4
Oxidation
A strong case can be made that staling is the most common defect in packaged beer found in the trade. In some sense, this predicament is to be expected with any natural organic food like beer.
Additives to cover the effects of staling have been used in the past, but since about 1990, there has been a declining interest in them. Potassium metabisulfite (K2S2O5) is an example. Although it still finds favor in wine making and various areas of food processing, the use of potassium metabisulfite has all but disappeared in brewing. Bisulfites work by binding with staling aldehydes to mask the presence of the latter. Unfortunately, these bonds are rather short-lived in beer, and when they are broken, the staling aldehydes fully reveal their presence. Worse still, the bisulfite component can undergo other reactions that produce unpleasant H2S and/or mercaptan notes.
Another type of additive includes the antioxidants that are neutral to beer flavor. Ascorbic acid (vitamin C) is an example, but unfortunately, it and analogous compounds are not very effective.
Therefore, it is obvious why “nonadditive brewing” is gaining favor. The effective additives tend to have unacceptable side effects, and the neutral additives rarely seem to work. As a consequence, the best course for brewers is to master the fundamental mechanisms that lead to staling and then to use this knowledge to optimize equipment and procedures in order to achieve a respectable, albeit finite, shelf life. Methods for such optimization are covered in, for example, in the references by Bamforth (1999) and Fix (1998). Following C. D. Dalgliesch (1977), it is useful to characterize staling in terms of three basic stages as shown in Figure 4.1.
• Stage A is the period of stable, “brewery-fresh” flavor.
• Stage B is a transition period in which a multitude of new flavor sensations can be detected.
• Stage C products are the classic flavor tones involved in beer staling.
Stage A beer is pristine in flavor. During stage B, Dalgliesch described a decline in hop aroma, a decline in hop bitterness, an increase in “ribes aroma” (or sometimes “catty” flavor), and an increase in sweet, toffee-like, or caramel tones. The terms ribes (or currant) and catty are widely used in the United Kingdom and Scandinavia to recall overripe or spoiled fruit or vegetables. Some tasters cite a “black currant” tone (Hardwick, 1978). In truth, these terms describe a wide spectrum of negative flavors developed when beer is in stage B. Toffee or caramel flavors can come from many sources, but those associated with staling will invariably have unattractive cloying notes. These effects are enhanced by residual diacetyl and also by excess heat treatment of wort. Finally, stage C products range from papery or leathery to sherry- or vinegar-like notes.

Figure 4.1. The stages of beer flavor evolution.
Although it is not treated in the work by Dalgliesch (1977) or Hardwick (1978), a beer stage D also exists in which stage C flavors have evolved into a kaleidoscope of flavors that in very special formulations—Rodenbach’s Grand Cru comes to mind—recall the subtlety and complexity of great wines. It must be emphasized that this process takes years, not months, of maturation.
Barry Axcell and Phil Torline, in an interesting if provocative article, argued that most beers are consumed during stage B (Axcell and Torline, 1998). During this period, beer flavors undergo discernible changes, and the authors suggested that these changes are at the root of consumer dissatisfaction. Among other things, they cited the so-called import paradox as partial evidence of this theory, the paradox being that a definite proportion of the beer-consuming population actually prefers beers in stage C. (Here “import” means any beer consumed at a significant distance from where it is brewed.) These authors noted both the stability of flavor in stage C and “learned prejudices” (such as prestige of the beer and packaging) as the keys to this paradox.
M. C. Meilgaard, for one, has been sharply critical of stage C flavors because they are one-dimensional (Meilgaard, 1991). He stated, “I think it ranks as an all-American scandal that fully half of all the interesting and unusual packaged beers that are on the market get to us so oxidized that staleness and cardboard are the main flavor tones.” This is why most successful brewers try to produce beers in which stage A flavors are as stable as possible for as long as possible. If they, or their consumers, prefer stage B or C (or D for that matter), then putting the beer aside for the required time is all they need to do. Staling can go in only one direction!
It is useful to make a distinction between hot-side aeration and cold-side aeration. The hot side includes events from mashing through wort chilling. When the wort is cool, oxygen is added to the wort or, more commonly these days, to the yeasts, before the yeasts are pitched. At this point in the process, oxygen serves as an valuable yeast nutrient. Once the fermentation has started, however, oxygen returns to being a negative element. At this point, what is referred to as the cold side starts and continues until the beer is consumed.
As a general rule, cold-side aeration involves auto-oxidative processes, i.e., processes in which staling products are produced by the direct attack of molecular oxygen on beer constituents, including aldehydes, hop constituents, phenolic compounds, and, most important, ethanol and other alcohols. Cold-side aeration can arise during the processing of fermented beer; air pickup during filtration causes a particular concern. Thus, the most critical time associated with cold-side aeration is during the packaging of beer. The negative effect of high air levels has been well documented (see, e.g., Fix, 1998). However, it cannot be overemphasized that thermal and mechanical abuse during the storage of packaged beer can greatly accelerate staling even with low headspace air levels, as shown by Fix and Fix (1997).
Hot-side aeration is fundamentally different because of the speed of redox reactions that occur at elevated temperatures. For example, introducing 1 mL of oxygen per liter of wort at 70 °C will start reactions that consume the free oxygen in seconds. The oxidized wort constituents will ultimately cause flavor problems in packaged beer via the chemical mechanisms described in the next section. Doing the same for wort at 20 °C will have virtually no effect. The free oxygen will remain an inert gas for days and, in practical brewing situations, will either be consumed by yeasts or removed by CO2 evolution during fermentation before it can oxidize wort constituents.
The most deleterious effect of hot-side aeration is associated with long-chain staling aldehydes like trans-2-nonenal. Because of the practical importance of these compounds, an entire section later in this chapter is devoted to them.
BASIC MECHANISMS
This section considers two basic mechanisms that are major factors in beer staling. The first is auto-oxidation in which molecular oxygen directly acts on a compound to produce an oxidized product:

Auto-oxidation reaction
The second mechanism is more subtle, and it is sometimes called oxidation without molecular oxygen. It involves electron transfer during which an oxidized compound is reduced in conjunction with a reduced compound becoming oxidized.
The simplest example of auto-oxidation is the oxidation of ethanol to acetaldehyde via

Oxidation of ethanol to acetaldehyde
This reaction reverses the reduction step at the end of the EMP pathway discussed in chapter 3. Other alcohols, e.g., the fusel alcohols also discussed in chapter 3, can be oxidized by a similar mechanism:
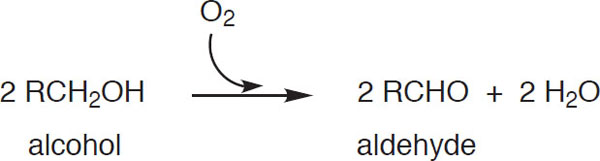
Oxidation of other alcohols to yield an aldehyde
The reappearance of these aldehydes invariably leads to unpleasant flavors (Fix, 1998). In addition, the aldehydes can further oxidize to an acid via
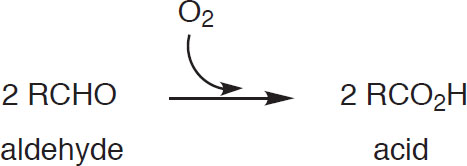
Oxidation leading to acid formation
The so-called vinegar process involves acetaldehyde and acetic acid whereby
![]()
Such reactions generally occur on the cold side of the brewing process, i.e., they are an important part of flavor disorders due to cold-side aeration.
The case of iso-α-acids is very important because beer staling invariably involves transformations of hop flavors and these transformations tend to be a signal that staling is underway. The most common mechanism involving iso-α-acids removes or cleaves the acyl side chain R

Acyl side chain R in an iso-α-acid
from the iso-α-acid. This reaction leads to the formation of fatty acids having unmistakable stale and cheesy tones. For isohumulone, whose structure is shown in chapter 2, the structure of the side chain R is as follows:

Side chain R in isohumulone
Splitting off this side chain yields isovaleric acid as the oxidation product. Cleavage of the side chain R from isocohumulone yields isobutyric acid. The structure of the side chain R for isocohumulone is as follows:

Side chain R in isocohumulone
Acyl side-chain splitting is also involved in the “sun-struck” effect mentioned in chapter 2. When iso-α-acids are exposed to light, photochemical cleavage takes place, yielding 3-methyl-2-butene-1-thiol. This compound is sulfur based and has a pronounced skunky character. It is interesting that the sun-struck phenomenon competes with oxidation, in the sense that beers with high oxygen levels (or with a large amount of oxidized components) tend to be more resistant to photochemical transformations than beers in a reduced state.
Another class of beer-staling constituents consists of fatty acids. In beer, fatty acids come from two sources, namely, unsaturated fatty acids from wort trub and saturated ones from yeast metabolism. As discussed in chapter 3, the saturated fatty acids can react with alcohols to form esters. The unsaturated fatty acids, on the other hand, are major players in beer staling. They tend to be fairly resistant to oxidation and spill over into the finished beer where they tend to produce “fatty or goaty notes.”
Fatty acids undergo auto-oxidation by the nonenzymatic pathway. The fate of oleic acid in the nonenzymatic mechanism is typical. This unsaturated fatty acid has the structure
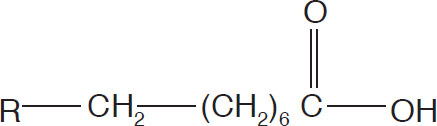
Oleic acid structure
The presence of molecular oxygen causes oleic acid to oxidize through loss of hydrogen; the oxidized product is
![]()
Oxidized oleic acid structure
This oxidation process can occur on both the hot and cold side of brewing. Whatever oxidized oleic acid spills over to the finished beer will tend to impart fatty and/or soapy notes. Melanoidins undergo auto-oxidation by the same mechanism, which is denoted by

Auto-oxidation of a melanoidin
However, this reaction definitely requires heat and, hence, is induced by molecular oxygen present in wort production. The melanoidins oxidized as a part of hot-side aeration are held in check during fermentation and most of maturation by the strong reducing effects of yeasts. In packaged beer, they can start further oxidation by the mechanism of oxidation by electron transfer without molecular oxygen. The melanoidin-induced oxidation of fusel alcohols is a common reaction:

Oxidation without molecular oxygen
In this reaction, oxidized melanoidin and alcohol yield reduced melanoidin and aldehyde.
The staling aldehydes tend to have a broad range of off-flavors, although metallic and/or grain astringent notes are often detected. Conversely, in a beer that already contains reduced melanoidins, they can act as flavor protectors by absorbing molecular oxygen and preventing it from reacting with alcohols or other easily oxidizable beer constituents. This effect is why dark beers, prepared with low hot-side aeration and high-melanoidin malts, can have extraordinary flavor stability.
Finally, malt- and hop-based phenols react much in the same way as unsaturated fatty acids and melanoidins. The auto-oxidation products, which are generated during hot-side aeration, have a characteristic harsh, grainy character. Once formed, phenolic compounds, melanoidins, unsaturated fatty acids, and iso-α-acids can interact in a highly complex electron-exchange system. The following diagram is an example in which a phenol is oxidized as oxidized fatty acids and melanoidins are reduced. The center circle refers to the liquid medium in which these reactions take place.

Electron-exchange reaction
Observe that molecular oxygen does not play a role in this system, although the beer flavors that result will invariably be perceived as associated with staling.
Oxidized phenols also display a tendency to polymerize via further oxidation, and this process produces harsh and astringent flavors. Various haze-forming mechanisms accompany this process. One typical mechanism is called Baeyer condensation. Here, wort-derived phenols like catechin react with aldehydes to form a complex.

D-catechin

Acetaldehyde
Both haze and the formation of harsh flavors accompany this reaction. The process is one instance in which haze formation and staling are coupled. Such a mechanism is called an oxidation haze, and it is a good indicator of staling. It, unlike chill hazes, is permanent and will remain even if the beer is warmed.
The role played by hop iso-α-acids in the electron-exchange system is also significant. Most important is the role they play as natural antioxidants (Hashimoto and Kuroiwa, 1981). One such mechanism involves the donation of electrons to melanoidins. This results in the acyl side-chain cleavage discussed above, but it also results in the reduction of oxidized melanoidins. This effect then inhibits the melanoidin-mediated oxidation of alcohols.
Most important, however, is the reduction of unsaturated fatty acids by this same mechanism. This process inhibits the mechanisms (discussed in the next section) that lead to trans-2-nonenal and is the main reason why carefully brewed beers with elevated hop levels are less prone to develop cardboard and/or papery notes compared to other beers. This phenomena is often incorrectly attributed to hop flavors masking the flavors of staling compounds. If anything, the reverse is more common in most beers.
THE SPECIAL CASE OF TRANS-2-NONENAL
The most feared of the staling compounds are a class of long-chain unsaturated aldehydes, of which trans-2-nonenal (T-2-N) is a major example. It has very powerful paper- and/or cardboard-like flavors with a threshold of a mere 0.00010 mg/L (i.e., 0.1 parts per billion). Unlike other staling compounds, there are very few beer drinkers who are taste blind to these compounds, and their presence is universally seen as a major defect. The striking lack of brand loyalty for “import beer” (as defined previously in this chapter) is very likely a reflection of this phenomenon.
Short-chain aldehydes of the form

Short-chain aldehyde
are precursors to T-2-N. They, plus whatever T-2-N is present, are called the T-2-N potential. These short-chain precursors are somewhat neutral in flavor, but are readily transformed to a longer-chain (and much stronger-flavored) version by aldol condensation (Narziss, 1989). The long-chain aldehydes have the form
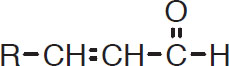
Long-chain aldehyde
with T-2-N being the special case, as shown in the following diagram:
![]()
Side chain in trans-2-nonenal
It was once thought (see, e.g., Chapon, 1981; Liebermann, 1984; MacFarlene, 1970) that just about all of the oxidative defects, including the presence of T-2-N, were attributable to air in the headspace of beer packages. An early fundamental paper (Hashimoto, 1975) showed that air in the headspace was not the cause of the T-2-N problems and, in particular, that the total T-2-N potential was not affected by the presence of molecular oxygen. If anything, it tends to reverse aldol condensation, although this effect is weak in packaged beer (Narziss, 1989). Later work (Hashimoto, 1986) showed that the T-2-N potential was set by the wort production and changed little from that point forward. These findings were confirmed by Narziss (1990) (see also Huige, 1993; Fix, 1992), who emphasized the elimination of hot-side aeration.
More recent studies (see, e.g., Cantrell and Griggs, 1996; Forster et al., 1998; Lermusuary et al., 1999) have shown that eliminating hot-side aeration is not the entire answer because there were other pathways leading to T-2-N. Confirmation of its presence can be detected during formal tastings of prestigious European beers produced in very modern facilities that have been completely optimized with respect to cold-side aeration and hot-side aeration. On occasion (see Fix and Fix, 1999), the “nonenal problem” with these beers can still be severe.
Dutch researchers (Barker, 1983) showed that lipase and lipoxygenase enzymes present in barley are the key to an enzymatic pathway leading to T-2-N. This pathway along with the hot-side aeration (nonenzymatic pathway) is shown here:

Enzymatic and nonenzymatic pathways
Both pathways occur in wort production and involve unsaturated fatty acids in a fundamental way. It has been shown (Barker, 1983; Nyborg et al., 1999) that in fresh beers, both T-2-N and its precursors are bound up with natural sulfur compounds from yeast metabolism. However, after a lag, which is reduced if thermal or mechanical abuse occurs, the effects of T-2-N start becoming discernible.
Note that the enzymatic pathway does require some oxygen in conjunction with the action of lipoxygenase. However, this requirement is small (typically in the range of parts per billion) and thus virtually impossible to avoid in any type of brew house, even modern high-tech configurations that have been completely optimized with respect to hot-side aeration. As a consequence, the practical control of T-2-N has focused on wort lipid levels and the malts used. First of all, achieving a reasonable wort clarity in lautering is important. It is interesting that some of the biggest nonenal problems have been in breweries who have stepped up to a 12-brew-a-day schedule, and with this increase, their wort has undergone a reduction in clarity (Fix and Fix, 1999).
That malt and malting are involved in the formation of T-2-N was first suggested by Doperer et al. (1991). It is true that lipase and lipoxygenase are thermally unstable and thus 96% of these enzymes are lost on kilning of pale malts (Cantrell and Griggs, 1996) in a process called blanching. Even more is lost for Munich and Vienna types. Unfortunately, because of the very low flavor threshold of T-2-N, not much is needed before the deleterious flavor appears. As a consequence, careful malt selection (and reasonable wort clarity) may be the best way to minimize the effects of T-2-N, assuming of course that hot-side aeration is not a factor.