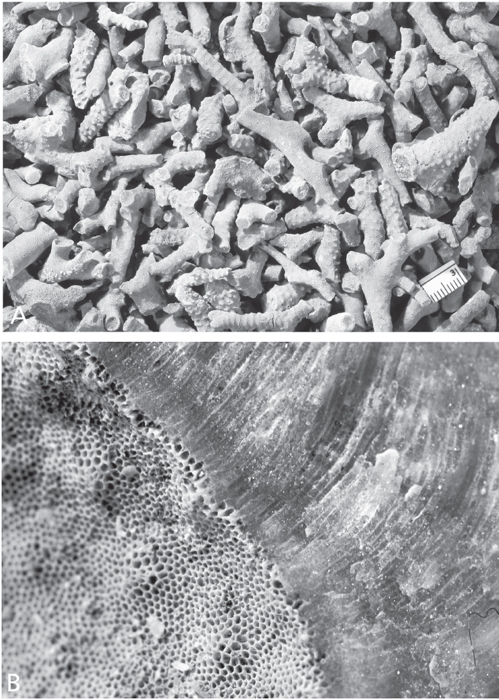
Figure 7.1. A. Fragments of bryozoan colonies are the most abundant fossils in the type-Cincinnatian. Parvohallopora ramosa (d’Orbigny), CMC IP 27957, Bellevue Limestone, Cincinnati, Ohio. Scale in mm. B. Surface of bryozoan colony showing minute openings (zooecia) on the left and a cross-section through a broken surface on the right. Each of the openings leads to a tube that was home to a tiny, individual animal. Trepostome bryozoan, Monticulipora mammulata d’Orbigny, CMC IP 51107, Bellevue Limestone. Cincinnati, Ohio. Diameter of individual openings (zooecia) about 0.2 mm.
The rocks in the Cincinnati region are loaded with fossils. Visitors to the area commonly are struck by all the “things” in the rock that look like small twigs, or, with a stretch of the imagination, small pieces of bones (Figure 7.1A). They are the most common fossils in the bedrock of the area. Indeed, if you were to pick up a fossil in the Cincinnati region at random, chances are that it would be one of these objects. But they are neither twigs nor bones. They are, in fact, the remains of a group of organisms called bryozoans (Plates 3D, E).
If you look at an unbroken surface of your bryozoan fossil with your trusty hand-lens, you see that it is replete with tiny holes (Figure 7.1B). If you shift your field of view to a broken surface, the tiny holes are revealed to be minute tubes. Each one of those minute tubes was once home to an equally minute animal. Thus, the fossil in your hand was constructed by a colony of tiny creatures. A bryozoan colony is reminiscent of a piece of coral found on a present-day beach in that coral reefs also are made by myriads of individual animals. Despite the superficial resemblance of bryozoan colonies and coral colonies to one another, the animals involved are very different, indeed. Corals are members of phylum Cnidaria, commonly called coelenterates. Each coral animal is basically sac-shaped with a single aperture serving as both mouth and anus.
A bryozoan animal is more complexly organized (Figure 7.2). There is an alimentary canal, with a distinct mouth on one end and a distinct anus on the other. Surrounding the mouth is a ring of tentacles called a lophophore. The lophophore serves as a food-gathering structure and for gas exchange between the animal and the surrounding water (in other words, it is also a respiratory structure). The anus is located outside of the lophophore. This is what gives the taxon of the bryozoans its technical name—phylum Ectoprocta. The “procta” part of the word means “opening,” and the “ecto” means “outside of.”
Frankly, most people do not call these animals ectoprocts. In former years, when the creatures were less well understood than they are today, people spoke of phylum Bryozoa. Eventually it was recognized that so-called phylum Bryozoa lumped together animals that are not-at-all closely related to one another. Hence, the name “Bryozoa” should be abandoned, in the technical sense. However, like the use of the term “glasses” to refer to items made of plastic, the term “bryozoans” persists in common parlance. Bryozoans sometimes are said to comprise phylum Polyzoa, especially in Great Britain. In some respects, “Polyzoa” is an appropriate name for the group. “Poly” means “many,” and “zoa,” “animals.” The word “Bryozoa” literally means “moss animals,” presumably because a living bryozoan colony, with its surface of many minute animals, might be thought to resemble a rock coated with many of the tiny plants we call moss. Potential confusion can ensue, however, if one forgets the fact that bryozoans are decidedly animals, whereas mosses are just as decidedly plants. Regardless of the technical terms and the reasoning behind them, most people refer to fossil ectoprocts colloquially as bryozoans, and they have done so for generations. We will follow that hoary tradition here.
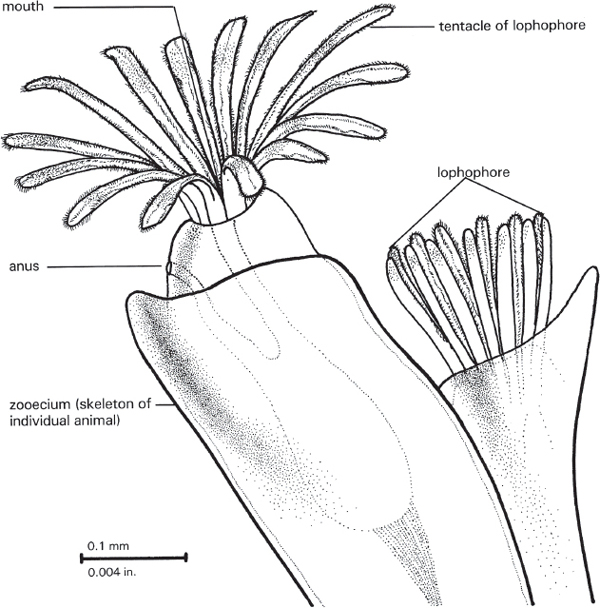
Figure 7.2. Living bryozoan, showing one zooid with tentacles extended in feeding position (left) and the other partly retracted (right). Drawing by Kevina Vulinec.
Bryozoans are animals. All animals derive the energy they need to grow, reproduce, and, indeed, to live, by consuming other organisms, or, at least, organic matter produced by living organisms. A parasite, for example, a tape-worm living within the alimentary canal of another animal, may absorb organic-rich fluids from within its host. A mosquito eats its victim one tiny drop of blood at a time. But bryozoans are neither parasites nor mosquito-like. So what do bryozoans eat, and how do they eat it?
Bryozoans are aquatic. Depending on the kind, some bryozoans live in fresh water, but most live in salt water. In either case, the bryozoan subsists on minute organisms (protozoans and so on) and tiny bits of organic matter suspended in the water. The bryozoan does not just wait passively for such food to fall into its mouth; it literally filters the food from the water. Bryozoans are active filter feeders. The individual animal spreads the tentacles of its lophophore into a funnel-, bowl-, or vase-like configuration (Plate 3E; Figure 7.2), and cilia that line the tentacles move in such a way that food particles are carried down to the mouth. Not only does the individual polypide generate feeding currents for itself, but the colonies of at least some kinds of bryozoans generate currents that enhance feeding in the colony as a whole. In at least some kinds of bryozoans there are particular areas on the colony that have polypides that direct currents away from the colony. These excurrent chimneys (Plate 3E) carry water that already has been filtered by the lophophores away from the zoarium, and thereby draw unfiltered, nutrient-laden water across polypides elsewhere in the colony (McKinney and Jackson 1989). Moreover, the very topology of the colony may facilitate the passage of nutrient-filled water through the colony and across its polypide-lined, and, hence, lophophore-lined, and, hence, food-gathering surfaces.
Fossil bryozoan colonies come in a wide variety of sizes and shapes (Figures 7.3, 7.4). Some are lumps the size and shape of a gum-drop. Some are stony masses larger than your fist. Some grow up from a shell or shell fragment on the sea floor as a small blade or delicately branched structure resembling a miniature version of a present-day “stag-horn coral.” Such colonies can exceed the size of a basketball, although what we mostly see are twig-shaped fragments. Many bryozoans grow as thin crusts on brachiopod shells or mollusc shells (see Figure 9.2D). A small number even grow within the shell matter of the brachiopod or mollusc, forming tiny dendritic or anastomosing canals (Figures 7.3B, C). Whatever its size and shape, the hard parts of a bryozoan colony comprise what is called a zoarium.
So zoaria exhibit a tremendous variety in overall shape. They also offer a tremendous variety in details. The surface of the colony may be smooth. Many, however, bear regularly spaced bumps, termed monticules, or regularly spaced depressions, called maculae. Monticules may be equidimensional in map-view, or they may be elongated, even ridge-like. They may be disposed in a seemingly random array, or they may have a distinct pattern. For example, specimens of the aptly named genus Constellaria are veritable constellations of star-shaped bunches of monticules (Figure 7.3D). Some students of bryozoans have concluded that monticules and maculae are simply manifestations of the same phenomenon, so do not differentiate the two from one another; they therefore call all of them, whether bumps or depressions, maculae (McKinney and Jackson 1989). Regardless of what one calls these elevations and depressions, they apparently were the places on the colony where the excurrent chimneys were generated.
All this variety would seem to offer a fertile field for the taxonomist—a new name for each morphologic variant. Indeed, a whole slew of bryozoan genera and species have been recognized by a whole slew of paleontologists. But there is a problem. Sometimes, in a single colony, the characteristics of one “species,” or even “genus,” give way to those of another—all in the space of a centimeter or two. Presumably, everyone in a single colony is of the same species. Thus, the conclusion is inevitable: overall colony shape and details on its surface may not always be reliable indicators as to who is related to whom. Indeed, there is ample evidence provided by present-day bryozoans that environment can play a significant role in colony shape, at least in some taxa.
Figure 7.3. Cincinnatian bryozoans. A. Colonies of the bryozoan genus Spatiopora characteristically form a thin coating on shells of orthoconic cephalopods, MUGM uncatalogued, Cincinnatian, scale in mm. Note that raised lumps on colony surface (monticules) are elongated and aligned with the axis of host nautiloid shell. B, C. Ctenostome bryozoan, Ropalonaria venosa Ulrich. B. Colony on shell of brachiopod Rafinesquina, CMC IP 40061, Waynesville Formation, Butler Co., Ohio, × 9. Arrow indicates sac zooid. C. Scanning electron micrograph of polyester cast of cavities excavated by zooids into host shell, BMNH D.52264, × 22. B and C from Pohowsky (1978, plate 1, figures 5, 7) and reprinted by permission of the Paleontological Research Institution. D. Cystoporid bryozoan, Constellaria florida Ulrich, CMC IP 51108, Fairview Formation, Kenton Co., Kentucky. Inset, enlargement showing characteristic star-shaped monticules, scale in mm. E. Cryptostome bryozoan, Escharopora sp., CMC IP 51110, Fairview Formation, Boone Co., Kentucky. Escharopora has zooecia on both sides of the thin, bladelike zoarium. Inset, enlargement showing zooecia, scale in mm. F. Basal surface of trepostome bryozoan that has grown on the pedicle valve of the brachiopod Rafinesquina and overgrown it, Richard Arnold Davis collection, Bellevue Limestone, Cincinnati, Ohio, scale in mm. G. Trepostome bryozoan colony that has grown on shell of pelecypod Ambonychia and overgrown it, Richard Arnold Davis collection, Cincinnatian, horizon and locality unknown, scale in mm. H. Ring-shaped bryozoan zoarium (“Weichold donut”) presumably encrusting nautiloid, CMC IP 51109, Richmondian, Hamilton Co., Ohio, × 0.7. I. Cross-section of ring-shaped bryozoan zoarium (“Weichold donut”) encrusting nautiloid, Richard Arnold Davis collection, Upper Maysvillian–Lower Richmondian, Butler Co., Ohio, scale in mm. Dark, elliptical zone on right and left sides of ring are calcitic replacement of nautiloid shell.
Well, if the shape of the zoarium is not an incontrovertible taxonomic indicator, what, if anything, is? Is there an “inner truth” in bryozoan taxonomy? It turns out that there is just that, namely, the internal structure of the colony. Each zoarium consists of the hard parts of all of the animals that comprise the colony. An individual bryozoan animal is called a zooid, and the hard parts of that individual animal constitute a zooecium (Figure 7.2). If one examines the holes in a zoarium with a hand-lens or low-power microscope, one commonly sees that the holes are not identical (Figure 7.1B). There may be size classes of larger holes and smaller holes; there may even be spine-like projections in addition to holes. It would appear that, in at least some colonies, not all the zooids were identical. In some present-day colonial animals, there is polymorphism, with different-shaped or different-sized individuals performing different tasks, for the good of the colony, the species, or both. That seems to have been the case among at least some now-extinct bryozoans. As mentioned previously, the surface of a zoarium may be marked by regularly spaced bumps or depressions (Figures 7.3A, D). In general, these monticules and maculae, respectively, consist of zooecia of sizes and natures different from those between them, and, hence, seem to represent areas of the colony in which the zooids performed particular functions for the colony as a whole.
As we noted previously, some bryozoan colonies exceed the size of basketballs. How do we know that? The answer is painstakingly simple, with the emphasis on the word “painstaking.” In general, bryozoans are found only as small fragments scattered throughout the rock. Very occasionally, however, one finds that all the fragments of a colony are lying together, as part of a single layer of rock. That’s the good news: the complete colony is there! The bad news, however: although the colony may be complete, it is not whole. That’s where the “painstaking” comes into the picture. One must oh-so-carefully collect each fragment, paying meticulous attention to just where the fragment was in the rock and adjacent to what other fragments. Then one must play three-dimensional bryozoan jigsaw puzzle and glue the tens, or hundreds, or thousands of pieces each in its proper place.
According to the old nursery rhyme, “All the King’s horses and all the King’s men couldn’t put Humpty together again” (Opie and Opie 1955). As it happens, some of the local fossil collectors and other paleontologists have been more clever, or at least more persistent. For example, amateur paleontologists Jerry Rush and, more recently, Ron Fine have spent countless hours collecting and reconstructing bryozoan colonies from the type-Cincinnatian rocks. It is to the perseverance of such folk that we know that local bryozoan colonies did, in fact, sometimes exceed the size of basketballs. Indeed, one colony re-assembled by Mr. Fine is some 66 cm by 35 cm by 15 cm (Figure 7.4E), a whopping 26 inches by 14 inches by 6 inches (Cuffey and Fine 2005). Similar efforts by Erickson and Waugh (2002), Waugh and Erickson (2002), and by Waugh et al. (2004) provided new information about the form and patterns of water flow through complete colonies (Figure 7.5).
Figure 7.4. Large bryozoan colonies from the type-Cincinnatian. A. Intact colony of a branching trepostome bryozoan, Parvohallopora ramosa d’Orbigny, University of Cincinnati collections, Corryville Member of Grant Lake Limestone, Hamilton Co., Ohio. Scale in mm. B. Intact colony of an unidentified branching trepostome bryozoan. CMC IP uncatalogued, Cincinnatian, no horizon or locality data. Scale in mm. C. Intact colony of trepostome bryozoan, Monticulipora mammulata d’Orbigny, CMC IP uncatalogued, Cincinnatian, no horizon or locality data. Scale in mm. D. Trepostome bryozoan encrusted on probable nautiloid shell that has disappeared, Monticulipora mammulata d’Orbigny, University of Cincinnati collections, Corryville Member of Grant Lake Limestone, Hamilton Co., Ohio. Scale 2 cm in length. Note borings into bryozoan. E. Intact colony of trepostome bryozoan, Heterotrypa frondosa d’Orbigny, CMC IP, Corryville Member of Grant Lake Limestone, Kenton Co., Kentucky. Colony about 65 cm in width. This colony was excavated and reassembled by Ron Fine. See Cuffey and Fine (2005).
Figure 7.5. Reconstruction of the lower part of the zoarium of Heterotrypa sp., diameter about 28 cm. From Waugh et al. (2004). The open structure of the zoarium would provide exposure of interior feeding surfaces to water flow, while the downward-arching fronds would provide stabilization and attachment to the substratum. Reprinted by permission of Sigma Gamma Epsilon.
Another spectacular growth of bryozoans was found in a roadcut several miles south of Maysville, Kentucky. Here were discovered two broad mounds of bryozoans, each between three and three and a half meters wide by about one-third meter tall (10 feet by 1 foot). Like Mr. Fine’s specimen, these mounds grew on the sea floor, but, unlike his, each of the Maysville mounds consists of numerous individual colonies and may have taken a thousand years to grow to that size (Cuffey 1998).
When organisms produce elevations on the sea floor, such constructs are technically termed bioherms (“bio” means “life,” and “herm” means “mound”). The Great Barrier Reef is the most spectacular example of this phenomenon in today’s oceans. It must be admitted that in comparison to the string of coral reefs that stretch more than 2000 km (1260 statute miles) parallel to the east coast of Australia, the mounds in Northern Kentucky are less than miniscule. However, to the fossil collector used to bryozoan fragments much smaller than pieces of blackboard chalk, the bryozoan mounds are gargantuan. On a jocular note, Roger Cuffey, one of the most productive bryozoan workers alive today, long has referred to the Maysville mounds and such as “bryoherms.” Alas! This paragraph must close on a sad note: sometime during the 1990s, road widening destroyed the Maysville mounds. There is, of course, some solace in the possibility that even bigger bryoherms still may be buried somewhere in the local rocks, awaiting discovery.
Associations
In most localities and in most strata, bryozoans are the most common fossils one encounters. In sheer abundance alone, they must be reckoned to have been truly important denizens of the Cincinnatian sea floor. This impression is nothing but enhanced when one focuses in on the details of just where and with whom they occur.
At many times and in many places, the Cincinnatian sea bottom appears to have been soft mud. Most of the kinds of animals that we generally find preserved as fossils do not seem to have “liked” soft, muddy bottoms, presumably because the mud did not provide solid footings upon which to build a stable life. It was all too easy to be engulfed by the ooze. Moreover, the fine sediment was too easily swirled up into the water and clogged respiratory and food-gathering apparati. Even a centimeter or two above the mud was more hospitable. Bryozoans ordinarily did not grow their colonies directly on the surface of the mud. But let a storm drop a few shells or fragments of shells onto the goo, and the sea floor was open for colonization. So often, when one is able to examine the actual base of a colony—where growth commenced—one discovers that the colony was founded on a fragment of a shell of a brachiopod or pelecypod, if not a complete or nearly complete shell (Figures 7.3F, G, 7.4D). Once the sea floor was a bit stabilized, the bryozoans colonized and grew in earnest.
Once established, the bryozoans themselves added to the stability of the sea floor in their immediate vicinity. First, as colonies grew, a certain proportion of them toppled over, and their skeletal material became incorporated as part of the sea floor, thus increasing the stability of the bottom and making it more hospitable for other creatures. Moreover, the little “thickets” of bryozoan colonies provided places for other organisms to hide from potential predators or, in the case of the predators, places from which to orchestrate ambushes of potential prey. In addition, some animals scrambled up the stalks and branches of bryozoan colonies to avoid the muddy water immediately adjacent to the sea floor. And larvae that happened to attach up in a bryozoan “thicket” would not only have escaped the worst of the turbid water, but also might have had a better chance of latching onto minute particles of food suspended in the water. Moreover, even as trees ameliorate the effects of wind on the land, bryozoan colonies must have moderated the currents on the sea floor. This would not only have made life easier for some organisms, but would have resulted in entrapping sediment, thereby further helping to stabilize the sea floor.
One particularly intriguing example of how bryozoan colonies were used by other creatures was documented by Douglas Shrake of the Ohio Division of Geological Survey in his master’s thesis. Trilobites, like other arthropods, are enclosed within a hard exoskeleton. Because this “suit of armor” cannot expand as the animal grows, the trilobite periodically must shed its exoskeleton, expand in size, and harden up a new protective shield. This is an especially trying time for the trilobite, first because it can be difficult to wriggle and squirm out of the old armor, and, second, because until the new suit hardens, the animal is “naked”—a soft, tempting morsel for any passing predator. Shrake found evidence that individuals of the trilobite genus Primaspis resorted to lowly bryozoans to make the time of trial a bit less trying (Shrake 1987, 1989).
When the time for shedding was at hand, the trilobite apparently climbed its way up into a suitable part of a bryozoan colony and wedged the projections of its exoskeleton into the bryozoan (see Figures 11.6E, F) This enabled the trilobite to pull itself out of the old exoskeleton and commence the hardening of the new one, all the while being hidden amongst the bryozoan fronds from the eyes of would-be predators. Although this must have been a convenient arrangement for the trilobite, it may have been less so for the bryozoans. Shrake found that, in some instances, there was pathologic growth in the colony as the bryozoans grew up and around the trilobite exoskeleton they had no way to dislodge.
The trilobite/bryozoan association described by Doug Shrake is just one of a host of examples of the interactions of bryozoans and myriad other creatures. On the one hand, a bryozoan larva would attach to almost anyone, given suitable circumstances, and a colony would sprout. Bryozoan colonies have been documented as attached to, encrusting, overgrowing, or etched into articulate brachiopods, inarticulate brachiopods, cephalopods, corals, cornulitids, crinoids, foraminifers, hydrozoans, monoplacophoran molluscs, pelecypods, trilobites, and, of course, other bryozoans, both of the same and of different species (see chapter 16, Table 3). On the other hand, a number of other organisms have been found attached to or bored into bryozoans: corals, articulate brachiopods, inarticulate brachiopods, cornulitids, and pelecypods, along with a number of organisms of uncertain affinities, including Catellocaula, Sanctum, Sphenothallus, and Trypanites (see chapter 16, Table 3).
In some cases, it is obvious that both the “guest” and the “host” were alive at the time of the association. In other cases, the “guest” was merely using a dead shell, exoskeleton, or whatever as a handy site for attachment on the sea floor. In other words, it commonly is a tough task to unravel in-life association from post-mortem happenstance. Nonetheless, it is abundantly obvious that the Cincinnatian sea floor of the ancient past, and, hence, the Cincinnatian rocks and fossils we find today would have been drastically different without the bryozoans.
Ordovician Doughnuts
Occasionally a lucky collector will find in the rocks of the Cincinnati area a stone object that looks rather like a doughnut (Figures 7.3H, I). Closer examination reveals that this toroid fossil consists of bryozoan zooecia; indeed, it is a ring-shaped zoarium.
The bryozoan rings have been known for a long time. Years ago, when Kenneth E. Caster, the eminent paleontologist at the University of Cincinnati, was shown one of them by a local fossil collector, he quipped, “Ah! Yes! A Weichold Doughnut.” He then went on to explain that Weichold was one of the old-time collectors in the Cincinnati region, and that these unusual fossils had been dubbed “Weichold doughnuts” or “Weichold rings,” although he did not know the specific connection between Weichold and the rings.
So why would a bryozoan zoarium grow in the shape of a ring? Weichold doughnuts tend to be some 5 or 6 cm in diameter (2–2½ in). As it happens, that diameter is comparable to that of the shells of some of the orthoconic cephalopods in the local rocks. Could there be a connection? Within some of the Weichold doughnuts, there is a ring of what might be recrystallized cephalopod shell (Figure 7.3I). Perhaps the apertural part of the tube that comprises the shell of an orthoconic nautiloid cephalopod broke off and came to rest on the sea floor. Then, one or more bryozoan larvae settled on this hard object protruding above the ooze. As the zoarium grew, it assumed the ring-shape of the “segment” of cephalopod shell.
Such rings of cephalopod shells have been described and figured in the scientific literature (Teeter 1978), and similar things have been found in the local rocks. However, the story may not be quite so straightforward. The problem is that some of the Weichold doughnuts seem to be bryozoan hard parts all the way through—with no obvious remnants of cephalopod shell.
Frank McKinney, the well-known bryozoan worker, has seen a ring-shaped colony of the bryozoan genus Constellaria, with mud in the center. His interpretation was that the colony had slowed the water and caused mud to precipitate to such an extent that growth of the colony was able to proceed only at the periphery (McKinney, pers. comm.). However, this colony was some 8 to 10 inches across (20–25 cm)—more than twice as big as the largest Weichold rings. Obviously, the phenomenon needs some serious scientific study.
Ordovician Hitch-Hikers
As mentioned above, many bryozoan colonies in the rocks of the Cincinnati region originally grew on shells on the sea floor. In some cases, the shell no longer sheltered its maker, but was merely a lifeless, hard object lying on the mud. In other cases, both the bryozoans and the organisms on which the zoarium grew were alive. In these instances, the attachers are called epizoa, and the attachee is the host (Davis et al. 1999).
Some bryozoans carried epizoism to a higher level.
Not too uncommonly, an observant fossil collector will find a fossil that has the size and shape of an orthoconic cephalopod. However, unlike the case of an ordinary nautiloid, the surface bears the tell-tale apertures of zooecia (Figure 7.3A)—and looks are not deceiving. The specimen is an orthoconic nautiloid, but one that bears a thin coating that consists of a bryozoan colony. It is obvious that the cephalopod shell was not just lying around on the sea floor dead and empty, because its entire exterior is covered by the encruster—with neither gaps nor seams. Moreover, the picture is enhanced by the surface features of the zoarium. Instead of being equidimensional bumps, the monticules are decidedly elongate, and their longest dimension is aligned with the length of the orthoconic shell. It is almost as though the bryozoan colony was carried through the Cincinnatian sea on the swimming cephalopod. The whole cephalopod/bryozoan assemblage looks so streamlined that even the monticules are disposed so as to minimize the friction of slipping through the water.
This bryozoan/cephalopod association has been known for well over a century (Ulrich 1883). In fact, at least one taxon of bryozoans, Spatiopora, seems to be known only as encrustations on cephalopods (Baird et al. 1989). Early on, the association was interpreted as a parasite/host relationship. However, in parasitism, the parasite consumes part of the host. In the bryozoan/cephalopod association, it is not likely that the bryozoans were “eating” the cephalopod. It is possible that they were deriving nutrition from leftovers and organic debris generated when the cephalopod, itself, fed. It could be that the bryozoans picked up suspended matter from the sea water as the cephalopod swam from place to place.
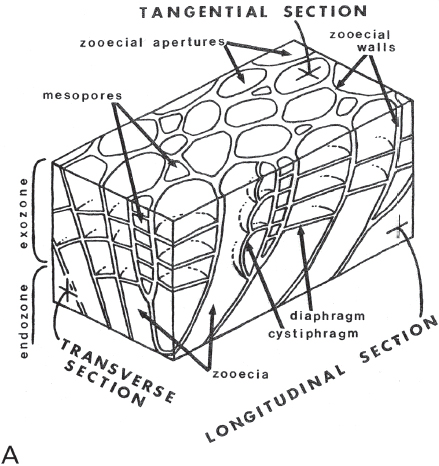
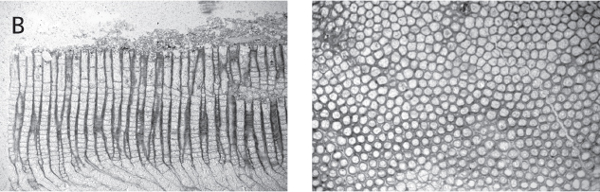
Figure 7.6. A. Detailed studies of bryozoans require carefully oriented thin-sections or acetatepeels. Diagram shows orientation of sections and terminology of internal skeletal structures used to identify species. From Arens and Cuffey (1989, figure 5), reproduced by courtesy of Roger J. Cuffey, with permission of the Pennsylvania Academy of Science. B. Left, longitudinal thin section of Heterotrypa frondosa (d’Orbigny), CMC IP 40336, Bellevue Limestone, Cincinnati, Ohio, R. J. Singh Collection. Right, tangential thin section of same. Both approx. × 10.
At first glance, one might worry that the weight of a “stony bryozoan” would have impeded significantly the swimming of the cephalopod. However, the bryozoan colony is just one zooecium thick and would have been mostly soft parts. Moreover, like present-day Nautilus, the Ordovician cephalopod may have been able to compensate for the extra weight of the bryozoans by means of the gas in its camerae (see chapter 9). In addition, the bryozoan coating might have increased the hydrodynamic drag on the cephalopod.
There even may have been some advantages to having a coating of bryozoans. Present-day “decorator crabs” are camouflaged by the load of anemones and such like that they carry. Indeed, the crabs deliberately “plant” other creatures on their dorsal surfaces. Perhaps the bryozoan epizoa helped conceal the Cincinnatian cephalopods that bore them. (Of course, the zoarium would have covered, and thereby made visually useless, any color patterns that the cephalopods had; but that is a story that does not belong in the chapter about phylum Bryozoa.)
Studying Bryozoans
Most bryozoan specimens can be identified only on the basis of internal structures, at least definitively so. This means that, except for Superman, it is necessary to cut them open—no small feat for the ordinary fossil collector. It is necessary to use a rock saw to make precisely oriented cuts through an individual specimen. Because the bryozoan colony is preserved as part of the rock, it will not let enough light through to see internal details. This can be overcome in two ways. The zealous and well-equipped paleontologist can cut and grind the specimen into slices so thin that they become transparent. These are called thin-sections, and they are what generally are used in studying bryozoans.
Depending on the nature of the specimen, alternatively, it may be possible to use what are called acetate peels. Like a thin-section, a peel starts with a carefully oriented cut through the specimen. The cut surface is then carefully ground flat and then etched in an appropriate acid of appropriate “strength.” If the specimen is suitably preserved, certain of its features will be a bit more resistant to dissolution by the acid. Hence, they will stand out slightly from the surface. If one takes a thin sheet of plastic (the acetate) and uses acetone to allow the acetate to adhere to the etched surface, it may be possible to pull away the sheet, along with enough of the specimen, to reveal internal details.
The use of thin-sections is generally considered to be “industry standard,” but, regardless of which study technique is used, both require equipment that may be beyond the budget, or desire, of the ordinary fossil collector. Moreover, both require definite safety precautions and safety equipment; for example, acids can etch more than just rock, and acetone not only is flammable, but its vapor is unhealthy to breathe.
As indicated, both thin-sections and peels must be carefully oriented within the zoarium. This is to maximize the information that may be derived about the internal structure of the colony. Because there are different kinds of zooids in a colony, it is important to be able to study the different sizes, shapes, and natures of the zooecia as viewed in a plane perpendicular to the individual tubes. This is best done in a tangential section, which is cut parallel to the surface of the colony and near its surface (as opposed to near its center, or axis) (Figure 7.6). On the other hand, a longitudinal section is cut parallel to the length of the individual tubes and can provide important information on both the growth of the zoarium and of the zooecia of which it consists. A transverse section is oriented at right angles to the other two, for example, across a branch of a given colony. In short, one needs longitudinal, tangential, and transverse sections of a colony to get a complete picture of its internal structure, and this complete picture is essential to an understanding of just what kind of bryozoan is at hand and how it grew and was constituted.
After the properly oriented thin-sections or peels are made, the only way they can be studied adequately is under the microscope. This, again, is a piece of equipment that may challenge one’s budget.