2
Wort Boiling
Wort boiling is the one step that is used by all brewers, from beginners working with malt extracts to experts working with sophisticated equipment. It is certainly the easiest part of brewing to manage and, until recent years, has not been given a great deal of attention other than the technological issues associated with equipment. It is ironic that from a purely scientific point of view, it is by a wide margin the most sophisticated part of brewing. The complex redox and Maillard reactions that take place make the microbiological systems in the fermentation look like child’s play in comparison. Moreover, there is growing awareness that these reactions do affect finished beer flavors in nontrivial ways, and hence, this area of brewing should not be ignored.
The next section discusses hops and focuses on the fundamental issues that have undergone a considerable evolution since the first edition of this book. For a review of all of the varieties available, see Fix and Fix (1997).
The last two sections discuss the need to establish a sufficiently vigorous boil so that sulfur constituents, most notably dimethyl sulfide (DMS), are appropriately reduced, while at the same time, production of certain Maillard products is minimized. Some Maillard products have less than impressive flavors, particularly when they arise from excess thermal loading during the boil. Successful brewing, like gourmet cooking, is very much a balancing act, and these two aspects of wort boiling are but one example of this point.
HOPS
Hops are botanically classified as Humulus lupulus. Only the flowers, or cones, of the female plant are of interest to brewers. Their brewing value comes from the resins and essential oils found in the lupulin glands (Neve, 1991; Garetz, 1994).
RESINS
There are two types of resins. Hard resins are those that are insoluble in hexane. In fresh hops, they consist of at most 5% to 6% of the total resin content (measured by weight) although this percentage generally increases as the hop deteriorates while aging in storage. The brewing value of the hard resins is negative.
The soft resins are those that are soluble in hexane. They have been subdivided into α-acids, ß-acids, and uncharacterized soft resins.
α-Acids. The bulk of the hop bitterness in beer is due to α-acids (Verzeli and de Keukeleire, 1991). These are phenolic-like compounds (i.e., they contain aromatic rings) that consist of humulone and its analogues. The α-acid structure is
α-acid
In this structure, R stands for a side chain that defines the humulone analogue, as follows:
|
Name |
R (= Acyl side chain) |
|
Humulone |
COCH2CH(CH3)2 |
|
Cohumulone |
COCH(CH3)2 |
|
Adhumulone |
COCH(CH3)CH2CH3 |
Observe that cohumulone has one less carbon atom than the other analogues, which among other things means that after isomerization (a chemical process in which an organic compound is transformed into another organic compound—an isomer—having the identical chemical composition and molecular weight, but a different structure), cohumulone is more soluble than the other isomerized analogues. Isomerization of cohumulone is described later in this section. It is now generally acknowledged that cohumulone gives a harsher bitter than the other analogues (Rigby, 1972). Hops like Brewers Gold, Clusters, Eroica, and Galena invariably have cohumulone levels in excess of 35% of the total α-acids and are indeed known for their very pungent bitter. In contrast, noble varieties like Hallertau, Saaz, and Tettnang always have cohumulone levels below 25%.
It was once feared that high cohumulone levels were endemic to high-α hops, i.e., hops like Eroica and Galena whose α-acid levels are in excess of 10%. This concern was initially countered by brewers using Columbus, a high-α and moderate–cohumulone hop that recalls Cascade and Centennial, yet because of its fine bitter and aroma, has been widely used by ale brewers both as a kettle and dry hop. Even more striking is Horizon, a new high-α hop whose cohumulone levels are at or slightly below those in noble varieties. Many lager brewers who have a long tradition of commitment to low-α hops are showing interest in this variety.
How a hop is used is also important. Because of the high solubility of cohumulone, brief contact times with wort can lead to undesirably high cohumulone levels even with noble hops. Late hopping in a whirlpool is an example (Mitter, 1995) that is discussed later in this chapter.
A point that cannot be overemphasized is that the characteristics of hops, like grapes, depend on where they are grown. The dramatic difference between the Saaz varieties grown in the United States and those from the Saaz region in Bohemia is but one example of this point. This assertion is not to imply that the former is an inferior hop, but rather that it is different from its European counterpart and should be evaluated on its own merit.
ß-Acids. The ß-acids have the following structure:
ß-acid
Again, the R stands for a side chain. In ß-acids, the R is one of the following three analogues:
|
Name |
R (= Acyl side chain) |
|
Lupulone |
CH2CH(CH3)2 |
|
Co-lupulone |
CH(CH3)2 |
|
Adlupulone |
CH(CH3)CH2CH3 |
These ß-acid compounds generally do not play a major roll in fresh hops. However, as hops age they oxidize to more soluble compounds that form an important part of the nonhumulone bitter of beer (Wackerbauer, 1993). The desirability of these compounds depends very much on their concentration and on the type of beer being brewed.
The ratio of α-acids to ß-acids is often cited—along with the percentage of cohumulone—as an important quality indicator. Lager brewers in particular are generally sensitive to this index; however, explanations showing why this index should be important have not been developed. All of the noble varieties have α/ß ratios in the range 0.8 to 1.2. Varieties like Chinook and Eroica, on the other hand, have ratios in excess of 2.5 and generally near 3. It is also striking that Horizon, a high-α hop that is attracting the attention of many lager brewers, has an α/ß ratio below 2. This is the lowest ratio among the major high-α varieties.
Hops with poor storage stability can lose significant amounts of their α-acids in just one year. For example, the α-acid level of refrigerated Cascade pellets dropped from 7.6% to 4.6% after 12 months (Peacock et al., 1981). In the same study, the α-acid level of Hersbrucker pellets dropped from 7.4% to 4.7%. Other varieties showed lower rates of α-acid reduction.
The end products of the oxidation of humulones during storage (and in the kettle boil) comprise a complex array of different compounds (Verzeli and de Keukeleire, 1991). The reaction involves the uptake of oxygen atoms, the following being typical:

α-acid
Oxidized product
The acyl side chain R can also be cleaved via oxidation, giving valeric, butyric, and 2-methyl butyric acids via the mechanisms described in chapter 4. All of these have unmistakable “cheesy” tones.
ESSENTIAL OILS
Although the resins provide the primary hop bitterness, the essential oils are responsible for the overall hop presence, particularly the hop aroma. The essential oils include hydrocarbon components, oxygen-bearing components, and sulfur-containing components.
Hydrocarbon components. The hydrocarbon components form slightly less than 75% of the essential oils (Kunze, 1996) and consist of a monoterpene (e.g., C10H16) or a sesquiterpene (e.g., C15H24). Terpenes are a structurally diverse family of compounds with carbon skeletons like those of humulene and myrcene, the two main hydrocarbon compounds that have been identified in beer. Their structures are shown here.

Humulene
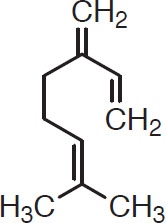
Myrcene
Humulene has a delicate and refined flavor that is often described as “elegant.” Myrcene has a greater flavor intensity, frequently characterized as “pungent.” Because of its structure, myrcene is usually classified as a monoterpene, whereas humulene is classified as a sesquiterpene. Two other sesquiterpenes of interest are farnesene and caryophyllene:

Farnesene

Caryophyllene
Distinctions between noble and more aggressively flavored hops can be seen in their content of essential oils. The noble hops typically have very high sesquiterpene/monoterpene ratios, generally 2.5–4. On the other hand, the pungent hops are typically dominated by myrcene, which can constitute almost 60% of their essential oils (thus yielding a sesquiterpene/monoterpene ratio near 0.67).
Another widely cited index is the H/C ratio, i.e., the ratio of humulene to caryophyllene. The H/C ratio of noble varieties will generally exceed 3.5, although there are variations. Thus, this index is probably a better discriminator among noble hops than a discriminator in general. The same is true for the farnesene level. For example, Saaz-type hops (Saaz, Tettnang, Spalt) tend to have much higher values than the Hallertau types (Peacock, 1992). A summary of the various indices cited above for selected hop types is given in Tables 2.1 to 2.3. These data were taken from Garetz (1994), Peacock (1992), and Haunold (1998).
The data cited in Tables 2.1–2.3 are for fresh-leaf hops. Oxidation will alter their composition, especially the essential oils, as described later in this chapter. Also, heat treatment such as pelletization can alter hop composition. For example, high-farnesene hops like Saaz and Tettnanger tend to lose the farnesene oil component during pelletization. Such farnesene reduction is likely the main reason why there are clear differences in the character of beer aroma depending whether leaf or pelletized versions of these hops are used.
Oxygen-bearing components. The oxygen-bearing components form approximately 25% of the hop essential oils (Kunze, 1996). Nevertheless, in selected varieties, these oil constituents can play a major role in defining the hop’s basic flavor characteristics. Two alcohols in this category, namely linalool and geraniol, have been extensively studied (Peacock et al., 1981). In isolation, linalool has a pleasing hoppy smell, not as elegant as humulene, but quite attractive. Geraniol, in isolation, tends to recall floral or herbal cheap perfume. Nevertheless, there is a synergy between these oils. Very floral or herbal hops like Amarillo, Cascade, Centennial, and Columbus tend to have very high concentrations of both oils, which is likely responsible for their very special aroma (Peacock et al., 1981).
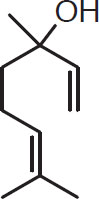
Linalool (C10H18O)
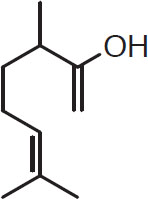
Geraniol (C10H18O)
| Table 2.1 Selected German and Czech Hops | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Name |
α |
α/β |
CoH |
MYR |
HUM |
F/H |
H/C |
Comments |
|
Czech Saaz |
3–4.5 |
1 |
20–25 |
30–35 |
25–30 |
1.5–2 |
3.5–4 |
Traditional noble hops |
|
Hallertauer Mittelfruh |
3–4.5 |
1 |
20–25 |
30–35 |
35–40 |
0 |
3.5–4 |
Traditional noble hops |
|
Hersbrucker |
3–5.5 |
1 |
20–25 |
35–40 |
30–35 |
0 |
3–3.5 |
Traditional noble hops |
|
Spalt |
4–5.5 |
1 |
25–30 |
15–25 |
15–20 |
1.5–2.0 |
1.5–2 |
Traditional noble hops |
|
Tettnanger |
3.5–5.5 |
1 |
20–25 |
30–35 |
20–25 |
1.5–2 |
3.5–4 |
Traditional noble hops |
|
Hallertauer Tradition |
5–7 |
1–1.5 |
25–30 |
20–25 |
45–55 |
0 |
.3–3.5 |
New |
|
Spalter Select |
5–6 |
1–1.5 |
20–25 |
15–25 |
15–25 |
1.5–2.0 |
2–2.5 |
Low-α hops |
|
Northern Brewer |
7–10 |
2–2.5 |
30–35 |
30–35 |
25–30 |
0 |
3–3.5 |
Other |
|
Perle |
7–10 |
1.5–2.0 |
30–35 |
45–55 |
30–35 |
0 |
2.5–3 |
Other |
|
NOTE: α = α-acid level (% of the total acids), α/ß = ratio of α-acid to ß-acid, CoH = cohumulone (% of the total a-acids), MYR = myrcene (% of the total essential oils), HUM = humulene (% of the total essential oils), F/H = farnesene/humulene ratio, and H/C = humulene/caryophyllene ratio. Data from Garetz (1994), Peacock (1992), and Haunold (1998). |
||||||||
| Table 2.2 Selected U.K. Hops | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Name |
α |
α/β |
CoH |
MYR |
HUM |
F/H |
H/C |
Comments |
|
East Kent Golding (EKG) |
4–5.5 |
1.5–2.5 |
20–25 |
45–50 |
25–30 |
0 |
3.5 |
Traditional U.K. aroma hops |
|
Fuggles |
4.5–5 |
1.5–2 |
20–25 |
25–30 |
35–40 |
1.5–2 |
3.3 |
Traditional U.K. aroma hops |
|
First Gold |
7–10 |
1.5–2 |
30–35 |
25–30 |
20–25 |
0.1–.2 |
3–3.5 |
New EKG replacement |
|
Admiral |
11.5–14.5 |
2–3 |
40–45 |
40–50 |
25–30 |
0.1–.15 |
3–3.5 |
High α-hops |
|
Phoenix |
8–11.5 |
1.5–2 |
30–35 |
25–35 |
25–35 |
0 |
2.5–3 |
High α-hops |
|
Wye Target |
10–13 |
2.2 |
35–40 |
60–65 |
10–15 |
0 |
2.4 |
High α-hops |
|
NOTE: α = α-acid level (% of the total acids), α/ß = ratio of α-acid to ß-acid, CoH = cohumulone (% of the total a-acids), MYR = myrcene (% of the total essential oils), HUM = humulene (% of the total essential oils), F/H = farnesene/humulene ratio, and H/C = humulene/caryophyllene ratio. Data from Garetz (1994), Peacock (1992), and Haunold (1998). |
||||||||
| Table 2.3 Selected U.S. Hops | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Name |
α |
α/β |
CoH |
MYR |
HUM |
F/H |
H/C |
Comment |
|
Crystal |
3.5–5 |
0.5–1 |
20–25 |
40–50 |
20–30 |
0 |
3.5–4 |
Low-α hops |
|
Liberty |
3–5 |
1–1.5 |
25–30 |
35–40 |
35–40 |
0 |
3.5–4 |
Low-α hops |
|
Mt. Hood |
5–8 |
1 |
20–25 |
55–65 |
15–25 |
0 |
2–2.5 |
Low-α hops |
|
Santiam |
6–7 |
0.8–1.0 |
15–20 |
30–35 |
20–25 |
0.5 |
3.2–3.5 |
Low-α hops |
|
Tettnanger |
4–5 |
1–1.5 |
20–25 |
35–45 |
20–25 |
1.0–1.5 |
3–3.2 |
Low-α hops |
|
Cascade |
4.5–7 |
1 |
35–40 |
45–60 |
10–15 |
1.0–1.5 |
3.5–4 |
Moderate-α hops |
|
Amarillo |
4.5–8 |
1 |
30–40 |
40–50 |
10–20 |
1–1.5 |
3.5–4 |
Moderate-α hops |
|
Centennial |
9.5–11.5 |
2.5–3 |
30–35 |
45–55 |
15–20 |
0 |
2.2 |
Moderate-α hops |
|
Northern Brewer |
8–10 |
2–2.5 |
20–30 |
50–60 |
20–30 |
0 |
3–3.5 |
Moderate-α hops |
|
Brewers Gold |
5.5–8.5 |
2–2.5 |
40–45 |
60–65 |
10–15 |
0 |
2–2.5 |
Pungent; moderate-α hops |
|
Cluster |
5.5–8.5 |
1–1.5 |
35–45 |
45–55 |
15–20 |
0 |
2–2.5 |
Pungent; moderate-α hops |
|
Columbus |
14–16 |
3–4 |
25–30 |
25–30 |
15–25 |
0 |
2–3 |
Special high-α hops |
|
Horizon |
11–13 |
1.7 |
15–20 |
40–50 |
10–15 |
0.3 |
1.5–2 |
|
|
Chinook |
12–14 |
3.5–4 |
30–35 |
35–40 |
20–25 |
0 |
2–2.5 |
High-α hops |
|
Eroica |
11–13 |
2.5–3 |
35–45 |
55–65 |
0 |
0 |
0 |
High-α hops |
|
Galena |
12–14 |
1.5–2.0 |
40–45 |
55–60 |
10–15 |
0 |
3 |
High-α hops |
|
Nugget |
12–14 |
2.5–3 |
25–30 |
50–60 |
10–20 |
0 |
2 |
High-v hops |
|
NOTE: α = α-acid level (% of the total acids), α/ß = ratio of α-acid to ß-acid, CoH = cohumulone (% of the total a-acids), MYR = myrcene (% of the total essential oils), HUM = humulene (% of the total essential oils), F/H = farnesene/humulene ratio, and H/C = humulene/caryophyllene ratio. Data from Garetz (1994), Peacock (1992), and Haunold (1998). |
||||||||
As noted, the hydrocarbons undergo alterations of their oil structure during storage. For example, one study reported that the myrcene level of Cascade hop pellets decreased from 329 mg/L to 7 mg/L after 12 months of refrigerated storage (Haley and Peppard, 1983). For this reason, many now believe that the oxidized products of hop oils are actually more important than primary hydrocarbons like humulene and myrcene.
Sesquiterpenes such as those listed below are known to be important oxidation products. Most studies show that they consistently increase during hop storage as the primary hydrocarbons decrease (Haley and Peppard, 1983). Five sesquiterpenes derived from humulene are humuladienone, humelene, epoxide I, humelene epoxide 2, humelol, and humulenol. Their structures are key shaped:

Humuladienone
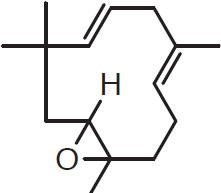
Humulene epoxide 1
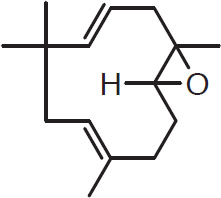
Humulene epoxide 2

Humelol
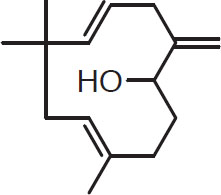
Humulenol
Oxidation of myrcene leads to the analogous products myrcene epoxide and myrcenol:

Myrcenol
The oxidation products from humulene tend to have sensory characteristics that recall hay or sagebrush; sometimes the term grassy is used. Flavor panels tend to be sharply divided over the desirability of such flavors (Stucky and McDaniel, 1997). The products derived from myrcene, on the other hand, retain the pungency of their precursor, but at a slightly lower intensity.
Sulfur-containing components. Beers containing excessive sulfury tones are generally regarded as defective. Thus, a good deal of attention has been given to the origin of these flavors. It is believed that malt and the fermentation are the major sources of sulfury flavors, but hops are known to contribute to them as well.
Of the various mechanisms that are possible, two are well understood. One is the “light-struck phenomenon” described by S. Bickham (1998). Light of wavelength from 400 to 500 nm can cause photochemical rearrangement of hop resins, resulting in a sulfury mercaptan compound with a pronounced skunky character. The other mechanism arises from excessive use of sulfur compounds in the field to protect the hops from fungus infestation before harvest. Most of the residual sulfur, however, is removed in the kiln (i.e., during hop drying after harvest), and residual sulfur in hops added early in the kettle boil is generally evaporated and not passed on to the finished beer. High-sulfur hops used in late kettle additions or dry hopping can still produce unacceptable levels of thioesters (thio means sulfur-bearing; esters are a class of typically aromatic organic compounds). High thioester production became a serious problem for brewers in England in the 1970s. However, greater caution has been used in fungicidal spraying since that time (Seaton et al., 1981).
Aside from these well-understood mechanisms, there is also the poorly understood role of sulfur-based radicals (i.e., parts of complex compounds) that are natural to hop constituents. Hop oils in particular are known to contain various thiols, sulfides, thioesters, thiophenes, and episulfides. Their concentrations are small, yet so are their flavor thresholds. As a consequence, their overall relevance to beer is yet to be determined.
HOP TRANSFORMATIONS DURING WORT BOILING
Isomerization of hop resins. The α-acids in hops are poorly soluble in wort; under boiling conditions, however, the α-acids are rearranged to more soluble iso-α-acids (iso- indicates that the compound is an isomer of another compound). This transformation is called isomerization. As an example, consider the humulone structure (for purposes of discussion, the six carbon atoms in the phenolic ring are numbered):
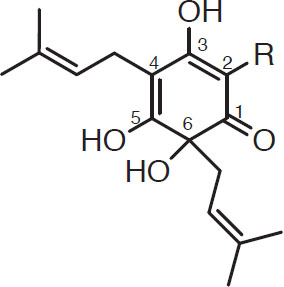
Humulone
Under boiling conditions, the bond between the first and sixth carbon atoms is broken, and a new bond is formed between carbon atoms one and five. The result is isohumulone:
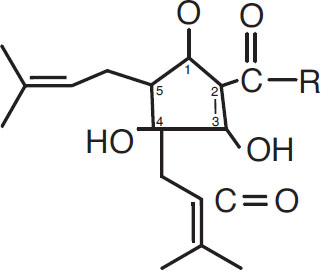
Isohumulone
Similar transformations take place for the humulone analogues cohumulone and adhumulone.
Not all of the α-acids added to the brew kettle will be converted to iso-α-acids. In addition, some of these hop resins are lost during both the hot break and the cold break. It is customary to define the efficiency of this process in the following way as a kettle utilization rate (KUR):
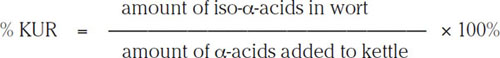
(2.1)
Kettle utilization rates vary with many factors. However, for a given brewing configuration, it is the hop type (whole, pellet, or extract), contact time, temperature vs. pressure profile, wort gravity, and pH that cause batch-to-batch variations (Fix and Fix, 1997). With these factors under control, brewers can make accurate estimates of their KUR from measurements of test brews. After that, the wort iso-α-acid content can be directly computed from the α-acid content of the hops used. In metric units (i.e., SI—International System of Units), this calculation is trivial. For example, using 200 g of 5% α hops per hectoliter is equivalent to an α-acid level of
![]()
and, through standard conversion of units,
![]()
If the KUR is 25%, then the iso-α-acid level of wort is
![]()
For those working in oz./bbl or oz./gal., it is easiest to convert values in these units to metric units via the following conversion relationships:
Then do the rest of the calculation in metric units.
The term bitterness unit (BU) typically refers to a number measured by a spectrophotometer (see Methods of Analysis, American Society of Brewing Chemists, 1987). It measures selected soft resins as well as iso-α-acids, and the result is typically 5%–10% higher than the actual iso-α-acid level. The iso-α-acid level in finished beer is typically less than that of wort. For example, losses in the fermentation occur when hop constituents get trapped in the bottom yeast layer (Garetz, 1994). These losses, however, tend to be constant in any given brewing configuration, so they can be estimated from measurements of test brews. Garetz (1994) also has given some useful rules for determining approximate losses.
It must be emphasized that the perception of hop flavor (taste and smell) is far too subtle to be captured by one single number. It is not uncommon for one beer to be rated as having a milder hop bitter than another in blind tastings, even though the former has a higher BU (see, for example, Preis and Mitter, 1995). Moreover, there is absolutely no correlation between BUs and the quality of the hop flavor. Thus, BUs should be seen as a rough estimate of the overall intensity of a beer’s hop profile, but at the end of the day, the human palate reigns supreme as the ultimate arbitrator.
Oxidation and/or polymerization of hop resins and oils. As noted above, the reaction mechanisms in wort boiling are by far the most sophisticated to be found in brewing. This fact is illustrated by complicated transformations involving humulones and deoxyhumulones (Shannon et al., 1978; these transformations have been further elaborated on by Verzeli and Keukeleire, 1991). Ironically, the overall relevance of these mechanisms is still unresolved.
There are, however, some established facts about transformations of cohumulone. As noted earlier, isocohumulone is the most soluble of the humulone analogues. Moreover, it is ultimately transformed into milder products. For example, M. Mitter (1995) compared the isocohumulone concentrations of two brews. The brews were identical in composition and brewing method except for one thing: the hops were added with the first wort (first-wort hopping), but in the second brew, the hops were added at the very end of the boil. A low–cohumulone hop (Tettnanger) was used in both brews. It was found that the isocohumulone level of the first-wort-hopping brew was 20% lower, and blind tasting of the finished beers showed a clear preference for it. Additional studies have shown that actually as little as 15 minutes of wort boiling will reduce the isocohumulone level to within 5% of the value of first-wort hopping.
Transformations of the hop oils are even more important as far as finish beer flavor is concerned. The lovely aroma that occurs after hops are added to boiling wort suggests that perhaps there is a considerable loss of hop oils. Moreover, it would seem that the most desirable components are the first to go. This problem has led to a distinction between “bitter hops” and “aroma hops.” The former are used solely for their α-acids and are added early into the boil. Aroma hops, on the other hand, are valued for their fine gastronomic qualities and are usually used in late hopping. De Clerck (1957) questioned these assumptions. He asserted that beer always retains the smell of hops no matter when they were added.
Direct evidence of this assertion is given by Preis and Mitter (1995). They compared the two extremes—namely, a late-hopped beer (the “reference” beer) and a first-wort-hopped beer (FWH)—by using both chromatography and blind tasting with a professional taste panel. The comparison was done at two breweries (A and B), and the procedures described in Table 2.4 were followed. The chromatographs of the reference and FWH brews were dramatically different (see Figs. 1 and 2 in Preis and Mitter, 1995). They suggested that there was not so much of a loss of hop oil in the FWH brew, but rather a different spectrum of oil components. There was, however, a noticeable drop in the oxygen-bearing components of the hop aroma substances (see Table 2.5). In addition, there was a predictable increase in the iso-α-acids recovery with FWH (see Table 2.4).
| Table 2.4 Hop Additions | ||
|---|---|---|
|
Brewery A |
Brewery B |
|
|
Hop type |
Tettnang and Saaz pellets |
Tettnang pellets |
|
Total α-acid added |
13 g/L |
13 g/L |
|
Reference brew |
||
|
Middle addition |
63% |
48% |
|
Late addition |
37% |
52% |
|
FWH brew |
||
|
First wort |
37% |
52% |
|
Middle addition |
63% |
48% |
| Table 2.5 Iso-α-Acid Recovery | |||
|---|---|---|---|
|
Brewery A |
Brewery B |
||
|
Wort iso-α-acid |
|||
|
Reference |
47.6 |
32.6 |
|
|
FWH |
55.0 |
44.8 |
|
|
Beer iso-α-acid |
|||
|
Reference |
40.9 |
27.4 |
|
|
FWH |
42.7 |
35.1 |
|
What is striking is the strong preference that the professional panels of both breweries showed for the FWH beer. The sensory assessments found that the FWH beer had “a finer and rounded hop aroma” with a “finer and more pleasant” bitter in spite of the higher iso-αacid concentration. The latter conclusion could possibly be explained by the lower cohumulone content. The former came as a surprise to the investigators, and they conjectured that the reduction in the oxygen-bearing components of the hop oils (Table 2.6) may be the reason. The results of the professional tasting panel in the Preis and Mitter (1995) study are shown in Table 2.7.
| Table 2.6 Hop-Oil Concentrations (µg/L) | ||
|---|---|---|
|
Brewery A |
Brewery B |
|
|
Linalool |
||
|
Reference |
29.0 |
34.1 |
|
FWH |
8.1 |
6.4 |
|
Geraniol |
||
|
Reference |
18.8 |
14.6 |
|
FWH |
10.7 |
13.7 |
|
Humulene epoxide |
||
|
Reference |
32.7 |
10.8 |
|
FWH |
19.6 |
9.8 |
| Table 2.7 Results of the Professional Tasting Panel | ||
|---|---|---|
|
Brewery A |
Brewery B |
|
|
No. of tasters |
12 |
13 |
|
No. passing triangle test |
11 |
12 |
|
Preference for FWH beer |
8 to 3 |
11 to 1 |
After the Brauwelt study (Preis and Mitter, 1995) was published, FWH was tried by a large number of homebrewers. Some came to the same conclusions as those in the Brauwelt study, but not everyone agreed (Home Brewers Digest [hbd.org]—Electronic Discussion Group on Beer), which is not very surprising given the highly heterogeneous nature of homebrewing. Experimentation with FWH has also been done by commercial craft brewers. Preliminary results have been favorable; however, additional research is needed into how well the procedures work with the type of ales that are brewed by craft brewers. The Brauwelt study was confined to Pilseners, as were follow-up studies (Fix, 1997).
PRODUCTION AND REDUCTION OF DIMETHYL SULFIDE
As noted in chapter 1, dimethyl sulfide (DMS) is the most important sulfur constituent in beer. Aside from bacterial action, the most common reaction system is production of DMS via heat in malt kilning, wort boiling, and wort cooling. The DMS precursor of greatest interest is S-methyl methionine (SMM), which, during heating, breaks down to DMS in the following way:
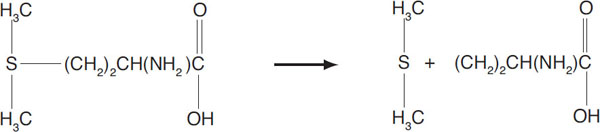
Breakdown of SMM to form DMS
Even SMM itself is not neutral to finished-beer flavor. For example, free SMM can bond with heterocyclics to form some rather nasty compounds (see the next section).
SMM is formed in malt during germination. Moreover, as the nitrogen content of SMM suggests, its formation closely follows protein levels and protein modification. As a consequence, high-protein malts (e.g., those made from six-row varieties) tend to have higher SMM levels than low-protein malts. For example, English pale malts tend to have 1 to 2 μg of SMM per gram of malt, whereas some North American six-row varieties can run as high as 8 to 10 μg of SMM per gram of malt.
As the malt is dried during the kilning, some SMM is lost by heat decomposition into DMS and other products. These are removed in later processing and are unimportant to DMS levels in the finished wort. It is for this reason that malts produced at high kilning temperatures (e.g., ale and color malts) have lower SMM levels than lager malts.
During wort boiling, SMM is converted to DMS via a first-order reaction mechanism. Under isothermal conditions, this reaction is well approximated by a linear ordinary differential equation of the form
![]()
(2.2)
where x is the SMM concentration at time t and c is a positive constant. Equation 2.2 states that the rate of reduction of SMM (i.e., the negative of the derivative dx/dt) is proportional to the instantaneous concentration x. It is customary to write this equation in terms of the half-life H of SMM. By using the concept of half-life, the constant C can be written
![]()
(2.3)
where 21/H represents 2 taken to the power of (1/H) and ln indicates the natural logarithm (Hughes-Hallet et al., 1998). The solution for the concentration x at any time t is
![]()
(2.4)
where x0 is the initial SMM concentration. Careful measurements have shown that the half-life H of SMM is 40 min for an isothermal boil at T = 100 °C and ambient pressure.
To illustrate the implications of this model, suppose one is using malt that averages 5 μg of SMM per gram of malt, and suppose that the malt concentration is 20 kg/hL = 200 g/L (which is typical of 12 °P wort). If any DMS produced during mashing is ignored, the SMM content of wort at the start of the boil is
![]()
Suppose a boil at 100 °C for t = 90 min is used. Then
![]()
of SMM survives the boil, and the rest is transformed to DMS, which means
of DMS was created. Reasonable kettle ventilation allows the DMS so formed to be removed with the vapor. The residual, i.e., 210 μg/L of free SMM, is considered as DMS potential, since the transformation SMM → DMS can still occur after wort boiling.
However, the most obvious place where this transformation occurs is during wort cooling, particularly if this is done in a closed environment. The half-life of SMM (HSMM) is approximately related to temperature T by an inverse relationship. As the temperature falls below 100 °C, the HSMM will increase from its value of 40 min at 100 °C. If T = T(t) denotes the temperature profile over time t, then measurements indicate that the half-life at any given time H(t) obeys (to a first approximation) the inverse relationship
![]()
(2.5)
for 50 °C < T(t) < 100 °C. At temperatures below 50 °C, the number 4000 must be replaced with a function of time t, which rapidly increases below 40 °C. This implies that the changes in the SMM concentration over time satisfy a time-dependent differential equation (equation 2.2), where C is now a function of time via equations 2.3 and 2.5. An approximate solution is given by
![]()
(2.6)
where
![]()
(2.7)
Observe that if T = 100, then equations 2.6 and 2.7 reduce to equation 2.4 and H = 40 min.
A couple of numerical examples may clarify this topic. Suppose the wort cools from 100 °C to 10 °C in t = 60 min. Taking
![]()
then
![]()
This result means that of the 210 μg of SMM per liter remaining after the 90 min boil, the concentration of SMM left after cooling to 10 °C in t = 60 min is
![]()
and the concentration of DMS created during the cooling is
![]()
Practically all of the DMS will be dissolved in wort if a closed cooling system is used. Note that this concentration is slightly over three times the DMS threshold of 30 μg/L.
On the other hand, if the cooling time is cut in half to t = 30 min, then
![]()
and so
![]()
of SMM remains, giving a dissolved DMS concentration of
![]()
Chapter 3 shows that fermentation at ambient temperatures will cause an approximately 50% reduction in both SMM and DMS due to CO2 evolution. For cold fermentations at 10 °C, however, SMM and DMS will only be reduced by 25%. Thus for lager brewers, the first scenario will have an objectionable concentration of DMS according to Kunze’s rule of no more than 60 μg/L (see chapter 1), but the short (t = 30 min) cooling procedures will likely lead to a normal DMS level between 30 to 50 μg/L. Nevertheless, many lager brewers worry about the SMM residuals in both cases, and as a consequence, take extra steps. The largest brewery in the United States sprays the hot wort with nitrogen gas, which removes virtually all DMS and SMM. However, the procedure generally removes some desirable compounds as well, leaving a finished beer with an inordinately low malt profile. Another procedure that is used for reducing (as opposed to eliminating) SMM and DMS levels consists of holding wort at 90–95 °C, allowing for maximum ventilation. Equations 2.6 and 2.7 allow one to determine the time period required.
For ales, these sulfur-compound issues tend to disappear. For example, ale malt with only 1 μg of SMM per gram of malt is common. This low initial concentration means that the SMM level at the start of the boil is
![]()
This initial SMM concentration leaves a SMM residual of
![]()
at the end of the boil. As noted above, 50% of the sulfur-containing compounds (be they SMM or DMS) is removed in the fermentation. Thus, the finished beer will have subthreshhold levels of DMS no matter how the wort is cooled. Indeed, any hint of DMS in ales is likely from technical brewing errors, most notably contamination (see chapter 3). This fact probably explains why many ale aficionados react very negatively to DMS in any type of beer.
NONENZYMATIC BROWNING
The Maillard reactions discussed in chapter 1 in the context of malting also occur in wort boiling. Schematically, the chemical process involved is represented as follows:
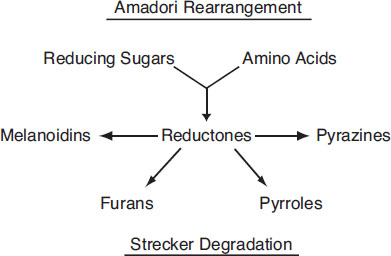
Nonenzymatic browning
This process, which is sometimes called nonenzymatic browning (NEB), is remarkably efficient. For example, the carbohydrate profile is modified only in trivial ways, and amino acids used in the process account for only 1% of the total, yet the wort color can typically double. The heterocyclic compounds formed in the process can be assigned to one of the three following levels of complexity:
• Level 1—Simple melanoidins
• Level 2—Sulfur-bearing heterocyclic compounds
• Level 3—Strongly flavored heterocyclics
As the term reductones suggests, they are natural antioxidants. These compounds are found in abundance in well-executed decoction mashes. Of all wort reductones, the simple melanoidins in their reduced state are the most desirable. They have an attractive “toasty” flavor in amber and dark beers. Similar results can be achieved with worts produced from infusion mashes if boiling is properly conducted. On the other hand, excessive thermal loading (e.g., uncontrolled high-temperature and/or high-pressure boils) can transform the simple melanoidins into less desirable heterocyclics such as described next (Narziss, 1992).
The second level of complexity consists of sulfur-bearing heterocyclics. Heterocyclic and methionine products have a flavor that strikingly recalls cooked cabbage. The potential presence of these compounds in beer is responsible for the sensitivity of many brewers to free SMM in wort. Nevertheless, it is excessive heat treatment—not inadequate boiling—that is responsible for their formation (Heath, 1988).
The third and final level of heterocyclic compound complexity is generally regarded as giving off-flavors except for very special beer styles. Pyrazines are the most studied class of nitrogen heterocyclics formed via NEB (Heath, 1988), and it is also the most relevant to wort that has been subject to heavy thermal loading. The alkyl pyrazines are common; their structures were described in chapter 1. An example commonly found in wort produced by boiling under pressure is 2-acetonyl pyrazine, which has a sharp toasted or burnt flavor. Its structure is shown here:

Structure of 2-acetonyl pyrazine
Pyrroles constitute another class of nitrogen-bearing heterocyclics relevant to wort boiling. An important example is 2-formyl pyrrole, which has a sweet-corn-like flavor. It has the following structure:

Structure of 2-formyl pyrrole
It is often confused with DMS, yet the mechanisms responsible for their formation are completely different. The heterocyclic increases with increasing thermal loading, whereas increasing thermal loading encourages the reduction of DMS by vaporization (Herrmann et al., 1985).
Furanones are the most characteristic of the level-three heterocyclics and have flavors that have been variously described as burnt, caramel-like, nutty, or smoky. Furan derivatives are also often found in bread and coffee (Heath, 1988).
Because of the potential for heterocyclic-compound formation, wort boiling poses a dilemma for brewers. Thermal loading on wort should be sufficiently high to expel DMS, yet not so high as to produce undesirable off-flavors. Even for dark beers, it is usually better to use the malt to define the malt flavor profile than to rely on heterocyclics formed in wort boiling. This dilemma is aggravated by the fact that during wort boiling, the system is very far from equilibrium, and hence standard pressure-temperature-volume relationships from equilibrium thermodynamics are not valid. As a consequence, select parameters are used as indicators. These are not fundamental in themselves, but they do tend to track thermal loading within a given system. The most widely used indicator is the percent evaporation that takes place in the boil (Narziss, 1992). With standard boiling systems, a general rule is that the volume reduction be at least 7%. However, it has been shown that evaporation rates above 12% may produce level 2 heterocyclics, leaving vegetal malt tones that are accompanied with some astringency. A wide range of level 2 and 3 heterocyclics is possible once evaporation rates exceed 15%. As already stated, the flavor of the finished beer will determine the extent to which this effect is relevant.
Protein coagulation also occurs during wort boiling and is highly desirable as far as finished beer is concerned. Protein coagulation primarily constitutes the so-called “hot break” and is evidenced just after the boil where clear wort has protein complexes suspended in it. The “cold break” refers to sediment formed after wort cooling. The stability of beers with respect to haze requires the sufficient removal of both cold and hot breaks. Not much in the way of thermal loading is needed to get good breaks (hot and cold) with modern malts. Some studies have concluded that a little as a 2% volume reduction is adequate (Buckee et al., 1982).