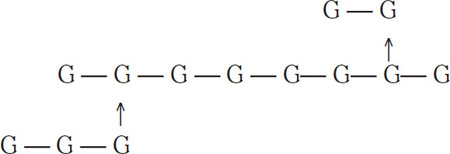1
Malting and Mashing
Malting and mashing should be considered as closely related aspects of the same process. In effect, both involve the breakdown of key grain constituents—most notably starch and proteins—into simpler units. Brewing yeasts are an elementary type of microbe in the sense that they can only metabolize simple sugars and proteins. Hence, degradation that occurs in malting and mashing is crucial for beer. Water plays a role in both malting and mashing. The former starts with steeping, a process in which grains are immersed in water. The resulting moisture uptake into the barley kernels marks the beginning of the modification process. As a consequence, chapter 1 starts with a discussion of brewing water, which is usually called brewing liquor. Waters of highly diverse composition have been successfully used in brewing, and even with a particular beer style, there are a number of reasonable options to adjust the water composition. Therefore, the fundamental issues are discussed to provide brewers a base from which they can decide among the diverse water-treatment options.
Logically the next subject is a review of the key grain constituents, namely, carbohydrates and proteins. Also included are other grain constituents, most notably phenolic and sulfur compounds, which play a major role in finished beer flavor. Acquiring a good grasp of the structural properties of these compounds can be of great value to brewers when deciding on the type of brewing program that will be used for a given beer style.
In the rest of this chapter, the point of view taken is that both malting and mashing are in effect major enzyme-induced transformations in the presence of water. This theme will reemerge throughout this book, and it is not farfetched to see most of brewing as a series of enzyme-induced transformations. Water-borne minerals affect enzymatic activity in the mash. Also important to malting and, to a lesser extent, to mashing, are the heat-induced Maillard (i.e., the brown-pigment-producing) reactions. As these are topics of great practical importance, they are taken up again in chapter 2.
BREWING LIQUOR
Most beers are between 90% and 94% water; hence, it is by a wide margin the most abundant beer constituent. Certain properties of water are especially relevant to beer. The primary application of a discussion of these properties is to help the brewer decide how a given source of raw water can be transformed into “brewing liquor,” i.e., water that has been suitably treated so that it can be advantageously used in the brewing process for a particular type of beer.
Water is an excellent solvent, a property that is crucial to brewing. Otherwise, the brewer’s efforts to dissolve grain constituents in the mash and hop constituents during wort boiling would be for naught. The key to the solvency of water lies in its polarity. In particular, a water molecule (H2O) can be represented as follows:

Water molecule
This molecule is electrically neutral because the oxygen ion O2− has two negative charges, whereas each of the two hydrogen ions H+ has one positive charge. Nevertheless, near the oxygen vertex of the H2O molecule, the overall-neutral molecule has a partial negative charge. The hydrogen side of the H2O molecule, on the other hand, is positively charged. The existence of a negative region and a positive region on an electrically neutral molecule is called polarity.
To see how this works, consider calcium chloride (CaCl2), a salt that is widely used in brewing to treat water. Once added, this salt tends to (partially) dissociate into three ions: one Ca2+ ion and two Cl− ions. These ions are held in solution by the polar charges of the H2O molecule as follows:

Calcium and chloride ions in solution
In this book, dissociation is represented by using the following notation, for example:
![]()
(1.1)
The double arrows indicate that the reaction can go both ways; i.e., added calcium chloride will dissociate into ions; however, there could also be undissociated calcium chloride molecules. Calcium sulfate (sometimes called gypsum; CaSO4) is another salt used to treat brewing water. The dissociation reaction for it is
![]()
(1.2)
where SO42− is the sulfate ion. The primary source of magnesium ion, Mg2+, in brewing water typically comes from magnesium sulfate (sometimes called Epsom salt; MgSO4) via the dissociation reaction
![]()
(1.3)
Calcium carbonate (CaCO3) is often used to treat water for dark beers. It partially dissociates into calcium and carbonate ions:
![]()
(1.4)
A different situation is presented by common table salt (NaCl), which is also used in brewing. It tends to completely dissociate, and hence the dissociation reaction for it is written with only a single arrow that points toward the ionic side of the reaction:
![]()
(1.5)
In fact, most water-treatment procedures tend to promote full dissociation (by stirring and other means) of any salt that is added to the water. Thus, it can be assumed, unless otherwise noted, that the right side of reactions 1.1 to 1.4 is favored. Hence these dissociation reactions can be represented as follows:
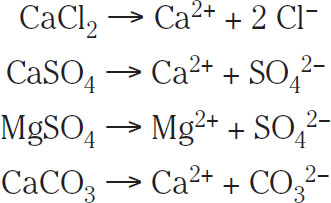
(1.6)
Water-borne ions play an important role in brewing, and calcium ions are used as an example. These ions react with malt phosphates (primarily K2HPO4) and hence decrease pH (i.e., make the water more acidic; pH is a measure of the acidity—lower values indicate a more acidic solution compared to higher values) via
![]()
(1.7)
The second term on the right is a calcium phosphate and is essentially insoluble in wort. Kolbach’s (1960) studies clearly show that this reaction does not go to completion, and his data indicate that 5 calcium equivalents are needed to produce 2 equivalents of hydrogen. Moll (1994), using improved instrumentation, found that approximately 7 calcium equivalents are needed. This 7:2 ratio is much more than the 3:2 ratio expected from a 100% utilization in reaction 1.7. The implications of this finding for water treatment are discussed later in this chapter.
Calcium ions also tend to afford thermal protection for mash enzymes (Comrie, 1967). In addition, they continue to interact with malt phosphate during wort boiling, and the ongoing reaction between calcium and phosphate is the primary reason that the pH decreases in the kettle boil. Calcium ions also tend to inhibit color formation during the boil and facilitate protein coagulation. Finally, calcium ions also influence beer fermentation. For example, they favorably affect yeast flocculation and beer clarification during maturation (Harrison et al., 1963; Saltukoglu and Slaughter, 1983; Taylor, 1990).
Magnesium also plays a role in mash acidification by a mechanism similar to that described in reaction 1.7. However, these effects are small and are usually ignored. The major role magnesium plays is in the fermentation where magnesium is an important cofactor in various metabolic activities. Actually, malt supplies sufficient magnesium for this purpose, and in addition, at too high of a concentration, magnesium ions will contribute a harsh bitterness (Krauss et al., 1983). As a consequence, magnesium salts are rarely used for treating water in modern brewing.
Carbonate (CO32–) and bicarbonate (HCO3–) ions are very important to wort production. They work in the manner opposite to Ca2+ ions in the sense that they tend to increase pH by binding H+ ions to other compounds:
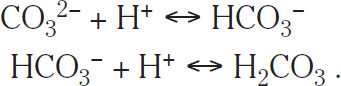
(1.8)
In water, these reactions are equivalent to the liberation of hydroxyl ions (OH−):
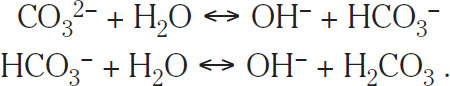
(1.9)
The last compound, carbonic acid (H2CO3), is neutral and thus does not change the pH of wort. In fact, it decomposes to carbon dioxide (CO2) via
![]()
(1.10)
and the CO2 for the most part evolves as a gas under normal mashing conditions. The proportions of reactants and products that reactions 1.8 to 1.10 yield depend on the pH of the media, as can be seen in Table 1.1.
A widely accepted rule in brewing is to have calcium concentrations of at least 50 mg/L, and values in the range of 100–150 mg/L are very common. Such a range of concentration is denoted
![]()
(1.11)
where the angle brackets < > indicate ionic concentration in mg/L. In most practical brewing situations, the available water is calcium deficient. To achieve the criterion specified in equation 1.11, calcium-based salts are typically added. Calcium chloride (CaCl2) is widely used for this purpose. It usually contains water of hydration, and CaCl2•2H2O is the most common version found in brewing. The effect of the addition of this salt can be understood by computing its molecular weight:
|
Species |
Molecular weight (g/mol) |
|
Ca2+ |
40 |
|
2Cl− |
70.9 |
|
2H2O |
36 |
|
146.9 |
The total of 146.9 g/mol means that 1 mol (mole, a scientific unit that describes a set quantity of entities, such as identical molecules) of CaCl2•2H2O has a mass of (i.e., “weighs” in the common parlance) 146.9 g. Moreover, each mole of CaCl2•2H2O contains 40 g of calcium. Therefore, to increase the calcium content of 1 L of water by 50 mg, it is necessary to add
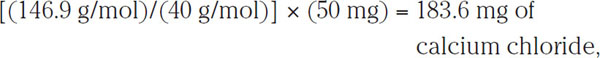
which amounts to 0.025 oz./gal. Conversely, adding 100 mg of calcium chloride to 1 L of water gives an increase of
![]()
Similar calculations can be used for gypsum (CaSO4•2H2O), whose molecular weight is 172. Thus,
![]()
is needed to increase the calcium level of 1 L of water by 50 mg, and 100 mg of gypsum added to 1 L of water increases the calcium level by
![]()
Calcium carbonate (CaCO3) represents a special case in that not only is it added to increase calcium levels, but also the carbonate ions are used to counteract the acidity in mashes that have significant amounts of dark grains. The relevant molecular weights are
|
Species |
Molecular weight |
|
Ca2+ |
40 |
|
CO32− |
60 |
|
100 |
Note that adding 100 mg of calcium carbonate to 1 L of water will potentially contribute
![]()
Also, adding 100 mg of CaCO3 to 1 L of water will potentially contribute
![]()
The exact amount will depend on pH (see reactions 1.8 to 1.10 and Table 1.1).
| Table 1.1 Carbonate, Bicarbonate, and Carbonic Acid Proportion as a Function of pH | |||
|---|---|---|---|
|
pH |
CO32-% |
HCO3-% |
H2CO3% |
|
10 |
32 |
68 |
0 |
|
9 |
5 |
95 |
0 |
|
8 |
0 |
97 |
3 |
|
7 |
0 |
81 |
19 |
|
6.5 |
0 |
58 |
42 |
|
6 |
0 |
30 |
70 |
|
5.5 |
0 |
12 |
88 |
|
5 |
0 |
4 |
96 |
There are many circumstances in which acidification of the brewing water is advantageous. The most common example is with pale beers. Pale malts typically have a pH near 6.0, which is considerably higher than the desired maximum mash pH of 5.4. Therefore, with even mildly carbonate waters, it may be hard to hit mash pH targets without additional water-treatment measures like acidification.
For this purpose, the carbonate content of the raw water is usually monitored, and this content is typically expressed as an equivalent amount of CaCO3. This new unit is denoted with double angle brackets, e.g., <<HCO3−>>, to distinguish it from the standard unit <HCO3−> expressing concentrations in mg/L. The two units are related by a ratio of the molecular weight of CaCO3 (which is 100 g/mol) to that of the ion (61 g/mol in case of HCO3− and 60 g/mol in the case of CO32−); i.e.,

(1.12)
and
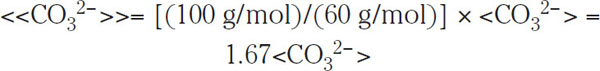
(1.13)
Given these relationships, alkalinity—the converse of acidity—can be expressed as
![]()
The factor of two in front of the last term reflects the potential of CO32−to react with two hydrogen ions via
![]()
Because of the double charge, CO32− is said to have an equivalence of 2.
Most water supplies have a pH value between 7.0 and 9.0. For such water, alkalinity is essentially the bicarbonate concentration expressed as an equivalent amount of CaCO3. Moreover, the traditional rule used by brewers of pale beers is that this concentration be below 25 mg of equivalent CaCO3 per 1 L of water, typically expressed as 25 mg/L as CaCO3 (Owades, 1985). To illustrate this relationship, suppose that the raw water has a pH of 8.0 and the alkalinity is 200 mg/L as CaCO3, i.e., <<HCO3−>> = 200. At pH = 8, the bicarbonate ions consist of 97% of the total carbonates (see Table 1.1). To reduce the bicarbonate ion concentration below 25 mg/L as CaCO3, it is necessary to decrease the HCO3−until it is
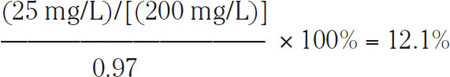
of the total carbonates. It follows from Table 1.1 that to achieve this concentration, the water pH should be reduced to at least 5.5.
The old practice of biological acidification has taken on a new life in recent years, not only in Germany, but also among some craft brewers in the United States. Since this procedure is used during the mash, a discussion of it is postponed until the end of this chapter.
An alternative is to use food-grade acids. For example, lactic acid (HC3H5O3) reacts with calcium carbonate (CaCO3) by transforming it into calcium lactate (Ca(C3H5O3)2) plus other products. The underlying reaction system is
![]()
Lactic acid and calcium carbonate form calcium lactate, water, and carbon dioxide
An analogous reaction is the liberation of hydrogen ions (and, hence, the reduction of pH) via
![]()
Mechanism for pH reduction
A similar mechanism holds for phosphoric acid (H3PO4), which is also widely used. Finally, strong acids like sulfuric (H2SO4) are used by large breweries for economic reasons. Regardless of the acid used, the preferred method is to add it incrementally, measuring the pH at each addition until the desired value is achieved. Table 1.2 gives the approximate amounts that will be needed.
| Table 1.2 Acid Treatment of Water | |||
|---|---|---|---|
| 50% Lactic Acid | |||
|
Alkalinity |
pH to 6.5 |
pH to 6.0 |
pH to 5.5 |
|
100 |
13 g/hL |
22 g/hL |
27 g/hL |
|
200 |
31 g/hL |
50 g/hL |
58 g/hL |
|
300 |
45 g/hL |
73 g/hL |
86 g/hL |
| 85% Phosphoric Acid | |||
|
Alkalinity |
pH to 6.5 |
pH to 6.0 |
pH to 5.5 |
|
100 |
7.8 g/hL |
15 g/hL |
20 g/hL |
|
200 |
17 g/hL |
32 g/hL |
41 g/hL |
|
300 |
26 g/hL |
50 g/hL |
61 g/hL |
| Sulfuric Acid | |||
|
Alkalinity |
pH to 6.5 |
pH to 6.0 |
pH to 5.5 |
|
100 |
4.8 g/hL |
7.4 g/hL |
9 g/hL |
|
200 |
10 g/hL |
16 g/hL |
19 g/hL |
|
300 |
14.4 g/hL |
24 g/hL |
28 g/hL |
|
NOTE: From Owades (1985). The unit hectoliter (hL) equals one hundred liters, which is about 22 imperial gallons or 26.4 U.S. gallons (Rabin and Forget, 1998). |
|||
A very effective alternative, particularly for small-volume brewers, is to boil alkaline water. Boiling causes bicarbonate ions to precipitate as calcium carbonate and evolve carbon dioxide (as a gas) via It must be emphasized that this reaction mechanism is quite reversible. Hence, some form of aeration (e.g., stirring) is needed to drive the process to the right side of reaction 1.14.

(1.14)
Finally, the old method of treating alkaline water with lime (Ca(OH)2, calcium hydroxide) is sometimes used. The reaction mechanism is
![]()
(1.15)
Calcium carbonate is precipitated in this process.
Another point to be emphasized is that calcium is removed in all of these methods, and its removal must be taken into account. In addition, none of these procedures fully removes carbonates. As a consequence, it is not uncommon for the mash pH to be higher than desired even with water treatment. This condition is particularly prevalent when treatment is applied to water whose alkalinity is in excess of 300 mg/L as CaCO3.
In such circumstances, the notion of a residual alkalinity can be useful. In effect, it gives an estimate of how much more water treatment is needed to reduce the mash pH by a given amount. According to Moll’s work that has already been discussed (Moll, 1994), 3.5 equivalents of Ca2+ ions are needed to neutralize 1 equivalent of HCO3− ions. This statement is the same as saying that

is the amount needed to neutralize 1 mol of HCO3−. If the contribution from magnesium is ignored, the residual alkalinity (RA) can be defined as follows:

(1.16)
This relationship expresses RA in terms of mg/L as an equivalent amount of bicarbonate. Kolbach’s (1960) work uses a different measure, German degrees of water hardness. These are obtained by multiplying the RA by 0.046 °German (mg•L−1):
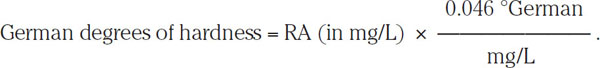
Kolbach showed that a reduction of residual alkalinity by 10 German degrees of hardness will reduce the mash pH by 0.3 pH unit. By using this proportion (i.e., 10 °German/0.3 pH unit), one can calculate that to reduce the pH of a 10 °German mash by 0.1 pH unit, the residual alkalinity RA must be decreased by
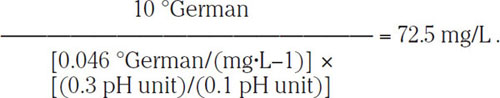
(1.17)
This relationship shows why direct mash acidification is generally preferred to salt additions for the reduction of the mash pH. For example, to reduce the pH of 1 L of mash by 0.1 pH unit requires
![]()
of additional calcium (see equation 1.16 for the source of the 0.87 value) or, equivalently,
![]()
of calcium chloride. This approach requires a lot of salt for a rather modest reduction in the mash pH.
In addition to the technical requirements associated with mashing, the salts discussed here are sometimes added to achieve stylistic goals. The guidelines are usually based on the composition of water found at historically important brewing centers. For example, the water associated with Munich and London—both of which have been noted for their dark, moderately hopped beers—is highly alkaline with low concentrations of sulfates, as shown by the following values:
|
Munich (mg/L) |
London (mg/L) |
|
|
<HCO3−> |
150 |
160 |
|
<Ca2+> |
75 |
55 |
|
<SO42−> |
10 |
30 |
These data reflect three aspects of water-borne minerals that are true in general. First, there is a positive synergism between carbonates from brewing liquor and dark malts. The latter contain complex Maillard products, some of which contribute a rather harsh and biting acidity. The carbonates tend to moderate this characteristic by giving a mellow and “fine malt” palate. Second, hop constituents tend to have the reverse effect. It is easy to demonstrate (Fix and Fix, 1997) that highly hopped beers made with highly alkaline water have a biting and crude bitterness. It is interesting, in this regard, that the London and Munich dark beers have had traditionally low hop levels. Finally, high sulfate levels and dark beers are not a particularly good marriage either. The effects are a drying and astringent afterfinish. From London’s and Munich’s popular dark beers, one could therefore predict that the sulfate levels of the water associated with London and Munich are low.
One must be careful, on the other hand, with indiscriminate use of historical models, for they can be misleading. A striking case is Dortmund whose water-ion concentrations are
|
<HCO3−> |
180 mg/L |
|
<Ca2+> |
225 mg/L |
|
<SO42−> |
120 mg/L |
The Dortmund water is very hard in the sense that it is loaded with minerals. According to M. Jackson (1997), however, Dortmund beers are generally “big and malty, with a clean, delicate sweetness.” These characteristics and the Dortmund water-ion concentrations are in direct conflict. Even the most cursory test brew will show that pale lagers made with water having these ion concentrations will have a mineral or salty taste with an underlying harsh aftertaste, totally uncharacteristic of authentic styles. This conflict is resolved in part by noting that Dortmund brewers have always been in the forefront of development of techniques for treating water. Even today, some of the best references on biological acidification come from there. In addition, data from Piendl’s (1970–1990) “Biere aus aller welt” columns tend to support the notion that extensive mineral reduction is used. For example, the mineral content of Dortmunder Actien–Export has a calcium ion content of 14 mg/L. This concentration is much lower than Piendl’s columns mention for just about all the Czech Pilseners, which range from 40 to 75 mg/L. There is of course no direct correlation between finished-beer mineral levels and the mineral content of brewing liquor. Yet, these data tend to support the notion that authentic Dortmunders are brewed with water that is as soft or softer than that used for Czech Pilseners. In short, using the Dortmund water-ion concentrations as a guide to desired water composition for this style is at best dubious. There are many other examples that illustrate analogous effects; Viennese style beers are a prominent example. Thus, instead of using historical examples as a guide, the best overall strategy is to first make sure the technical requirements of the mash are met (i.e., a proper pH) and then to adjust the mineral content by using the finished beer’s flavors as the guide.
COMPOSITION OF GRAINS AND MALT
The malt and grain constituents most relevant to brewing can be classified as follows: carbohydrates, nitrogen compounds, lipids, phenols, sulfur compounds, and miscellaneous constituents.
CARBOHYDRATES
A carbohydrate is a compound consisting of carbon, hydrogen, and oxygen molecules. As in any organic compound, carbon is the key ingredient. There is a large and diverse array of carbohydrates in barley, malt, and wort. They typically consist of 70%–85% of the weight of barley and malt and 90%–92% of wort solids. The same types of carbohydrates are also found in other grains such as maize or rice. Fortunately, “erector set models” greatly simplify the discussion of carbohydrate and other organic compound structures. Such models allow the representation of complicated carbohydrates, for example, as a few elementary units linked together in definite ways. Malting and mashing are processes that break down these links (in part) to provide elementary sugars for yeast metabolism.
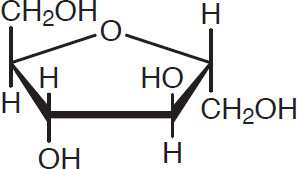
Glucose is the most important building block in brewing. It has the following structure:
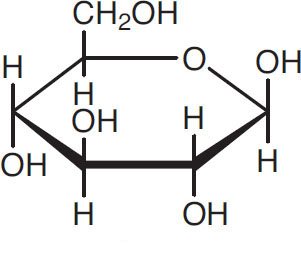
Glucose
One sometimes sees this structure called ß-D-glucose, to distinguish it from different but similar structures. These distinctions are not relevant to brewing and therefore are ignored in this book.
The following widely used shorthand notation omits some of the carbon atoms:
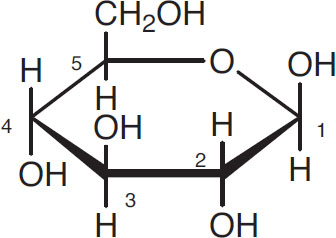
Glucose in shorthand notation
The numbers refer to the carbon atoms not displayed (1 through 5) and the one that is displayed (6). Since this numbering system is universally used, sometimes the numbers themselves are omitted.
One sometimes sees glucose described as a hexose compound, meaning that glucose has six (hex-) carbon atoms. In brewing, the greatest significance that glucose plays is its role as a basic building block for other carbohydrates. Because of its basic building block nature, glucose is classified as a monosaccharide, with mono- referring to a primary unit and saccharide referring to a sugar.
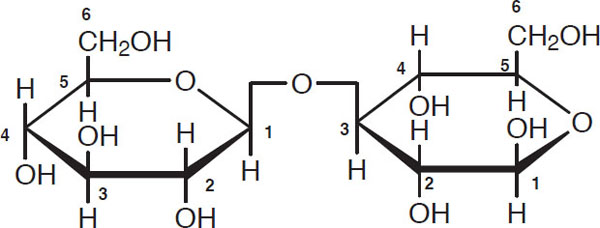
Glucose is a minor wort carbohydrate and typically makes up no more than 8%–10% by weight of wort sugars unless it is deliberately added. Of far greater importance are sugars obtained by linking glucose units. Maltose is such an example, and it generally makes up 46% to 50% by weight of the sugars in a grain wort. It has the following structure:
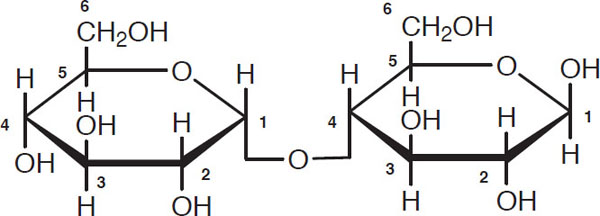
Maltose
Observe the carbon one atom on the left glucose molecule. The second glucose molecule is attached to it via the carbon four atom. Such a combination is given the descriptive name of “1-4 link.” A useful shorthand notation for maltose is
![]()
where G stands for a glucose unit and the horizontal bar stands for a 1-4 link.
Maltose is called a disaccharide because it consists of two elementary (glucose) units. Maltotriose is a trisaccharide that consists of three glucose units joined by 1-4 links:
![]()
It is an important wort constituent that typically makes up 12% to 18% of wort sugars. Chains like the following are called amylose sugars and are common in raw grains and malt:
![]()
A typical amylose molecule can have more than 1000 glucose units, which are largely separated during malting and mashing. Amylose sugars consisting of four or more glucose units are typically not fermentable by brewing yeasts because of their complexity.
Another important way glucose units can be joined is through a “1-6 link.” As the name suggests, two glucose units are linked from the carbon one atom of the first glucose molecule to the carbon six atom on the second glucose molecule in the following way:

Isomaltose
The shorthand notation for this compound is
![]()
Isomaltose in shorthand notation
The vertical arrow indicates a 1-6 link. Sugars that have both 1-4 and 1-6 links have traditionally been called dextrins, although in recent years the term α-glucans has become preferred. In biochemical literature, the term amylopectin is common. According to Meilgaard (1977), the following are examples of typical α-glucans groups found in wort:
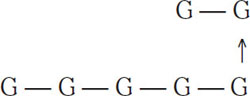
Example of group 1 (7 glucous units)
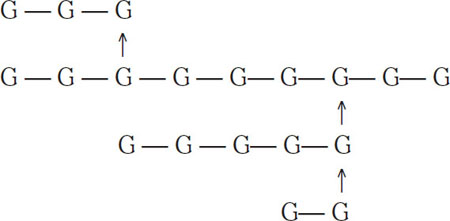
Example of group 3 (19 glucous units)
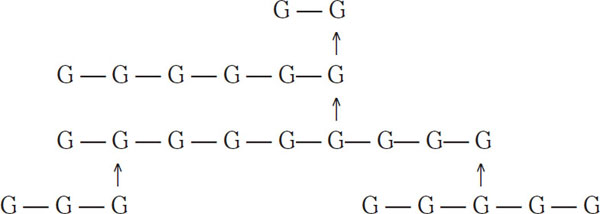
Example of group 4 (25 glucous units)
Here the bars represent 1-4 links, and the arrows represent 1-6 links. Because of their complexity, these four dextrin groups are not fermentable by normal brewing yeasts, and they are typically passed on to the finished beer. Low-calorie beers are an exception. For these beers, exogenous enzymes are added to the fermenter, and these enzymes break α-glucans down into fermentable sugars. Dextrins in groups 1, 2, and 3 generally will not color with iodine. Those in group 4 will produce a reddish hue when combined with iodine. More complex starch molecules will color a dark blue with iodine.
Carbohydrates more complicated than the four dextrin groups shown are quite common in unmalted grains and in malt. The term starch is sometimes used for such molecules. In barley, starch makes up 63%–65% of the dry weight. This proportion is normally reduced to 58%–60% in a properly modified malt. The majority of the “starch conversion” takes place in the mash, and the finished wort will generally have 25%–35% unfermentable carbohydrates.
The second monosaccharide found in wort is fructose. Again, as is customary, the carbon atoms (four in this case) in the “carbon ring” have been left out of the diagram. Unless deliberately added, fructose is a minor wort sugar and typically makes up only 1%–2% of wort carbohydrates.
Fructose
Sucrose, common table sugar, consists of a fructose unit joined with a glucose unit. It generally makes up 4%–8% of wort sugars (unless deliberately added) and, in the fermentation, is rapidly “inverted” into glucose and fructose units.
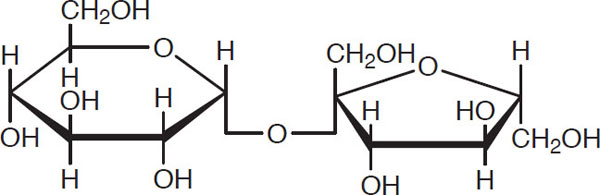
Sucrose
The other group of carbohydrates of interest are gums and cellulose compounds. In brewing, two types—namely, ß-glucans and pentosans—are most important. The ß-glucans consist of glucose units joined with 1-4 links as well as 1-3 links. They have the form
LAMINARIBIOSE
Many brewers view ß-glucans as a “wasteful” carbohydrate. They are known to greatly increase wort viscosity when not properly degraded, a situation that can lead to filtration and haze problems (Linemann and Krueger, 1997). For example, they are the major constituent of the sediment of frozen beer (Meilgaard, 1977).
The ß-glucans compounds are found in barley cell walls and can be enzymatically degraded with proper malting and mashing procedures. Their presence in raw barley is one of the main reasons that special enzymes are used when this material is added in appreciable amounts as an adjunct (Bourne et al., 1982). Rye and wheat also have high ß-glucans levels, and these tend to be present even when these grains are malted. A point that is sometimes overlooked is that some of the undegraded ß-glucans can react with iodine. This fact means that an iodine test might show a color reaction even when a satisfactory degradation of malt sugars has been achieved. This effect can be minimized to some extent by straining out grain kernels before adding iodine drops to the sample.
The other carbohydrates of interest are the sugars called pentosans that have five-carbon skeletons. These are formed through linkages of arabinose and xylose:
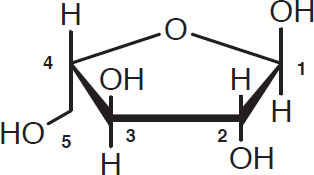
Arabinose
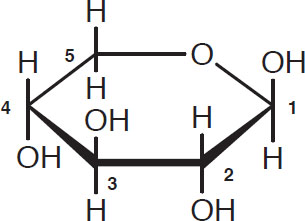
Xylose
Once again, the carbon atoms in the ring (four in arabinose and five in xylose) are customarily omitted from diagrams. As far as brewing is concerned, the pentosans and ß-glucans have similar properties.
It is important to note that ß-glucans levels differ significantly in barley varieties. In general, the cheaper six-row barleys will have a significantly higher ß-glucans content than the two-row varieties (Linemann and Krueger, 1997). Modern brewers have started to specify maximum ß-glucans levels as a part of their malt evaluation. In addition, research in genetic engineering is underway to produce barley with little or no ß-glucans content (Thomas, 1986).
Carbohydrates may also be classified as either reducing or nonreducing (Conn and Stumpf, 1976). Reducing sugars, which are the more common, contain a free (or potentially free) aldehyde (RCHO-type structures) or ketone (RCOCH2OH-type structures) in the molecule. One consequence of this type of structure is the ability to reduce copper salts, and most methods for the estimation of these sugars are based on this property. A typical reaction is
![]()
As far as brewing is concerned, attention has been focused on the reducing sugars present at the fermentation endpoint. De Clerck (1957) estimated that reducing sugars (as an equivalent amount of maltose) consist of 20%–30% of the extract in fermented beer. In addition, he found that the reducing sugars were primarily dextrins, trisaccharides, and pentosans. He concluded that the estimation of reducing sugars in beer was “not of such great importance in the determination of the attenuation limit” (De Clerck, 1957, 2:444–5).
Nevertheless, an assay of reducing sugars is useful in quality control on a comparative basis. For example, for a given beer formulation, there should not be significant batch to batch changes in the reducing sugars at the fermentation endpoint. Variations point to changes, often undesirable, in wort production or with the yeasts being used. The latter effect will be discussed in more detail in chapter 3.
NITROGEN COMPOUNDS
As far as brewing is concerned, the most important sources of nitrogen are amino acids and grain proteins, which in turn are a union of a number of different amino acids (Pierce, 1982). Like carbohydrates, the proteins are degraded into simpler units during malting and mashing.
There are, on the other hand, important differences between proteins and carbohydrates. Although most of the malt starch is converted to simple sugars in the mash, it is during malting that the most protein modification takes place. Also, although approximately the same types of carbohydrates are derived from a wide variety of grains, malted or unmalted, with proteins it is malt that makes the crucial contribution. Unmalted adjuncts such as rice or corn for all practical purposes do not contain proteins that are soluble in wort. Thus, their use will directly dilute the protein content of wort.
The basic building blocks of proteins are amino acids, compounds that have the following structure:
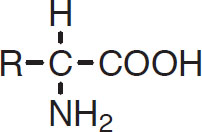
Amino acid
Here R denotes a side chain that will vary with different amino acids. For example, valine has the following structure:
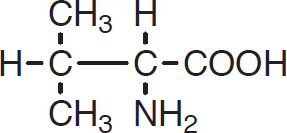
Valine
The importance of amino acids goes well beyond their role as elementary building blocks. For example, they play a crucial role in various Maillard reactions in the malt drying and kettle boil processes (see chapter 2). However, it is during the fermentation that their presence is most significant. As will be seen in chapter 3, yeasts will remove the amino nitrogen component NH3 (ammonia) via the following diagrammatically shown reaction:

Amino acids break down to ammonia and a carbon skeleton
The amino nitrogen is an essential component of yeast nutrition, and without it, a disordered fermentation results. The carbon skeletons play an important role later in fermentation, particularly in the way by-products like diacetyl and fusel alcohols are formed or repressed (see chapter 3).
Amino acids can be classified in two different ways. One classification is according to the rate they are assimilated by yeasts in a normal fermentation. The classification yields the following four groups:
• Group A. Amino acids that are absorbed very early and rapidly. They are generally eliminated from wort by the end of the growth period.
• Group B. Amino acids that are absorbed at a slower rate.
• Group C. Amino acids that are absorbed only after a considerable lag and only after all of the group A amino acids are utilized.
• Group D. Amino acids that are largely unabsorbed.
In a second classification, it is customary to categorize amino acids according to their roles in the fermentation, above and beyond their contribution of amino nitrogen to yeast nutrition.
• Class 1. Certain amino acids are unimportant because their carbon skeletons can be created by yeasts during normal metabolic activity. It is not necessary for these amino acids to be present in wort.
• Class 2. Other amino acids are vital. Yeasts can in part find replacements, but class 2 amino acids are of such importance that wort must contain them to ensure an adequate supply during the fermentation. Thus, their removal from wort would adversely alter beer flavors.
• Class 3. The last class of amino acids is crucial. Beer wort is the only source of these amino acids, and they are needed in the fermentation. Thus, their removal from wort would significantly alter the flavor of the finished beer.
There are some minor disagreements in the literature concerning the placement of various amino acids in these groupings. Moreover, there are differences in the uptake of amino acids by brewing yeast strains. This point has great practical relevance and is discussed in chapter 3. However, as a general guide, the assignments in Table 1.3 can be used.
| Table 1.3 Amino Acids in Brewing | ||||
|---|---|---|---|---|
| Absorption rate | ||||
|
Group A: Rapid |
Group B: Moderate |
Group C: Slow |
Group D: Largely unabsorbed |
|
|
Class 1: Unimportant amino acids |
Glutamic acid |
Proline |
||
|
Glutamine |
||||
|
Aspartic acid |
||||
|
Asparagine |
||||
|
Serine |
||||
|
Threonine |
||||
|
Class 2: Vital amino acids |
Valine |
Glycine |
||
|
Isoleucine |
Phenylalanine |
|||
|
Tyrosine |
||||
|
Alanine |
||||
|
Class 3: Crucial amino acids |
Lysine |
Leucine |
Tryptophan |
|
|
Arginine |
Histidine |
|||
Fortunately, worts that are prepared with reasonable percentages of malt tend to be rich in all amino acid groups. As a consequence, it has become customary to use a single number to characterize the total amino acid content of wort, analogous to the role that specific gravity serves as a measure of the carbohydrate content of wort. For amino acids, the relevant parameter is free amino nitrogen (FAN). This is a measure of the nitrogen contributed by the amino acids in the wort, irrespective of type, and is expressed in terms of mg/L.
There is universal agreement that low FAN levels are undesirable in wort (see chapter 3). However, there is some disagreement concerning the minimum desired level. The traditional rule is that serious problems (e.g., long lags in the start of fermentation, high diacetyl levels, etc.) can result if the FAN level goes below 150–175 mg/L (De Clerck, 1957). Differences in yeast strains as well as the presence or absence of other wort nutrients are confounding factors. Nevertheless, a wort in which malt contributes at least 12 g per 100 g of wort (i.e., 12 °P [degrees Plato]) to the carbohydrate pool will typically have FAN levels from 225 to 275 mg/L, which is ideal.
Too much of a good thing is rarely good in brewing, and this statement applies to wort FAN levels. For example, Pogh et al. (1997) reported a decrease in diacetyl as FAN levels increased until a minimum value was achieved. As FAN levels increased further, there was a linear increase in diacetyl with FAN (see also chapter 3). Fusel alcohols are produced from the carbon skeletons of amino acids, and the effect of fusel alcohols on finished beer flavors is quite negative if present above or near their flavor thresholds (see chapter 3). As a general rule, it is usually desirable to keep FAN levels below 350 mg per 1 L of wort, something that can be achieved with a suitable mashing program (Fix and Fix, 1997).
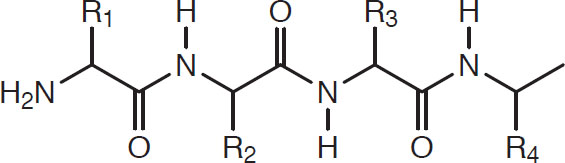
The first class of proteins in order of complexity is the peptides, which are combinations of 2 to 30 amino acids bound by peptide links. They have the following form:
That is, CH units are separated by the peptide link:
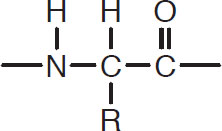
Peptide link
This pattern continues, and polypeptides with thousands of peptide linkages can occur.
It is customary to characterize the amount of protein in barley, malt, or wort either as “% protein” or “% nitrogen.” Although there are sources of nitrogen other than protein, the amounts they contribute are very small. Thus % protein is directly proportional to % nitrogen, and the following formula is used:
![]()
The factor 6.25 is based on the fact that the nitrogen content of protein averages 16%. Thus, multiplying % nitrogen by 100/16 = 6.25 gives a reasonably accurate approximation of the amount of protein.
Protein levels in different varieties of barley grown in different parts of the world vary widely. They typically range from 8% to 16% protein (1.3% to 2.6% nitrogen). The classic rule, developed by De Clerck (1957), is that barley used for malting should be in the range of 9% to 11% protein (1.4% to 1.8% nitrogen). Although this rule is not always followed, especially in North America, where malt protein levels can approach 13.5% (2.2% nitrogen), it remains a good guideline because excessive nitrogen levels can create a variety of brewing problems. Even in North America, use of high-nitrogen barley is invariably accompanied by the use of significant levels of unmalted grains and syrups that, in effect, dilute nitrogen levels.
During malting, the primary activity is protein breakdown. There is some utilization of amino acids and other loss of protein through precipitation, but for the most part, the protein content of malt is similar to that of the barley from which the malt was made. There is some continuation of protein breakdown in the mash, but the extent is greatly influenced by the mashing schedule used (Fix and Fix, 1997). In addition, there is precipitation of the higher-molecular-weight proteins. Thus wort nitrogen levels are generally lower than those of malt. In addition to amino acids, yeasts can also utilize some proteins, but these are only the simplest peptides having a few amino acid units. Some proteins are precipitated during wort chilling (the so-called “cold break”) as well as during fermentation and aging, but most are passed unaltered to the finished beer. The middle-molecular-weight proteins tend to make positive contributions to beer foam, as well as increasing the “maltiness” of beer in ways that are still not completely understood.
The last class of malt proteins, and perhaps the most important, is enzymes. In fact, brewing itself can be regarded as a series of enzyme-mediated reactions (where structures get broken into smaller units), interspersed with some synthesis (the reverse process) primarily mediated by yeasts. Enzymes are relatively high-molecular-weight proteins consisting of about 300–400 amino acids connected by the peptide links already described. Only about 20 different amino acids generally appear in enzymes relevant to brewing (Kunze, 1996). Enzymes are very sensitive to environmental factors—particularly temperature and pH—and tend to be very specific to the substrate on which they act. As a consequence, their names are typically derived from the substance they affect, with an -ase added at the end. For example, ß-glucanase is the enzyme responsible for the degradation of ß-glucans. Because of this specificity, enzymes are best discussed in the context of the reactions they promote.
LIPIDS
In brewing, the most important lipids are fatty acids. They come from three sources. One group is derived from yeast metabolism and is typically saturated, i.e., there are no double bonds like
![]()
Example of double bonds
Yeast metabolism and lipid formation are discussed in chapter 3. Oxidized hops are another source of unsaturated fatty acids; hops are discussed in chapter 2.
The group of interest here is the long-chain, unsaturated fatty acids that are derived from malt. They are typically found in wort trub (i.e., particles suspended in the wort), which can consist of as much as 50% lipids (Meilgaard, 1977). Cloudy wort can contain anywhere from 5 to 40 times the unsaturated fatty-acid content of clear wort, an important fact because unsaturated fatty acids can have a significant negative effect even at low concentrations.
On the positive side, fatty acids contribute to yeast viability via a number of mechanisms (see chapter 3), and they also inhibit the formation of some less pleasant acetate esters during fermentation (see chapter 3). On the negative side, they work against beer foam stability, as any fatty material does. Even more significantly, they play an important role in beer staling (see chapter 4).
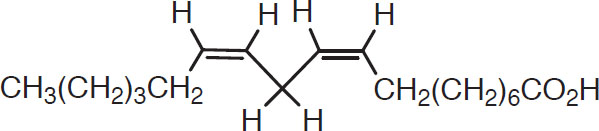
A number of fatty acids are derived from malt, but the most important are oleic and linoleic acids. These have 18 carbon atoms and one and two double bonds, respectively (Conn and Stumpf, 1976):
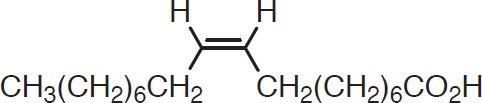
Oleic acid
These are distinguished from aldehydes and alcohols by the characteristic COOH tail. It is important to point out here that their presence in wort is at the center of the controversy concerning wort clarity. Unsaturated fatty acids have both positive and negative effects. Thus, some investigations have reported that wort clarity (via trub removal) is essential (Zangrando, 1979), whereas other investigators have found some carryover of unsaturated fatty acids in the trub to be beneficial (Hough et al., 1981). A traditional practice in North America involves using starting tanks to strike a compromise between these effects. The partially clarified wort is combined with yeasts in the starter tanks and left for 12 to 24 hours. It is then transferred into fermenters, leaving most of the trub behind in the cold break (Knudsen, 1977).
PHENOLS
Phenols have ring structures; the following diagram shows a typical structure:

Phenol
Here for simplicity, the carbon atoms have not been displayed. In addition, shorthand approaches like

Aromatic ring
are sometimes used for these compounds.
There are a large number of phenolic compounds, but the simplest ones relevant to brewing have two aromatic rings. Catechin (sometimes called catechol) is an example:

Catechin
More complicated structures are polymers (i.e., repeating structures) of such elementary phenols, exactly as carbohydrates are built from monosaccharides and proteins from amino acids. These complex phenols are called polyphenols in the brewing literature and flavonoids in some other disciplines (Dadie, 1980).
The term tannin is often used. Historically, tannins are phenolic materials applied to animal hides for tanning. In the modern brewing literature, the term generally refers to polyphenols that have molecular weights in the range of 500 to 3000. Of special interest are those having 4–25 aromatic rings (Dadie, 1980) since these can bond with high-molecular-weight proteins and form haze.
In addition to beer haze, phenolic compounds play a major role in other areas affecting the quality of the finished beer. The role of phenolic compounds in beer oxidation is of great interest. It has been shown that they can act as both oxidizing and reducing agents, depending on circumstances (discussed in detail in chapter 4). In addition, it is known that phenolic compounds in their reduced state tend to add an element of freshness to beer (Meilgaard, 1977), and this is considered an attractive part of the flavor of young beer. However, when oxidized, many phenolics contribute very astringent and harsh tones. The term herbstoffe has traditionally been used to describe the grain bitterness found in some beers, as contrasted with the more mellow bitterness that hop α-acids contribute.
Phenolic compounds derived from malt are typically found in the outer layers of the kernel, with the husk making notable contributions. Since two-row barley has a thinner husk than six-row barley, the malt these barleys produce contains different amounts of phenolics.
Extraction of phenolic compounds occurs to some extent during mashing, but it is in sparging that the issue becomes critical (Prechtl, 1964; Schneider, 1997). This is controlled primarily by the following factors: volume of sparge water used, the instantaneous pH of the runoff collected from the sparge, the alkalinity of the sparge water used, and the temperature of the sparge water.
Because of simple mechanical extraction, it is clear that phenols will increase with the volume of sparge water used. A parameter that correlates more directly with the grainy bitterness in the finished beer is the pH of the runoff collected. The first increases in direct proportion to the second. This relationship is why highly alkaline sparge waters must be avoided, unless there is balancing acidity from dark malts in the grist.
It should be pointed out that some phenolic compounds found in beer are derived from sources other than grains. Contributions from hops are discussed in chapter 2. Residues from halogen-based (e.g., chlorine or iodine) sanitizing agents if absorbed in beer can lead to very nasty flavored phenolic compounds, whose effect is far out of proportion to their concentration. Arguably, the most important contributor to phenolic compounds in finished beer comes from yeast metabolism. This very important topic is discussed in chapter 3.
SULFUR COMPOUNDS
The most important sulfur compound is dimethyl sulfide (DMS). It has the following structure:
![]()
DMS
As is typical of sulfur compounds, DMS has very powerful flavors. An appropriate flavor threshold is approximately 0.03 mg/L, and because of this low threshold, DMS levels are normally reported in μg/L (i.e., parts per billion; μ means micro- or one-millionth). In each liter of water there are 1000 g of water (a close approximation at room temperature and pressure), so 1 μg/L = 1 μg/L × [(1 L)/(1000 g)] × [(1 g)/(1,000,000 μg)] = 1/1,000,000,000 or 1 part per 1 billion parts). Note that 0.03 mg/L = 30 μg/L.
The most important DMS precursor is S-methyl methionine (SMM); it is formed in malt during germination. SMM has the following structure:

SMM
SMM is broken down to DMS via heat, mainly during the kettle boil and wort cooling period (see chapter 2).
Different types of malt have dramatically different SMM levels and thus produce dramatically different DMS levels in the finished beer. For example, ale malt has minimal SMM levels because this DMS precursor is largely removed by the high kilning temperatures used to produce ale malt (Fix, 1992). As a result, British ales brewed from these malts typically have DMS levels in the range of 0 to 20 μg/L, i.e., at most a very dilute secondary constituent (Fix, 1992). The same is typically true of ales brewed in North America, even those using domestic malt with higher SMM values than their British counterparts. This result is due to the strong volatilization of DMS that occurs in ambient-temperature ale fermentations.
Lager malt, in contrast, is produced with lower kilning temperatures and as a consequence has much higher levels of SMM. The extent to which this is converted to DMS in the finished beer depends on brewing conditions (see chapter 2). The cold fermentation and maturation also encourage the stability of DMS. Traditionally, DMS levels in lager beer have varied from 30 to 90 μg/L, although higher levels have been reported, particularly with German pale lagers and in the products of some of the regional breweries in the United States. During the 1990s, DMS levels in lagers have been considerably lower. Kunze has cited 60 μg/L as the “objectionable threshold” for German lagers (Kunze, 1996). Many large industrial brewers both in the United States and United Kingdom produce lagers with DMS levels well below 30 μg/L in order to obtain a beer with a cleaner finish. The descriptor “cat urine” (introduced by Bamforth, 1998) for DMS above its threshold of 30 μg/L gives some indication of the negative reaction that some have when DMS is detected. Lager aficionados, on the other hand, tend to be critical of low-DMS beers because of their very low malt profiles. A good compromise is for DMS to be in the range of 30–60 μg/L, so that malty or slightly sulfury lager flavors will be present, without the distracting cooked-corn tones that are present with higher DMS levels.
DMS can also be created by bacterial action, the most common being wort-spoiling coliform bacteria (see chapter 3). When bacteria produces DMS, its levels can be much higher than the range of 30–60 μg/L (Owades and Plam, 1988) and thus can impart sulfury flavors reminiscent of overcooked sulfur-bearing vegetables like broccoli or cabbage. It was once believed that DMS could be created in the fermentation by normal brewing yeasts. The precursor in malt is dimethyl sulfoxide (DMSO):
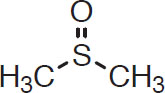
DMSO
Although DMSO can be reduced to DMS by brewing yeasts in synthetic media (White, 1977), the minor sulfur constituents (e.g., methionine sulfoxide) in beer wort block this pathway for the most part (Gibson and Bamforth, 1982). Therefore, in fermentations free of bacterial infections, DMS levels are actually reduced when this sulfur constituent is scrubbed out of beer by evolving CO2 gas.
The second most important sulfur constituent is hydrogen sulfide (H2S). An appropriate threshold has been reported as 10 μg/L in lagers and 30 μg/L in ales (Fix, 1992). It is produced by yeasts during the fermentation, but is rapidly removed by evolving CO2 gas. Thus, it is rare to find its characteristic rotten-egg flavor tones in beer. Normal H2S levels are usually reported in the range of 0 to 0.9 μg/L (Fix, 1992). Various bacteria, especially the wort spoilers, can create very high H2S levels, which are normally accompanied by large DMS levels. The net flavors so produced are highly sulfury and have been variously described as reminiscent of parsnip, celery, cooked corn, cooked cabbage, or black currant. Finally, elevated pitching rates (e.g., initial yeast-cell concentrations above 100 million cells per milliliter) can lead to offensive sulfur compounds that spill over in part to the finished beer and have a negative impact on beer flavor (Nyborg et al., 1999).
Mercaptans or thiols are sulfur compounds having H2S as a base; they have the following form:
![]()
Mercaptan or thiol structure
Some have very powerful flavors. For example, amyl mercaptan has a threshold near 0.00007 μg/L = 0.00000007 mg/L! Some of these compounds are derived from malt, hops, and additives like bisulfites. The extent to which they are formed in the fermentation is strongly dependent on the yeast strain used (see chapter 3).
MISCELLANEOUS CONSTITUENTS
Barley and malt are rich sources of several vitamins. These include the B-complex vitamins, which are important growth factors for yeasts during fermentation (see chapter 3). In this group, biotin, inositol, and pantothenate are important. Fortunately, worts with a high malt content tend to supply these vitamins in at least a twofold excess of what yeasts need (Meilgaard, 1977). The vitamins left after the fermentation add to the overall nutritional value of beer.
Yeasts also require trace quantities of copper (0.012 ppm), iron (0.075 ppm), and zinc (0.5 ppm), although levels in excess of these can lead to other problems. Most grain worts are sufficient in copper and iron, and all-malt worts tend to be sufficient in all three metals (Meilgaard, 1977). Many of the so-called “yeast nutrients” contain mixtures of these metals. However, some brewers directly add zinc to correct deficiencies that typically arise from high concentrations of unmalted grains.
Magnesium ions also play an important role in yeast growth, primarily as a cofactor in metabolic reactions. Malt generally will provide sufficient magnesium for these purposes, even when the brewing water is low in this ion. Corrections with MgSO4 additions are needed only with very high adjunct worts. It is interesting that calcium ions, which are highly beneficial in the mash, tend to inhibit yeast growth if they are at excessive levels (Saltukoglu and Slaughter, 1983).
There are numerous trace elements derived from grains that are difficult to measure and whose effect on beer flavor is uncertain. Among these are nucleic acids and amines. There are also trace elements that are known to be important. Products of the browning reaction, also called the Maillard reaction, certainly fall into this category. This reaction is important not only in beer, but also in any perishable food. The reaction, described in detail next and further in chapter 2, occurs in brewing during the malt kilning as well as during the kettle boil. Products of this reaction—typically called melanoidins—can be found in malt, particularly dark malts.
TRANSFORMATIONS DURING MALTING AND MASHING
BASICS OF MALTING
In its simplest terms, malting is the controlled germination of cereal grains. The basic goals of this process are the following:
• Enzyme development. The appropriate enzymes are generally not found in unmalted grains and must be formed during malting.
• Cytolysis. The grain cell walls must be broken down so that enzymes can start modification.
• Modification. Modification involves the appropriate degradation of protein and starch so that they can be used advantageously in mashing and in the fermentation.
• Maillard products. These products are responsible for the coloring potential of malt and also for the special flavors that are associated with these products.
Malting starts with steeping so that the grain kernels can absorb water. This is needed for growth of the acrospire into the embryo (see Fig. 1.1). Also, foreign material is washed from the kernels. The moisture level is normally increased to 38%–42%, but can go as high as 46%–47% for problematic barley and as a way to reduce the malting times. High-moisture steeps, however, do not usually produce high-quality malts.
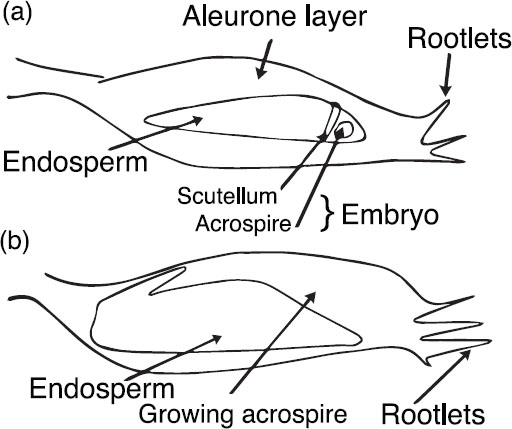
Figure 1.1. (A) Raw barley and (B) germinating (sprouting) barley.
Since the kernel is not yet a green plant, the oxygen needed for growth and respiration is supplied by the maltster during steeping (Sebree, 1997). Respiration consumes part of the barley carbohydrates via the standard respiration pathway:
![]()
(See also chapter 3 for a discussion of the basic metabolic pathways.) As carbohydrates in the embryo are depleted, the scutellum tissue begins to produce various hormones (gibberellic acid and related compounds), which in turn stimulate enzyme formation. Exogenous additions of gibberellic acid during malting are sometimes used as a short cut; however, this practice is controversial and banned in Germany and other countries.
The following are the four basic enzyme systems that are crucial to both malting and mashing:
• Starch-degrading enzymes consist of α- and ß-amylase as well as select debranching enzymes (i.e., enzymes capable of breaking 1-6 links connecting glucose in grain carbohydrates).
• Cytolytic enzymes are responsible for degrading cell-wall structures. The most important of these enzymes are ß-glucanase and cytase.
• Protein-degrading enzymes consist mainly of proteinase (which works on a wide spectrum of proteins) and peptidase (which mainly degrades medium-molecular-weight proteins to peptides and amino acids). These proteolytic enzymes are also active in mashing.
• Acid-forming enzymes are needed for control of malt and mash pH. An important example is phosphatase, which breaks down phosphoric acid.
Once steeping is complete, the kernels are removed from the steeping water. Here, germination—which in fact has already started during steeping—is encouraged. Oxygen is still supplied to promote respiration.
Cytolysis is crucial to all aspects of grain modification. The kernel cell walls are essentially composed of ß-glucans and other gums already discussed. They are responsible for the hardness (friability) of the endosperm in unmalted barley, and the cell walls must be degraded by the action of hydrolytic enzymes in order for the carbohydrases and proteolytic enzymes to attack the starch and protein molecules. The extent of cytolytic modification is usually measured in two ways. Friability (as measured by a friabilimeter) is a widely accepted malt-quality index, and the usual rule is for it to be no less than 80%. Brewers are also sensitive to the viscosity of standardized worts, and values in the range of 1.4–1.5 mPa•s are considered desirable.
The second most important aspect of malt modification is the degradation of proteins. Actually, proteins—in addition to gums—are structural components of the endosperm cell walls. Thus, cytolysis and protein modification are not independent. During germination, 35% to 40% of the protein is degraded to soluble compounds (Kunze, 1996). The ratio of soluble protein to total protein—called the Kolbach index in Europe and the S/T ratio in the Americas—gives a reasonable index of the extent of protein degradation. Traditional lager malt used to fall into the range 36%–38%, although today, all malt types are typically over 40%. Values in excess of 45% are generally unacceptable since they imply overdegradation of protein, which tends to have a negative impact on the finished beer head stand and malt flavor. Another interesting index, but unfortunately one that is rarely found on malt-analysis sheets, is the malt FAN level. In modern highly modified malts, the FAN levels can fall into the range 150–250 mg/L, which is more than adequate for wort.
Carbohydrate modification is considerably less extensive. Kunze (1996) reported typical values, which are shown in Table 1.4. From these, it can be seen that barley begins with a starch level of 63%; during malting, some of the barley starch is degraded into simple sugars with the result that the malt’s starch content is only 58%. Compared to barley, the malt’s sugar content is higher, however.
| Table 1.4 Starch Content of Barley and Malt | ||
|---|---|---|
|
Starch (%) |
Sugar (%) |
|
|
Barley |
63 |
2 |
|
Malt |
58 |
8 |
|
NOTE: From Kunze (1996). |
||
As far as malting is concerned, the most important carbohydrase enzymes are the debranching types. These are able to break the 1-6 links already discussed.
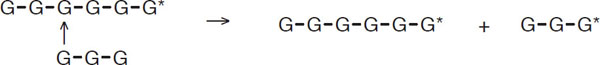
Reaction involving debranching enzymes
G* denotes the reducing end of the chain. As a result of the action of the debranching enzymes, the average length of α-glucans (amylopectins) is reduced, and the amount of straight-chain amylase starches is increased.
The second most important enzyme is ß-amylase. The enzyme breaks 1-4 links near the reducing ends and produces maltose units:
![]()
Production of maltose units by ß-amylase
The ß-amylase does not attack 1-6 links nor will it break 1-4 links at reducing ends that are near a 1-6 link:
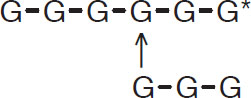
Blockage of ß-amylase at a 1-6 links
The enzyme α-amylase is generally more important to the mash than in malting. This enzyme will break 1-4 links at random:

Action of ß-amylase
The α-amylase and ß-amylase enzymes are active in mashing, where the bulk of the carbohydrate modification takes place.
A widely used index for starch degradation is the fine- to coarse-grind extract difference. The values normally fall in the range 1.5–2.0. Values in excess of 2.2 are a cause of concern—particularly with respect to the uniformity of grain modification. It must be emphasized that such a situation can be present in malt whose other modification indices are in acceptable ranges. The total extract (fine grind or coarse grind) is usually quoted on malt-analysis sheets. It is usually expressed as yield, i.e., the percentage of the extract recovered as a proportion of the grain’s weight. These values are obtained under laboratory conditions, and it is not possible or desirable to achieve them in a full mash.
Malting terminates in the kiln, where moisture is removed by drying. This is a crucial step, since malt with a high moisture level does not store well. In extreme cases, mold and/or unattractive aromas (e.g., those recalling damp, cut grass) can be detected. In less extreme cases, there will still be negative effects on the quality of malt flavor. A widely accepted rule is for the moisture level of malt to be below 4% by weight. Because of its importance, it is almost always quoted on malt-analysis sheets. It should be emphasized that a malt’s moisture level is not stable in storage and that the malt will invariably take on the ambient moisture level. This process can undo all of the effects of the kiln and lead to a wide range of deterioration products. It is highly advisable that malt that is stored over an extended period of time have its moisture level checked on a regular basis. Effective techniques for checking the moisture level can be found in Methods of Analysis (American Society of Brewing Chemists, 1987).
The color of malt is also determined during the kilning, and this process in effect defines the malt type. Different units to describe malt color are used in Europe and the United States. The European Brewing Congress uses °EBC, and the American Society of Brewing Chemists uses °ASBC. (This unit is sometimes quoted as °SRM, Standard Reference Method or °Lovibond.) Between these two terms there are differences as well (Fix and Fix, 1997), but for the purpose of this discussion, they will be ignored.) Since these units are obtained from different determinations, they are not strictly comparable. Table 1.5 gives approximate correspondences. The errors are less than 5% in the lower color range of the data in Table 1.5 and increase to 20%–25% in the upper color range.
Malt color is controlled during the kilning by means of the temperature used. There are generally two phases (Kunze, 1996). The first is the drying phase, which is conducted at relatively low temperatures. Next is the curing phase during which the bulk of the color formation is achieved. There is also deactivation of enzyme systems during curing, and the extent of those that survive is inversely proportion to color. Another important factor is that high-protein malts tend to have more enzymes. The strength of a malt’s enzyme system is usually called diastatic power (DP). The °ASBC procedure described in Methods of Analysis (American Society of Brewing Chemists, 1987) is used in the Americas, and the units are called °Lintner (°L). Typical temperatures and color levels for the classical malt types are given in Table 1.6. A different but closely related determination is used in Europe, and the units are called °W-K (W-K = Windisch-Kolbach) (Kunze, 1996). These two units are related to a reasonable degree of accuracy (generally better than 1%) by the relationship (°W-K) = 3.5 × (°L) − 16.
| Table 1.5 Approximate Color Correspondences | |||
|---|---|---|---|
|
°ASBC |
°EBC |
||
|
1.5 |
2.5 |
||
|
2 |
4 |
||
|
3 |
6.5 |
||
|
4 |
9 |
||
|
6 |
14 |
||
|
8 |
20 |
||
|
10 |
24 |
||
|
15 |
30 |
||
| Table 1.6 Typical Malt Data | |||
|---|---|---|---|
|
Malt type |
Curing temperature (°C) |
Color (°ASBC) |
DP (°L) |
|
Czech Pilsener |
85 |
1.4–1.8 |
90–100 |
|
Domestic 2-row malt |
87.5 |
1.4–2.0 |
125–135 |
|
Pale-ale/Vienna |
90 |
3.0–4.0 |
50–70 |
|
Munich |
100–105 |
6.0–10.0 |
40–65 |
|
NOTE: From Fix and Fix (1997). |
|||
Not all enzyme destruction is harmful. Lipid-active enzymes (e.g., lipase and lipoxygenase) are major players in beer staling, so their removal is highly advantageous. These issues are discussed in chapter 4.
Important flavor compounds are formed during the kilning, and the strength and character of these increase with curing temperatures and hence color. These compounds are the Maillard products already mentioned; they are formed through a process called nonenzymatic browning. There are two steps in this process (Holtermand, 1963):
1. Amadori rearrangement is a combination of a malt sugar and low-molecular-weight proteins (typically an amino acid) to form a protein-sugar complex. This process requires heat and generally will not take place below 100 °C.
2. Strecker degradation is the breakdown of the Amadori complex into aldehydes such as furfural (isobutyraldehyde and isovaleraldehyde are also important). Heterocyclic compounds such as simple melanoidins are also formed. These have a toast-like or malty aroma and a reddish-brown color; they taste acidic.
Because of the temperature threshold of (approximately) 100 °C for nonenzymatic browning, these products are more relevant to Munich malts than to pale malts. However, the Maillard products can also be created during wort boiling, depending on the amount of thermal loading applied. Here (and elsewhere in this book), thermal loading refers to the area under the temperature vs. time curve that describes a given process. In malting, this term refers to the times and temperatures used during the kilning process. In chapter 2, analogous considerations arise with wort boiling (Herrmann et al., 1985).
Roasted malts have the largest concentrations of Maillard products because of the elevated temperatures used (Blenkinsop, 1991). In addition to simple melanoidins, higher heterocyclics are formed. Two examples are pyrroles and pyrazines. They have the following structure.

Structural pyrazine (left) and pyrrole (right)
These compounds usually take on burnt or sharp, acidic flavor tones. On the other hand, the lower heterocyclics, like furfural, recall caramel and toffee flavor tones. A survey of the roasted malts and their properties can be found in the reference by Fix and Fix (1997).
Sulfur compounds and their precursors (denoted DMS-P) are formed during malting and have a profound impact on finished beer flavor as already noted. The important DMS-P (SMM and DMSO) are formed during germination, and it is now established that the extent is proportional to modification (White, 1977). Barley variety is also an issue; for example, high-protein six-row barley tends to have elevated levels (Anners and Bamforth, 1982). The final factor is malt kilning during which DMS-P is converted to DMS. The latter is highly volatile and, by and large, is removed soon after it is formed. Therefore, DMS-P levels are not usually an important issue for highly modified malt if it is kilned at high temperature (e.g., pale ale, Vienna, Munich). It is, however, very much an issue for highly modified Czech Pilsener malts as well as high-protein pale malts. These issues are discussed in chapter 2 as there is additional DMS reduction during wort boiling.
So far, this discussion has focused on malting one grain, namely, barley. However, other grains can and have been malted for brewing. Most notable is wheat, but rye and sorghum are also important for special beer types. The first two grains (rye and wheat) have common characteristics including high protein levels and very high ß-glucans levels. Cytolytic activity is crucial, and both malt types challenge the skill of malting companies. Appropriate mashing procedures are also important. Malted sorghum is popular in Africa for that continent’s indigenous beer styles. These are beers are sour because of the way they are fermented. Yet, the malting issues associated with these grains are quite interesting, and important research projects have been devoted to this topic (Diefenbach, 1996).
Mashing is best seen as an extension of malting because at each step in the mashing process, decisions about the way to proceed cannot be made in isolation from the ways the grains were malted. The starting point is when the grains are milled. Ideally, individual husks are cracked open, exposing the starchy endosperm yet leaving the husk intact (Fix, 1994). The latter is needed as a filter medium during lautering (the process of straining out the spent grains from the sweet wort; Rabin and Forget, 1998). Moreover, husks contain astringent materials, which can be extracted during sparging. This effect is not subtle and can be demonstrated in test brews (Prechtl, 1964; Fix, 1992). Since quality malts have only a small difference in fine- and coarse-grind extract—less than 2%, as already noted—small-scale brewers tend to prefer coarser grinds, since the difference in the extract with a finer crush is small enough to be justified. Large-volume brewers, on the other hand, use hundreds of thousands of tons of malt each year, and in this context, a 2% swing in extract recovery is a major issue. Therefore, extremely fine grinds using hammer mills are finding favor (Kunze, 1996). In place of the traditional lauter tun (the specially designed container for straining out the spent grains), new technology—most notably the Mash Filter 2001TM (Rehberger and Luther, 1994)—is used. The combination is not only capable of producing normal wort with high extract efficiency, it also permits 10–12 brews per day.
Many mashing systems are used throughout the world. Given the high degree of modification of modern malts, brewers have a lot of options. The following key parameters can be controlled: time and temperature, pH, and mash thickness.
The effects of holding the mash in different temperature ranges—called rests—are summarized in Table 1.7. The lowest temperature range (35–40 °C) allows for extensive ß-glucans breakdown, the effect of this being improved lautering and beer clarification. Between 60 and 65 °C, α-amylase acts as a liquefaction enzyme and encourages the dispersal of grain carbohydrates, proteins, and enzymes into the mash. This activity greatly assists enzymatic activity at the higher temperatures; hence, rests in this range invariably give higher yields. Generally, 15 min is adequate to achieve all of these effects, and certainly, no more than 30 min is required (Fix and Fix, 1997). A rest at 35–40 °C was once called the “acid rest” since phosphatases and other acid-forming enzymes are active in this temperature range. However, practical experience has shown that the mash pH is quickly established and then is held in check from that point forward by strong buffering agents in the mash. Thus, other measures are needed to establish and control mash pH.
The proteolytic regime starts near 45 °C and continues up to 55 °C, with the strongest activity being near 50 °C. There is some activity outside this range, but it is small and not of practical significance. With malts having only a relatively small amount of protein degradation (e.g., traditional lager malts), a rest in this range is highly advantageous, particularly if unmalted cereals are used because these in effect dilute the FAN pool. Traditional lager malts are rare in modern times, and if the Kolbach index is 40% or more, rests in the proteolytic range can create negative effects, including poor head retention and/or dull malt flavors. As a consequence, in Germany, high-temperature, short-time mashing schemes (called Hochkurz Maischverfahren) are being used by many commercial brewers. These schemes usually have rests near 60 °C and 70 °C with a total mashing time under one hour.
| Table 1.7 Mashing Effects during Rests at Various Temperatures | |
|---|---|
|
Temperature (°C) |
Effects promoted during mash rest in given temperature range |
|
35–40 |
Grain liquefaction and ß-glucanase activity |
|
45–55 |
ß-glucanase activity |
|
47–52 |
Proteinase and peptidase activity |
|
55–60 |
Termination of ß-glucanase activity with minimal proteinase and peptidase activity |
|
60–65 |
a-amylase activity leading to maltose production |
|
65–70 |
ß-amylase activity leading to breakdown of starch to dextrins |
|
70–72 |
Formation of glycoproteins leading to stability and texture qualities of beer foam |
In general, the rests above 60 °C are by a wide margin the most important to mashing (Lewis, 1998). This is the regime in which the bulk of the extract is dissolved and the percent fermentability is determined. This process begins with gelatinization, in which carbohydrate granules are literally burst open, making them more accessible to enzymatic attack. This process starts at low temperatures, but goes to completion when temperatures of at least 60–65 °C are achieved. As a consequence, it will occur naturally with malted barley in just about any reasonable mash. In contrast, unmalted cereals require boiling or hot flaking to achieve proper gelatinization.
Temperature plays a crucial role in the type of carbohydrate profile achieved (see Table 1.8). For example, mashing at 60 °C will yield about 80% fermentable sugars. For beers of normal strength, the apparent attenuation (i.e., the degree of fermentation as measured by a hydrometer without alcohol corrections) is approximately a factor of 1.2 higher than the real attenuation (i.e., the actual amount of sugars fermented). For example, 80% real attenuation is equivalent to 96% apparent attenuation. German diat Pils are mashed at 60 °C (see data in Piendl, 1970–1990). At the other extreme, mashing at 70 °C will yield a real attenuation (actual fermentable sugar percentage) of 60%, which corresponds (approximately) to an apparent attenuation of (0.6 × 1.2 × 100%) = 72%. The difference in maltose produced follows the same pattern.
Another point that is a relatively new discovery concerns glycoproteins. These are polymers of dextrins and middle- to high-molecular-weight proteins. They are known to be major factors in the stability of beer foam (Melm et al., 1995). Moreover, the complexes are formed when the mash temperature falls into the range 70–74 °C with 72 °C being preferred (Ishibashi et al., 1997). Test brews have shown that this is a significant effect. Mash thickness is the concentration of grains in the mash. It is normally expressed as a liquor:grist ratio; i.e., thickness = liters of water per kilogram of grains. Many brewers quote thickness in terms of pounds per gallon.
| Table 1.8 Sugar Profiles | |||
|---|---|---|---|
| Percentages of Sugar Compounds at Various Temperatures | |||
|
60 °C |
65 °C |
70 °C |
|
|
Disaccharide |
61 |
55 |
41 |
|
Trisaccharide |
9 |
12 |
16 |
|
Monosaccharide |
10 |
9 |
8 |
|
Dextrins |
20 |
24 |
35 |
|
NOTE: Adapted from Fix and Fix (1997). |
|||
Mashes as thin as 3.5 L/kg (2.4 lb./gal.; note that the SI [metric] units are given in terms of volume per mass whereas the U.S. customary units are given in terms of weight per volume) have been reported as well as mashes as thick as 1.5 L/kg (5.5 lb./gal.) (Lewis and Young, 1995). Normally, mash thickness is near 2.67 L/kg (3.1 lb./gal.). It has been noted that thicker mashes favor proteolytic activity, whereas thinner mashes favor carbohydrase action because of the restraining influence sugar concentrations have on α- and ß-amylase (De Clerck, 1957). Measured data (Lewis and Young, 1995) show, however, that these effects are small over the 2–3 L/kg range.
The final control parameter in the mash is the pH, which has been known for a long time to be very important (De Clerck, 1957). The classic rule is for the chilled wort to have a pH of 5.0 to 5.2 and, to achieve this level, it is desirable to establish a mash pH in the range of 5.2–5.4 (Hind, 1950). This range, first of all, is favorable to enzymatic activity. Although the various enzymes have different optimum pH levels, in practical brewing conditions the enzymes’ activities do not decrease by much if the pH levels are more acid On the other hand, there is typically a sharp decrease in the enzymes’ activity if the pH becomes more basic. This factor is illustrated for enzymes, by using amylase as an example, in Table 1.9. Another equally important factor is that high-pH mashes (say, above 5.5) tend to lead to dull malt flavor that lacks definition (Narziss, 1992). Hop flavors are also negatively affected.
Biological acidification is an old method that is experiencing a rebirth. For example, L. Narziss in his article on brewing technology of the future cited biological acidification as a major item (Narziss, 1997). It was originally proposed as a method for controlling the mash pH that is natural to beer. It was targeted at brewers, such as those in Germany, whose compliance with purity laws excluded synthetic chemical additives. Today, many other attributes are associated with this procedure, including a cleaner aftertaste as well as a better definition of the malt and hop flavors (Oliver and Dauman, 1988a, 1988b; Bach and Fersing, 1997; Narziss, 1998). Personal test brews using this procedure support these claims.
| Table 1.9 Amylase Activity at 60 °C | |
|---|---|
|
pH |
Activity (%) |
|
4.8 |
98 |
|
5.0 |
99 |
|
5.2 |
100 |
|
5.4 |
95 |
|
5.8 |
85 |
|
6.2 |
65 |
|
NOTE: Adapted from Hind (1950). |
|
Crucial to this procedure is the use of special microbes whose only products are lactic acid. Lactobacillus delbruckii is a classical choice (De Clerck, 1957), but Lactobacillus amylolyticus is finding favor (Kunze, 1996). It must be emphasized that random contamination of the mash—for example, by overnight mashing—can lead to erratic results. Contamination can introduce undesirable microbes (e.g., butyric acid bacteria). These microbes are eliminated during wort boiling; however, their products have a pernicious way of surviving (at least in part) into the finished beer.
The best results in my test brews came from preparing the sour mash separately from the main mash. The sour mash, if pasteurized, can be stored at room temperature for weeks (and possibly longer). On the brew day, the sour mash is then added incrementally until the desired pH (5.2 to 5.4) is achieved. With specialty beers, e.g., Belgian or German “white” beers, the pH of the mash is reduced to the range 4.0–4.5. It is interesting that the same is being done in Germany with low-alcohol beers (Narziss, 1998). The strong buffering agents in the mash will keep this pH stable once it is established.
When starch conversion is complete, the mash is separated from the grains, a process that leaves residual extract in the grains. To remove that residue, the grains are washed (sparged) with hot water. From the point of view of efficiency, i.e., obtaining the most extract, the more sparge water, the better. Excess volumes can be reduced by appropriate evaporation in the kettle boil. However, it is rare in brewing for efficiency and beer quality to be in harmony. This adage is particularly the case in sparging, because in addition to removing extract, the sparge process also extracts a collection of undesirable compounds, traditionally called herbstoffe (astringents). In malt, these come from husk-derived phenols (tannins), silica ash, and other materials that themselves have astringent flavors. In addition, these undesirable elements contribute to beer staling and haze. Raw grains, corn more than rice, also contribute astringent compounds (Fix, 1992).
Thus, brewers must seek a compromise between the smoothness and elegance of a finished beer and the amount of extract they obtain. The traditional rule is to use approximately the same amount of water in the sparge as used in the mash.
In beer styles where smoothness is an important characteristic, brewers tend to augment the traditional rule with the following constraint: When the residual extract in the grains approaches 1.0 °P, then the sparge is terminated. Evaporation in the kettle boil can then be adjusted to get the proper volume for the fermenter. In modern practice, the focus has turned to the pH of the wort collected from the sparge because pH increases with the extraction of undesirable astringents. A general rule is to terminate the sparge when the pH of the collected wort increases much beyond 0.1 pH units higher than the mash pH. In any case, it should not exceed 5.5. Application of this general rule usually amounts to a 1–2 °P extract left in the grains.
If the sparge water has an alkalinity in excess of 50 mg/L as CaCO3, then the pH will quickly approach the critical point. High temperatures also increase the extraction of undesirable compounds, the critical temperature being about 77 °C. Thus, best results are generally obtained with a sparge in the range of 74 to 75 °C and with water whose alkalinity is as low as possible (25 mg/L or below).