Overweight and Obesity
Sheila Gahagan
Epidemiology
Obesity is an important pediatric public health problem associated with risk of complications in childhood and increased morbidity and mortality throughout adult life. Obesity is now linked to more deaths than underweight. In 2014, according to the World Health Organization (WHO), more than 1.9 billion persons ≥20 yr old were overweight or obese.
In the United States, 37% of adults are obese, and 35% are overweight. In children the prevalence of obesity increased 300% over approximately 40 yr. According to the National Health and Nutrition Examination Survey (NHANES), 2013–2014, 34% of children 2-19 yr old were overweight or obese, with 17% in the obese range. Risk for obesity in children 2-19 yr old varies significantly by race/ethnicity, with >20% for minority children compared with 15% for white children. Across all racial groups, higher maternal education confers protection against childhood obesity.
The first 1000 days, the period from conception to age 2 yr, are increasingly recognized as a modifiable period related to risk for childhood obesity. Parental obesity correlates with a higher risk for obesity in the children. Prenatal factors, including high preconceptual weight, gestational weight gain, high birthweight, and maternal smoking, are associated with increased risk for later obesity. Paradoxically, intrauterine growth restriction with early infant catch-up growth is associated with the development of central adiposity and adult-onset cardiovascular (CV) risk. Breastfeeding is modestly protective for obesity based on dose and duration. Infants with high levels of negative reactivity (temperament) are more at risk for obesity than those with better self-regulation.
Body Mass Index
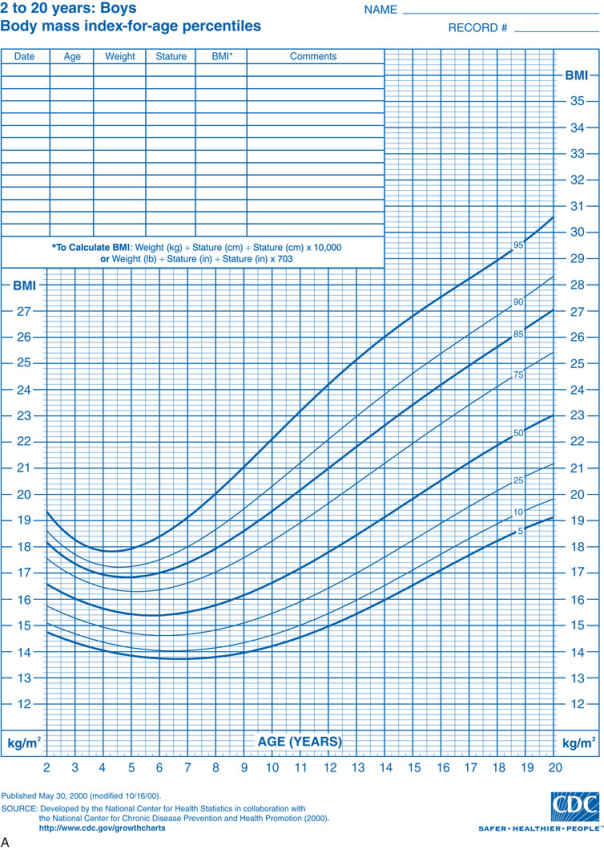
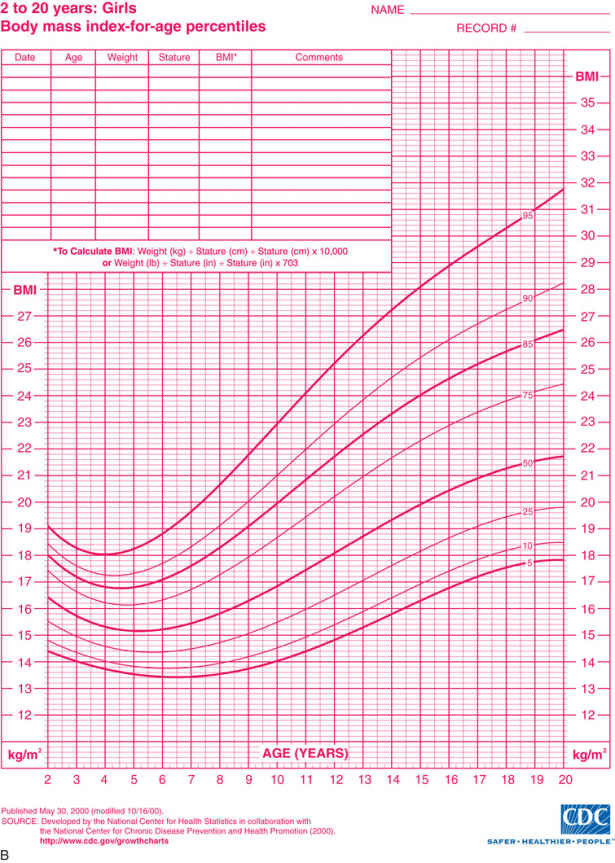
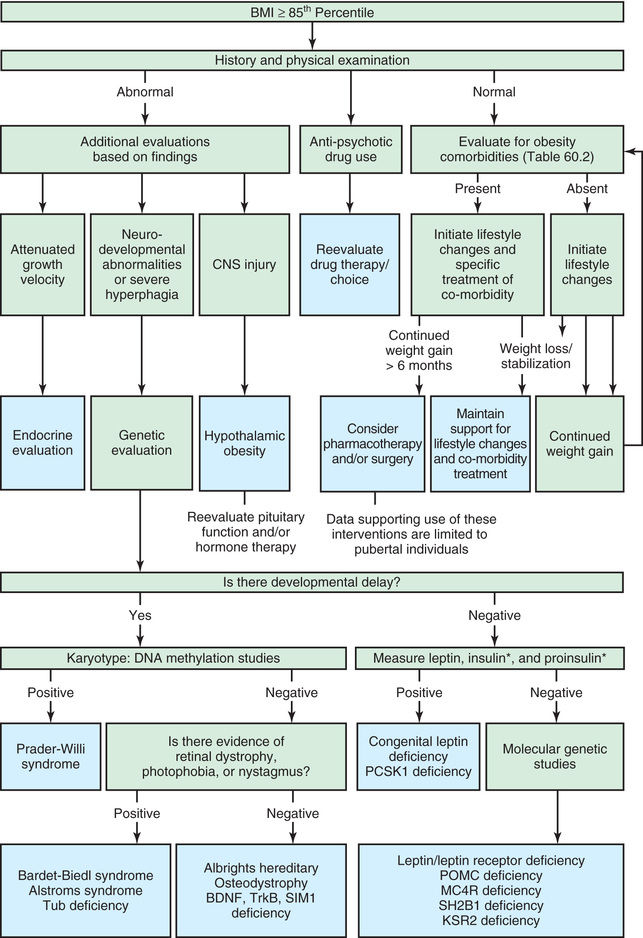
Obesity or increased adiposity is defined using the body mass index (BMI) , an excellent proxy for more direct measurement of body fat. BMI = weight in kg/(height in meters)2 . Adults with a BMI ≥30 meet the criterion for obesity, and those with a BMI 25-30 fall in the overweight range. During childhood, levels of body fat change beginning with high adiposity during infancy. Body fat levels decrease for approximately 5.5 yr until the period called adiposity rebound, when body fat is typically at the lowest level. Adiposity then increases until early adulthood (Fig. 60.1 ). Consequently, obesity and overweight are defined using BMI percentiles for children ≥2 yr old and weight/length percentiles for infants <2 yr old. The criterion for obesity is BMI ≥95th percentile and for overweight is BMI between 85th and 95th percentiles.
Etiology
Humans have the capacity to store energy in adipose tissue, allowing improved survival in times of famine. Simplistically, obesity results from an imbalance of caloric intake and energy expenditure. Even incremental but sustained caloric excess results in excess adiposity. Individual adiposity is the result of a complex interplay among genetically determined body habitus, appetite, nutritional intake, physical activity (PA) , and energy expenditure. Environmental factors determine levels of available food, preferences for types of foods, levels of PA, and preferences for types of activities. Food preferences play a role in consumption of energy-dense foods. Humans innately prefer sweet and salty foods and tend initially to reject bitter flavors, common to many vegetables. Repeated exposure to healthy foods promotes their acceptance and liking, especially in early life. This human characteristic to adapt to novel foods can be used to promote healthy food selection.
Environmental Changes
Over the last 4 decades, the food environment has changed dramatically related to urbanization and the food industry. As fewer families routinely prepare meals, foods prepared by a food industry have higher levels of calories, simple carbohydrates, and fat. The price of many foods has declined relative to the family budget. These changes, in combination with marketing pressure, have resulted in larger portion sizes and increased snacking between meals. The increased consumption of high-carbohydrate beverages, including sodas, sport drinks, fruit punch, and juice, adds to these factors.
Fast food is consumed by one third of U.S. children each day and by two thirds of children every week. A typical fast food meal can contain 2000 kcal and 84 g of fat. Many children consume 4 servings of high-carbohydrate beverages per day, resulting in an additional 560 kcal of low nutritional value. Sweetened beverages have been linked to increased risk for obesity. The dramatic increase in the use of high-fructose corn syrup to sweeten beverages and prepared foods is another important environmental change, leading to availability of inexpensive calories.
Since World War II, levels of PA in children and adults have declined. According to the 2012 NHANES survey, 25% of 12-15 yr olds met PA guidelines of 60 min of PA per day. Decline in PA is related to many factors, including changes in the built environment, more reliance on cars, lower levels of active transportation, safety issues, and increasingly sedentary lifestyles. Many sectors of society do not engage in PA during leisure time. For children, budgetary constraints and pressure for academic performance have led to less time devoted to physical education in schools. Perception of poor neighborhood safety also leads to lower levels of PA. Furthermore, screens (televisions, tablets, smartphones, computers) offer compelling sedentary activities that do not burn calories.
Sleep plays a role in risk for obesity. Over the last 4 decades, children and adults have decreased the amount of time spent sleeping. Reasons for these changes may relate to increased time at work, increased time watching television, and a generally faster pace of life. Chronic partial sleep loss can increase risk for weight gain and obesity, with the impact possibly greater in children than in adults. In studies of young, healthy, lean men, short sleep duration was associated with decreased leptin levels and increased ghrelin levels, along with increased hunger and appetite. Sleep debt also results in decreased glucose tolerance and insulin sensitivity related to alterations in glucocorticoids and sympathetic activity. Some effects of sleep debt might relate to orexins, peptides synthesized in the lateral hypothalamus that can increase feeding, arousal, sympathetic activity, and neuropeptide Y activity.
Genetics
Genetic determinants also have a role in individual susceptibility to obesity (Table 60.1 ). Findings from genome-wide association studies explain a very small portion of interindividual variability in obesity. One important example, the FTO gene at 16q12, is associated with adiposity in childhood, probably explained by increased energy intake. Monogenic forms of obesity have also been identified, including melanocortin-4 receptor (MC4R) deficiency, associated with early-onset obesity and food-seeking behavior. Mutations in MC4R are a common cause of monogenetic obesity but a rare cause of obesity in general. Deficient activation of MC4R is seen in patients with proopiomelanocortin (POMC) deficiency, a prohormone precursor of adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormone (MSH), resulting in adrenal insufficiency, light skin, hyperphagia, and obesity.
Table 60.1
Endocrine and Genetic Causes of Obesity
| DISEASE | SYMPTOMS | LABORATORY |
|---|---|---|
| ENDOCRINE | ||
| Cushing syndrome | Central obesity, hirsutism, moon face, hypertension | Dexamethasone suppression test |
| GH deficiency | Short stature, slow linear growth | Evoked GH response, IGF-1 |
| Hyperinsulinism | Nesidioblastosis, pancreatic adenoma, hypoglycemia, Mauriac syndrome | Insulin level |
| Hypothyroidism | Short stature, weight gain, fatigue, constipation, cold intolerance, myxedema | TSH, FT4 |
| Pseudohypoparathyroidism | Short metacarpals, subcutaneous calcifications, dysmorphic facies, mental retardation, short stature, hypocalcemia, hyperphosphatemia | Urine cAMP after synthetic PTH infusion |
| GENETIC | ||
| Albright hereditary osteodystrophy | Short stature, skeletal defects, PTH resistance | GNAS gene |
| Alström syndrome | Cognitive impairment, retinitis pigmentosa, diabetes mellitus, hearing loss, hypogonadism, cardiomyopathy | ALMS1 gene |
| Bardet-Biedl syndrome | Retinitis pigmentosa, renal abnormalities, polydactyly, syndactyly, hypogonadism | BBS1 gene |
| BDNF/TrkB deficiency | Hyperactivity, impaired concentration, limited attention span, impaired short-term memory and pain sensation | BDNF/TrkB gene |
| Biemond syndrome | Cognitive impairment, iris coloboma, hypogonadism, polydactyly | |
| Carpenter syndrome | Polydactyly, syndactyly, cranial synostosis, mental retardation | Mutations in RAB23 gene, located on chromosome 6 in humans |
| Cohen syndrome | Mid-childhood-onset obesity, short stature, prominent maxillary incisors, hypotonia, mental retardation, microcephaly, decreased visual activity | Mutations in VPS13B gene (often called COH1 ) at locus 8q22 |
| Deletion 9q34 | Early-onset obesity, mental retardation, brachycephaly, synophrys, prognathism, behavior and sleep disturbances | Deletion 9q34 |
| Down syndrome | Short stature, dysmorphic facies, mental retardation | Trisomy 21 |
| ENPP1 gene mutations | Insulin resistance, childhood obesity | Gene mutation on chromosome 6q |
| Fröhlich syndrome | Hypothalamic tumor | |
| FTO gene polymorphism, plus upstream regulatory and downstream activation genes | Dysregulation of orexigenic hormone acyl-ghrelin, poor postprandial appetite suppression | Homozygous for FTO AA allele |
| KSR2 deficiency | Mild hyperphagia and reduced basal metabolic rate, insulin resistance often with acanthosis nigricans, irregular menses, early development of type 2 diabetes mellitus | KSR2 gene |
| Leptin or leptin receptor gene deficiency | Early-onset severe obesity, infertility (hypogonadotropic hypogonadism), hyperphagia, infections | Leptin |
| Melanocortin 4 receptor gene mutation |
Early-onset severe obesity, increased linear growth, hyperphagia, hyperinsulinemia Most common known genetic cause of obesity Homozygous worse than heterozygous |
MC4R mutation |
| PCSK1 deficiency | Small bowel enteropathy, hypoglycemia, hypothyroidism, ACTH deficiency, diabetes insipidus | PCSK1 gene |
| Prader-Willi syndrome | Neonatal hypotonia, slow infant growth, small hands and feet, mental retardation, hypogonadism, hyperphagia leading to severe obesity, paradoxically elevated ghrelin | Partial deletion of chromosome 15 or loss of paternally expressed genes |
| Proopiomelanocortin (POMC) deficiency | Obesity, red hair, adrenal insufficiency due to ACTH deficiency, hyperproinsulinemia, hyperphagia, pale skin, cholestatic jaundice | Loss-of-function mutations of POMC gene |
| Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) | Often confused with congenital central hypoventilation syndrome (CCHS); presentation ≥1.5 yr with weight gain, hyperphagia, hypoventilation, cardiac arrest, central diabetes insipidus, hypothyroidism, GH deficiency, pain insensitivity, hypothermia, precocious puberty, neural crest tumors |
Unknown genes May be a paraneoplastic disorder |
| SH2B1 deficiency | Hyperphagia, disproportionate hyperinsulinemia, early speech and language delay that often resolves, behavioral problems including aggression | SH2B1 gene |
| SIM1 deficiency | Hyperphagia with autonomic dysfunction (characterized by low systolic blood pressure), speech and language delay, neurobehavioral abnormalities including autistic-type behaviors | SIM1 gene |
| TUB deficiency | Retinal dystrophy, deafness | TUB gene |
| Turner syndrome | Ovarian dysgenesis, lymphedema, web neck, short stature, cognitive impairment | XO chromosome |
ACTH, Adrenocorticotropic hormone; cAMP, cyclic adenosine monophosphate; FT4 , free thyroxine; GH, growth hormone; IGF, insulin-like growth factor; PTH, parathyroid hormone; TSH, thyroid-stimulating hormone.
In addition, evidence suggests that appetitive traits are moderately heritable. For example, some genes associated with appetite also relate to weight, and vice-versa. In addition, there are genetic conditions associated with obesity, such as Prader-Willi syndrome, which results from absence of paternally expressed imprinted genes in the 15q11.2–q13 region. Prader-Willi syndrome is characterized by insatiable appetite and food seeking. In the era of genomic medicine, it will be increasingly possible to identify risks according to specific genes and consider gene-environment interactions. Epigenetic environmental modification of genes may have a role in the development of obesity, especially during fetal and early life.
Microbiome
It is increasingly recognized the human gut microbiota play a role in regulating metabolism. This novel area of research raises questions about the role of antibiotics in the pathway to obesity and the possibility that probiotics could be therapeutic for certain individuals.
Endocrine and Neural Physiology
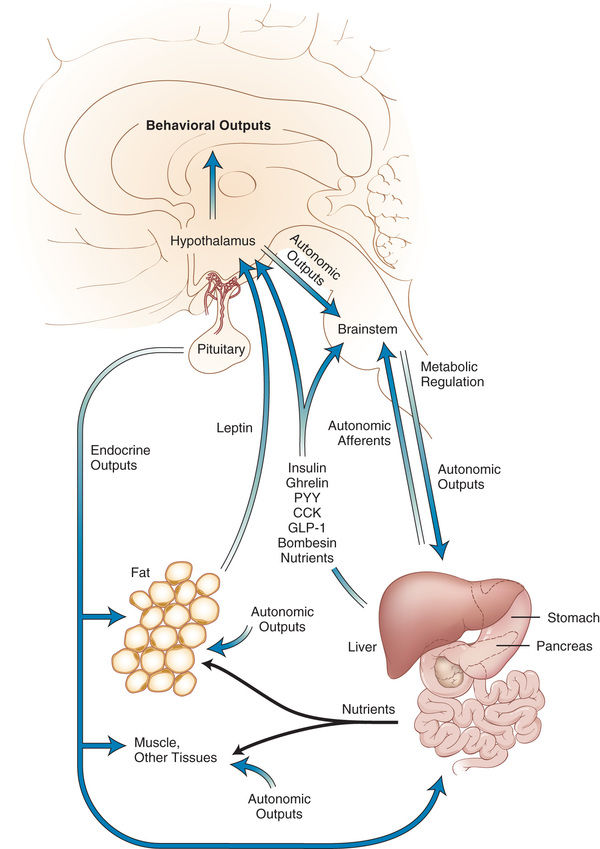
Monitoring of “stored fuels” and short-term control of food intake (appetite and satiety) occurs through neuroendocrine feedback loops linking adipose tissue, the gastrointestinal (GI) tract, and the central nervous system (CNS) (Figs. 60.2 and 60.3 ). GI hormones, including cholecystokinin, glucagon-like peptide 1, peptide YY, and vagal neuronal feedback promote satiety. Ghrelin stimulates appetite. Adipose tissue provides feedback regarding energy storage levels to the brain through hormonal release of adiponectin and leptin. These hormones act on the arcuate nucleus in the hypothalamus and on the solitary tract nucleus in the brainstem and in turn activate distinct neuronal networks. Adipocytes secrete adiponectin into the blood, with reduced levels in response to obesity and increased levels in response to fasting. Reduced adiponectin levels are associated with lower insulin sensitivity and adverse CV outcomes. Leptin is directly involved in satiety; low leptin levels stimulate food intake, and high leptin levels inhibit hunger in animal models and in healthy human volunteers. However, the negative feedback loop from leptin to appetite may be more adapted to preventing starvation than excess intake.
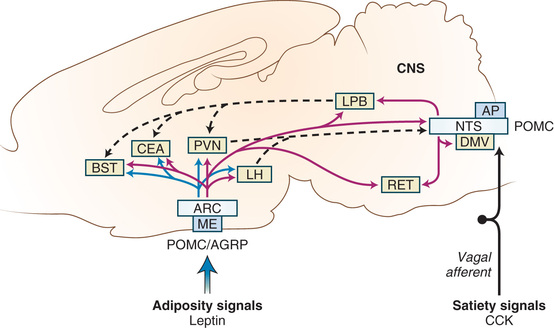
Numerous neuropeptides in the brain, including peptide YY (PYY), agouti-related peptide, and orexin, appear to affect appetite stimulation, whereas melanocortins and α-melanocortin–stimulating hormone are involved in satiety (Fig. 60.3 ). The neuroendocrine control of appetite and weight involves a negative-feedback system, balanced between short-term control of appetite and long-term control of adiposity (including leptin). PYY reduces food intake via the vagal-brainstem-hypothalamic pathway. Developmental changes in PYY are evident as infants have higher PYY levels than school-age children and adults. Obese children have lower fasting levels of PYY than adults. Weight loss may restore PYY levels in children, even though this does not happen in adults. In addition, patients homozygous for the FTO obesity-risk allele demonstrate poor regulation of the orexigenic hormone acyl-ghrelin and have poor postprandial appetite suppression.
Comorbidities
Complications of pediatric obesity occur during childhood and adolescence and persist into adulthood. An important reason to prevent and treat pediatric obesity is the increased risk for morbidity and mortality later in life. The Harvard Growth Study found that boys who were overweight during adolescence were twice as likely to die from CV disease as those who had normal weight. More immediate comorbidities include type 2 diabetes, hypertension, hyperlipidemia, and nonalcoholic fatty liver disease (NAFLD) (Table 60.2 ). Insulin resistance increases with increasing adiposity and independently affects lipid metabolism and CV health. The metabolic syndrome (central obesity, hypertension, glucose intolerance, and hyperlipidemia) increases risk for CV morbidity and mortality. NAFLD has been reported in 34% of patients treated in pediatric obesity clinic. NAFLD is now the most common chronic liver disease in U.S. children and adolescents. It can present with advanced fibrosis or nonalcoholic steatohepatitis and may result in cirrhosis and hepatocellular carcinoma. Insulin resistance is often associated. Furthermore, NAFLD is independently associated with increased risk of CV disease.
Table 60.2
Obesity-Associated Comorbidities
| DISEASE | POSSIBLE SYMPTOMS | LABORATORY CRITERIA |
|---|---|---|
| CARDIOVASCULAR | ||
| Dyslipidemia | HDL <40, LDL >130, total cholesterol >200 mg/dL | Fasting total cholesterol, HDL, LDL, triglycerides |
| Hypertension | SBP >95% for sex, age, height | Serial testing, urinalysis, electrolytes, blood urea nitrogen, creatinine |
| ENDOCRINE | ||
| Type 2 diabetes mellitus | Acanthosis nigrans, polyuria, polydipsia | Fasting blood glucose >110, hemoglobin A1c , insulin level, C-peptide, oral glucose tolerance test |
| Metabolic syndrome | Central adiposity, insulin resistance, dyslipidemia, hypertension, glucose intolerance | Fasting glucose, LDL and HDL cholesterol |
| Polycystic ovary syndrome | Irregular menses, hirsutism, acne, insulin resistance, hyperandrogenemia | Pelvic ultrasound, free testosterone, LH, FSH |
| GASTROINTESTINAL | ||
| Gallbladder disease | Abdominal pain, vomiting, jaundice | Ultrasound |
| Nonalcoholic fatty liver disease (NAFLD) |
Hepatomegaly, abdominal pain, dependent edema, ↑ transaminases Can progress to fibrosis, cirrhosis |
AST, ALT, ultrasound, CT, or MRI |
| NEUROLOGIC | ||
| Pseudotumor cerebri | Headaches, vision changes, papilledema | Cerebrospinal fluid opening pressure, CT, MRI |
| Migraines | Hemicrania, headaches | None |
| ORTHOPEDIC | ||
| Blount disease (tibia vara) | Severe bowing of tibia, knee pain, limp | Knee radiographs |
| Musculoskeletal problems | Back pain, joint pain, frequent strains or sprains, limp, hip pain, groin pain, leg bowing | Radiographs |
| Slipped capital femoral epiphysis | Hip pain, knee pain, limp, decreased mobility of hip | Hip radiographs |
| PSYCHOLOGIC | ||
| Behavioral complications | Anxiety, depression, low self-esteem, disordered eating, signs of depression, worsening school performance, social isolation, problems with bullying or being bullied | Child Behavior Checklist, Children's Depression Inventory, Peds QL, Eating Disorder Inventory 2, subjective ratings of stress and depression, Behavior Assessment System for Children, Pediatric Symptom Checklist |
| PULMONARY | ||
| Asthma | Shortness of breath, wheezing, coughing, exercise intolerance | Pulmonary function tests, peak flow |
| Obstructive sleep apnea | Snoring, apnea, restless sleep, behavioral problems | Polysomnography, hypoxia, electrolytes (respiratory acidosis with metabolic alkalosis) |
ALT, Alanine transaminase; AST, aspartate transaminase; CT, computed tomography; FSH, follicle-stimulating hormone; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LH, luteinizing hormone; MRI, magnetic resonance imaging; Peds QL, Pediatric Quality of Life Inventory; SBP, systolic blood pressure.
Obesity may also be associated with chronic inflammation. Adiponectin, a peptide with antiinflammatory properties, occurs in reduced levels in obese patients compared to insulin-sensitive, lean persons. Low adiponectin levels correlate with elevated levels of free fatty acids and plasma triglycerides as well as a high BMI, and high adiponectin levels correlate with peripheral insulin sensitivity. Adipocytes secrete peptides and cytokines into the circulation, and proinflammatory peptides such interleukin (IL)-6 and tumor necrosis factor (TNF)-α occur in higher levels in obese patients. Specifically, IL-6 stimulates production of C-reactive protein (CRP) in the liver. CRP is a marker of inflammation and might link obesity, coronary disease, and subclinical inflammation.
Some complications of obesity are mechanical, including obstructive sleep apnea and orthopedic complications. Orthopedic complications include Blount disease and slipped femoral capital epiphysis (see Chapters 697 and 698.4 ).
Mental health problems can coexist with obesity, with the possibility of bidirectional effects. These associations are modified by gender, ethnicity, and socioeconomic status. Self-esteem may be lower in obese adolescent girls than in nonobese peers. Some studies have found an association between obesity and adolescent depression. There is considerable interest in the co-occurrence of eating disorders and obesity. Obese youth are also at risk for bullying based on their appearance.
Identification
Overweight and obese children are often identified as part of routine medical care. The child and family may be unaware that the child has increased adiposity. They may be unhappy with the medical provider for raising this issue and may respond with denial or apparent lack of concern. It is often necessary to begin by helping the family understand the importance of healthy weight for current and future health. Forging a good therapeutic relationship is important because obesity intervention requires a chronic disease management approach. Intervention and successful resolution of this problem require considerable effort by the family and the child over an extended period in order to change eating and activity behaviors.
Evaluation
The evaluation of the overweight or obese child begins with examination of the growth chart for weight, height, and BMI trajectories; consideration of possible medical causes of obesity; and detailed exploration of family eating, nutritional, and activity patterns. A complete pediatric history is used to uncover comorbid disorders. The family history focuses on the adiposity of other family members and the family history of obesity-associated disorders. The physical examination adds data that can lead to important diagnoses. Laboratory testing is guided by the need to identify comorbidities.
Examination of the growth chart reveals the severity, duration, and timing of obesity onset. Children who are overweight (BMI in 85–95th percentile) are less likely to have developed comorbid conditions than those who are obese (BMI ≥95th percentile). Those with a BMI ≥99th percentile are more likely to have coexisting medical problems. Once obesity severity is determined, the BMI trajectory is examined to elucidate when the child became obese. Several periods during childhood are considered sensitive periods, or times of increased risk for developing obesity, including infancy, adiposity rebound (when body fat is lowest at approximately age 5.5 yr), and adolescence. An abrupt change in BMI might signal the onset of a medical problem or a period of family or personal stress for the child. Examination of the weight trajectory can further reveal how the problem developed. A young child might exhibit high weight and high height because linear growth can increase early in childhood if a child consumes excess energy. At some point the weight percentile exceeds the height percentile, and the child's BMI climbs into the obese range. Another example is a child whose weight rapidly increases when she reduces her activity level and consumes more meals away from home. Examination of the height trajectory can reveal endocrine problems, which often occur with slowing of linear growth.
Consideration of possible medical causes of obesity is essential, even though endocrine and genetic causes are rare (see Table 60.1 ). Growth hormone deficiency, hypothyroidism, and Cushing syndrome are examples of endocrine disorders that can lead to obesity. In general, these disorders manifest with slow linear growth. Because children who consume excessive amounts of calories tend to experience accelerated linear growth, short stature warrants further evaluation. Genetic disorders associated with obesity may manifest extreme hyperphagia, or they can have coexisting dysmorphic features, cognitive impairment, vision and hearing abnormalities, or short stature. In some children with congenital disorders such as myelodysplasia or muscular dystrophy, lower levels of PA can lead to secondary obesity. Some medications, such as atypical antipsychotics, can cause excessive appetite and hyperphagia, resulting in obesity (Table 60.3 ). Rapid weight gain in a child or adolescent taking one of these medications might require its discontinuation. Poor linear growth and rapid changes in weight gain are indications for evaluation of possible medical causes.
Exploration of family eating, nutritional, and activity patterns begins with a description of regular meal and snack times and family habits for walking, bicycle riding, active recreation, and screen time (TV, computer, video games). It is useful to request a 24-hr dietary recall with special attention to intake of fruits, vegetables, and water, as well as high-calorie foods and high-carbohydrate beverages. When possible, evaluation by a nutritionist is extremely helpful. This information will form the basis for incremental changes in eating behavior, caloric intake, and PA during the intervention.
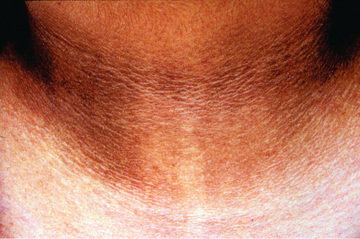
Initial assessment of the overweight or obese child includes a complete review of bodily systems focusing on the possibility of comorbid conditions (see Table 60.2 ). Developmental delay and visual and hearing impairment can be associated with genetic disorders. Difficulty sleeping, snoring, or daytime sleepiness suggests sleep apnea. Abdominal pain might suggest NAFLD. Symptoms of polyuria, nocturia, or polydipsia may be the result of type 2 diabetes. Hip or knee pain can be caused by secondary orthopedic problems, including Blount disease and slipped capital femoral epiphysis. Irregular menses may be associated with polycystic ovary syndrome. Acanthosis nigricans can suggest insulin resistance and type 2 diabetes (Fig. 60.4 ).
The family history begins with identifying other obese family members. Parental obesity is an important risk for child obesity. If all family members are obese, focusing the intervention on the entire family is reasonable. The child may be at increased risk for developing type 2 diabetes if a family history exists. Patients of African American, Hispanic, or Native American heritage are also at increased risk for developing type 2 diabetes. Identification of a family history of hypertension, CV disease, or metabolic syndrome indicates increased risk for developing these obesity-associated conditions. If the clinician helps the family to understand that childhood obesity increases risk for developing these chronic diseases, this educational intervention might serve as motivation to improve their nutrition and PA.
Physical examination should be thorough, focusing on possible comorbidities (see Table 60.2 ). Careful screening for hypertension using an appropriately sized blood pressure cuff is important. Systematic examination of the skin can reveal acanthosis nigricans, suggesting insulin resistance, or hirsutism, suggesting polycystic ovary syndrome. Tanner staging can reveal premature adrenarche secondary to advanced sexual maturation in overweight and obese girls.
Laboratory testing for fasting plasma glucose, triglycerides, low-density lipoprotein and high-density lipoprotein cholesterol, and liver function tests are recommended as part of the initial evaluation for newly identified pediatric obesity (Table 60.4 ). Overweight children (BMI 85–95th percentile) who have a family history of diabetes mellitus or signs of insulin resistance should also be evaluated with a fasting plasma glucose test. Other laboratory testing should be guided by history or physical examination findings. Fig. 60.5 provides a recommended approach to categorization, evaluation, and treatment.
Table 60.4
AST, Aspartate transaminase; ALT, alanine transaminase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
From Children's Hospital of Wisconsin: The NEW (nutrition, exercise and weight management) kids program (PDF file). http://www.chw.org/display/displayFile.asp?docid=33672&filename=/Groups/NEWKids/NewKidsReferral.PDF .
Intervention
Evidence shows that some interventions result in modest but significant and sustained improvement in body mass. Based on behavior change theories, treatment includes specifying target behaviors , self-monitoring , goal setting , stimulus control , and promotion of self-efficacy and self-management skills. Behavior changes associated with improving BMI include drinking lower quantities of sugar-sweetened beverages, consuming higher-quality diets, increasing exercise, decreasing screen time, and self-weighing. Most successful interventions have been family based and consider the child's developmental age. “Parent-only” treatment can be as effective as “parent–child” treatment. Because obesity is multifactorial, not all children and adolescents will respond to the same approach. For example, loss-of-control eating, associated with weight gain and obesity, predicts poor outcome in response to family-based treatment. Furthermore, clinical treatment programs are expensive and not widely available. Therefore, interest has grown in novel approaches such as internet-based treatments and guided self-help.
It is important to begin with clear recommendations about appropriate caloric intake for the obese child (Table 60.5 ). Working with a dietitian is essential. Meals should be based on fruits, vegetables, whole grains, lean meat, fish, and poultry. Prepared foods should be chosen for their nutritional value, with attention to calories and fat. Foods that provide excessive calories and low nutritional value should be reserved for infrequent treats.
Table 60.5
Recommended Caloric Intake Designated by Age and Gender
| LIFE-STAGE GROUP | AGE (yr) | RELATIVELY SEDENTARY LEVEL OF ACTIVITY (kcal) | MODERATE LEVEL OF ACTIVITY (kcal) | ACTIVE (kcal) |
|---|---|---|---|---|
| Child | 2-3 | 1,000 | 1,000-1,400 | 1,000-1,400 |
| Female | 4-8 | 1,200 | 1,400-1,600 | 1,400-1,800 |
| 9-13 | 1,600 | 1,600-2,000 | 1,800-2,200 | |
| 14-18 | 1,800 | 2,000 | 2,400 | |
| Male | 4-8 | 1,400 | 1,400-1,600 | 1,600-2,000 |
| 9-13 | 1,800 | 1,800-2,200 | 2,000-2,600 | |
| 14-18 | 2,200 | 2,400-2,800 | 2,800-3,200 |
Adapted from U.S. Department of Agriculture: Dietary guidelines for Americans , 2005. http://www.health.gov/DIETARYGUIDELINES/dga2005/document/html/chapter2.htm .
Weight reduction diets in adults generally do not lead to sustained weight loss. Therefore the focus should be on changes that can be maintained for life. Attention to eating patterns is helpful. Families should be encouraged to plan family meals, including breakfast. It is almost impossible for a child to make changes in nutritional intake and eating patterns if other family members do not make the same changes. Dietary needs also change developmentally; adolescents require greatly increased calories during their growth spurts, and adults who lead inactive lives need fewer calories than active, growing children.
Psychologic strategies are helpful. The “traffic light” diet groups foods into those that can be consumed without any limitations (green), in moderation (yellow), or reserved for infrequent treats (red) (Table 60.6 ). The concrete categories are very helpful to children and families. This approach can be adapted to any ethnic group or regional cuisine. Motivational interviewing begins with assessing how ready the patient is to make important behavioral changes. The professional then engages the patient in developing a strategy to take the next step toward the ultimate goal of healthy nutritional intake. This method allows the professional to take the role of a coach, helping the child and family reach their goals. Other behavioral approaches include family rules about where food may be consumed (e.g., “not in the bedroom”).
Table 60.6
| FEATURE | GREEN LIGHT FOODS | YELLOW LIGHT FOODS | RED LIGHT FOODS |
|---|---|---|---|
| Quality | Low-calorie, high-fiber, low-fat, nutrient-dense | Nutrient-dense, but higher in calories and fat | High in calories, sugar, and fat |
| Types of food | Fruits, vegetables | Lean meats, dairy, starches, grains | Fatty meats, sugar, sugar-sweetened beverages, fried foods |
| Quantity | Unlimited | Limited | Infrequent or avoided |
Increasing PA without decreasing caloric intake is unlikely to result in weight loss. However, aerobic exercise training has been shown to improve metabolic profiles in obese children and adolescents. Furthermore, it can increase aerobic fitness and decrease percent body fat even without weight loss. Therefore, increasing PA can decrease risk for CV disease, improve well-being, and contribute to weight loss. Increased PA can be accomplished by walking to school, engaging in PA during leisure time with family and friends, or enrolling in organized sports. Children are more likely to be active if their parents are active. As with family meals, family PA is recommended. When adults lose significant weight, they may regain that weight despite eating fewer calories. The body may adapt to weight loss by reducing the basal metabolic rate (BMR), thus requiring fewer calories. One approach to this phenomenon is to increase PA.
Active pursuits can replace more sedentary activities. The American Academy of Pediatrics recommends that screen time be restricted to no more than 2 hr/day for children >2 yr old and that children <2 yr old not watch television. TV watching is often associated with eating, and many highly caloric food products are marketed directly to children during child-oriented television programs.
Pediatric healthcare providers should assist families to develop goals to change nutritional intake and PA. They can also provide the child and family with needed information. The family should not expect immediate lowering of BMI percentile related to behavioral changes, but can instead count on a gradual decrease in the rate of BMI percentile increase until it stabilizes, followed by a gradual decrease. Referral to multidisciplinary, comprehensive pediatric weight management programs is ideal for obese children whenever possible.
Pharmacotherapy for weight loss in the pediatric population is understudied. Randomized controlled trials (RCTs) have evaluated many medications, including metformin, orlistat, sibutramine, and exanatide (Table 60.7 ). Available medications result in modest weight loss or BMI improvement, even when combined with behavioral interventions. Various classes of drugs are of interest, including those that decrease energy intake or act centrally as anorexiants , those that affect the availability of nutrients through intestinal or renal tubular reabsorption, and those that affect metabolism. The only U.S. Food and Drug Administration (FDA)–approved medication for obesity in children <16 yr old is orlistat, which decreases absorption of fat, resulting in modest weight loss. Complications include flatulence, oily stools, and spotting. This agent offers little benefit to severely obese adolescents. Because multiple redundant neural mechanisms act to protect body weight, promoting weight loss is extremely difficult. Thus there is considerable interest in combining therapies that simultaneously target multiple weight-regulating pathways. One example, approved for adults, combines phentermine, a noradrenergic agent, with topiramate, a γ-aminobutyric acid (GABA)–ergic medication. This combination resulted in a mean 10.2-kg weight loss vs 1.4 kg in the placebo group. Side effects are common and include dry mouth, constipation, paresthesias, insomnia, and cognitive dysfunction. Another promising example is the combination of amylin (decreases food intake and slows gastric emptying) with leptin, which has no anorexigenic effects when given alone. This combination requires injection and is in clinical trials in adults. Another FDA-approved drug for adults is lorcaserin, a selective serotonin 2C receptor agonist. Establishing long-term safety and tolerability in children is a challenge because medications of interest have CNS effects or interfere with absorption of nutrients. Teratologic effects must be considered for use in adolescent girls.
Table 60.7
Medications for Weight Management With Mechanism of Action, Availability, and Dosing
| MEDICATION | MECHANISM OF ACTION | AVAILABLE FOR CHRONIC USE | MEAN PERCENTAGE WEIGHT LOSS | ADVANTAGES | DISADVANTAGES | ||
|---|---|---|---|---|---|---|---|
| USA | European Union | Placebo | Drug | ||||
| Phentermine, 15-30 mg PO | Sympathomimetic | For short-term use | No | Not stated in label | Not stated in label | Inexpensive | Side effect profile; no long-term data* |
| Orlistat, 120 mg PO tid before meals | Pancreatic lipase inhibitor | Yes | Yes | −2.6% † | −6.1% † | Not absorbed; long-term data* | Modest weight loss; side effect profile |
| Lorcaserin, 10 mg PO bid | 5-HT2c serotonin agonist with little affinity for other serotonergic receptors | Yes | No | −2.5% | −5.8% | Mild side effects; long-term data* | Expensive; modest weight loss |
| Phentermine/topiramate ER, 7.5 mg/46 mg or 15 mg/92 mg PO indicated as rescue (requires titration) | Sympathomimetic anticonvulsant (GABA receptor modulation, carbonic anhydrase inhibition, glutamate antagonism) | Yes | No | −1.2% |
−7.8% (mid-dose) −9.8% (full dose) |
Robust weight loss; long-term data* | Expensive; teratogen |
| Naltrexone SR/bupropion SR, 32 mg/360 mg PO (requires titration) | Opioid receptor antagonist; dopamine and noradrenaline reuptake inhibitor | Yes | Yes | −1.3% | −5.4% | Reduces food craving; long-term data* | Moderately expensive; side effect profile |
| Liraglutide, 3.0 mg injection (requires titration) | GLP-1 receptor agonist | Yes | Yes | −3% | −7.4% (full dose) | Side effect profile; long-term data* | Expensive; injectable |
* Data from randomized controlled trials lasting >52 wk.
† Assuming the average patient in the orlistat and placebo groups weighed 100 kg at baseline.
Information is from U.S. product labels, except where noted. The data supporting these tables are derived from the prescribing information labeling approved by the US Food and Drug Administration.
ER, Extended release; SR, sustained release; PO, orally; bid, twice daily; tid, 3 times daily.
Adapted from Bray GA, Frühbeck G, Ryan DH, Wilding JPH: Management of obesity, Lancet 387:1947–1965, 2016, p 1950.
Hormone replacement therapy is available for patients with leptin deficiency and may become available for patients with POMC deficiency. Setmelanotide binds to and activates MC4R and may be useful for patients with POMC deficiency–associated obesity.
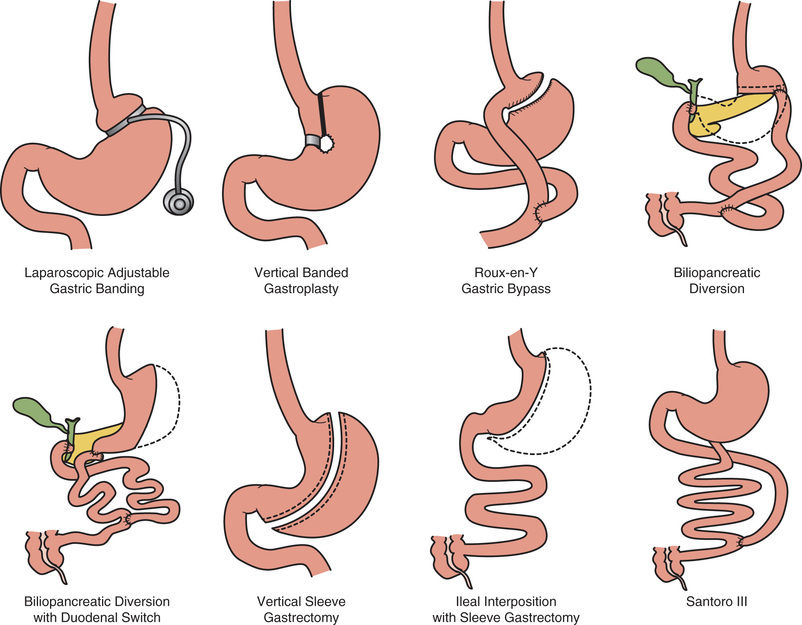
In some cases it is reasonable to refer adolescents for bariatric surgery evaluation. The American Pediatric Surgical Association guidelines recommends that surgery be considered only in children with complete or near-complete skeletal maturity, a BMI ≥40, and a medical complication resulting from obesity, after they have failed 6 mo of a multidisciplinary weight management program. Surgical approaches include the Roux-en-Y and the adjustable gastric band (Fig. 60.6 ). In obese adults, bariatric surgery reduces the risk of developing type 2 diabetes mellitus. In obese adult patients with existing type 2 diabetes, bariatric surgery improves diabetic control. Nutritional complications of bariatric surgery include malabsorption and vitamin (A, B1 , B2 , B6 , B12 , D, E, K) and mineral (copper, iron) deficiencies that require supplementation.
Prevention
Prevention of child and adolescent obesity is essential for public health in the United States and most other countries (Tables 60.8 and 60.9 ). Efforts by pediatric providers can supplement national and community public health programs. The National Institutes of Health (NIH) and U.S. Centers for Disease Control and Prevention (CDC) recommend a variety of initiatives to combat the current obesogenic environment, including promotion of breastfeeding, access to fruits and vegetables, walkable communities, and 60 min/day of activity for children. The U.S. Department of Agriculture (USDA) sponsors programs promoting 5.5 cups of fruits and vegetables per day. Incentives for the food industry to promote consumption of healthier foods should be considered. Marketing of unhealthy foods to children is now being regulated. Changes in federal food programs are expected, including commodity foods, the Women, Infant, and Children Supplemental Food Program (WIC), and school lunch programs, to meet the needs of today's children.
Pediatric prevention efforts begin with careful monitoring of weight and BMI percentiles at healthcare maintenance visits. Attention to changes in BMI percentiles can alert the pediatric provider to increasing adiposity before the child becomes overweight or obese. All families should be counseled about healthy nutrition for their children, because the current prevalence of overweight and obesity in adults is 65%. Therefore, approximately two thirds of all children can be considered at risk for becoming overweight or obese at some time in their lives. Those who have an obese parent are at increased risk. Prevention efforts begin with promotion of exclusive breastfeeding for 6 mo and total breastfeeding for 12 mo. Introduction of infant foods at 6 mo should focus on cereals, fruits, and vegetables. Lean meats, poultry, and fish may be introduced later in the 1st year of life. Parents should be specifically counseled to avoid introducing highly sugared beverages and foods in the 1st year of life. Instead, they should expose their infants and young children to a rich variety of fruits, vegetables, grains, lean meats, poultry, and fish to facilitate acceptance of a diverse and healthy diet. Parenting matters, and authoritative parents are more likely to have children with a healthy weight than those who are authoritarian or permissive. Families who eat regularly scheduled meals together are less likely to have overweight or obese children. Child health professionals can address a child's nutritional status and provide expertise in child growth and development.
Child health professionals can also promote PA during regular healthcare maintenance visits. Parents who spend some of their leisure time in PA promote healthy weight in their children. Beginning in infancy, parents should be cognizant of their child's developmental capability and need for PA. Because TV, computer, and video game time can replace health-promoting PA, physicians should counsel parents to limit screen time for their children. Snacking during TV watching should be discouraged. Parents can help their children to understand that television commercials intend to sell a product. Children can learn that their parents will help them by responsibly choosing healthy foods.
Because obesity is determined by complex multifactorial conditions, prevention will take efforts at multiple levels of social organization. Successful programs include EPODE (Ensemble Prévenons l'Obésité Des Enfants), a multilevel prevention strategy that began in France and has been adopted by more than 500 communities in 6 countries. Shape Up Somerville is a citywide campaign to increase daily PA and healthy eating in Somerville, MA, since 2002. The “Let's Move” campaign was championed by former First Lady Michelle Obama. Since community and environmental factors are related to pediatric obesity risk, changes in local environments, daycare centers, schools, and recreational settings can have a public health impact. Programs can empower families to adopt practices that promote healthy lifestyles for children and adolescents. The most successful programs are comprehensive and rely on 4 strategies: political commitment to change, resources to support social marketing and changes, support services, and evidence-based practices. Community-wide programs are important because neighborhood environmental factors (e.g., poverty) have been associated with obesity in its residents. There is considerable interest in focusing these efforts early in the life cycle. Beginning obesity prevention during pregnancy and engaging health systems, early childhood programs, and community systems to support healthier life cycles is an approach with great promise.
Rapid-Onset Obesity With Hypothalamic Dysfunction, Hypoventilation, and Autonomic Dysregulation (ROHHAD)
Sarah F. Barclay, Amy Zhou, Casey M. Rand, Debra E. Weese-Mayer
ROHHAD—rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation—is a rare, poorly understood disease of childhood onset, the first sign of which is sudden, rapid, and extreme weight gain in a previously healthy child. The acronym describes the presenting symptoms and the typical order in which they will manifest or unfold, as the condition evolves over months to years. Despite its rarity, ROHHAD must be considered whenever rapid-onset obesity is observed in a child, because in the absence of appropriate treatment, a high mortality rate is associated with the severe central hypoventilation that will invariably develop.
The diagnosis is initially considered after the observation of rapid-onset obesity (15-20 lb gain) after age 1.5 yr, accompanied by at least 1 additional sign of hypothalamic dysfunction. Central hypoventilation may not be present at diagnosis but will develop over time, and artificial ventilatory support will be required at least during sleep, if not 24 hr/day. Signs of autonomic nervous system dysregulation typically occur after the weight gain, hypothalamic dysfunction, and hypoventilation have been identified. Additionally, approximately 40% of ROHHAD patients will have a tumor of neural crest origin, typically ganglioneuroma or ganglioneuroblastoma.
ROHHAD is distinct from late-onset congenital central hypoventilation syndrome (LO-CCHS ; see later and Chapter 446.2 ). ROHHAD is primarily distinguished from LO-CCHS by the presence of obesity and other signs of hypothalamic dysfunction and by the absence of CCHS-related PHOX2B mutation. Approximately 100 cases of ROHHAD have been described in the literature to date.
Clinical Manifestations
Children with ROHHAD initially appear healthy, with an unremarkable history. The initial symptoms present between ages 18 mo and 7 yr. Typically, the 1st symptom observed is rapid-onset obesity , with weight gain of 15-20 lb in 6-12 mo. This is a sign of hypothalamic dysfunction (HD) in these patients. The 2nd common sign of HD, seen in most ROHHAD patients, is disordered water balance , including hyper- and hyponatremia and both adipsia and polydipsia. Growth hormone (GH) deficiency is also observed in most patients. In some this manifests clinically as slowed growth rate and short stature, whereas in others a failed GH stimulation test is the only evidence. Other symptoms of HD, occurring in >25–50% of ROHHAD patients, include hyperprolactinemia, poor thermoregulation, central hypothyroidism, adrenal insufficiency, and delayed or precocious puberty. The number of hypothalamic abnormalities that will be observed and the sequential order in which they will appear are variable, and some symptoms may not manifest for months to 1-2 yr after the initial diagnosis. However, all ROHHAD patients will present with at least 1 of these signs of HD.
Sleep-disordered breathing (SDB) is one of the key symptoms of ROHHAD, often manifesting as one of the most severe features of the phenotype, with the greatest potential for fatal complications. More than half of ROHHAD patients have initial obstructive sleep apnea (OSA) ; although SDB is known to be associated with obesity, and OSA is often seen in obese individuals, the extent that SDB is tied to obesity in ROHHAD patients is not yet well defined. However, over time, as the ROHHAD phenotype unfolds, SDB will evolve beyond what could potentially be explained as obesity related. All ROHHAD patients will eventually develop central alveolar hypoventilation , requiring artificial ventilatory support, even when the upper airway obstruction is relieved as an intervention for OSA. About half of ROHHAD patients will require artificial ventilation only during sleep, while half will require continuous artificial ventilation (during sleep and wakefulness). More than 40% of children with ROHHAD will have a cardiorespiratory arrest before their hypoventilation is identified and treated. Unfortunately, many ROHHAD patients die from cardiorespiratory arrest because of unrecognized or inadequately managed hypoventilation. Thus, if a ROHHAD diagnosis is suspected, it is crucial that a comprehensive respiratory physiology evaluation is performed, including overnight polysomnography and awake physiologic recording in activities of daily living (ADLs).
All ROHHAD patients have symptoms of autonomic nervous system (ANS) dysregulation , but as described for signs of HD, the exact symptoms and the order and timing of their appearance will vary between patients. The most common manifestations of ANS dysregulation in ROHHAD are ophthalmologic, including pupillary dysfunction, strabismus, and alacrima. Many ROHHAD patients will have gastrointestinal dysmotility, presenting as either chronic constipation or chronic diarrhea. Other signs of ANS dysregulation include altered sweating, decreased body temperature, decreased sensitivity to pain, and cold hands and feet indicating altered vasomotor tone. Bradycardia is seen in some ROHHAD patients, typically related to extreme hypothermia.
Neural crest tumors are observed in at least 40% of ROHHAD patients, most frequently ganglioneuromas and ganglioneuroblastomas of the chest or abdomen; rarely a neuroblastoma has been reported. These tumors can occur at any age, so proactive imaging evaluation to identify the tumors is essential.
Most patients do not have behavioral or psychologic disorders . For those who do, however, the disorders can be quite severe, including anxiety, depression, rage, lethargy, irritability, aggressiveness, psychosis, and obsessive-compulsive disorder. Developmental disorders described include neurocognitive delay, developmental regression, attention-deficit/hyperactivity disorder, and pervasive developmental disorder. These disorders are most likely caused by poorly managed hypoventilation because the majority of ROHHAD patients have no behavioral issues and a normal IQ.
Seizures have been reported in some ROHHAD patients, likely caused by episodes of hypoxemia, when hypoventilation either has not yet been diagnosed or has been inadequately managed.
Diagnosis
The diagnostic criteria for ROHHAD include r apid-onset obesity after 1.5 years of age, central hypoventilation beginning after age 1.5 yr, and ≥1 of the following signs of HD: disordered water balance, hyperprolactinemia, failed GH stimulation test, central hypothyroidism, corticotropin deficiency, and altered onset of puberty. Additionally, it should be confirmed that no CCHS-related PHOX2B gene mutation is present, to rule out a diagnosis of CCHS or LO-CCHS.
Since no single diagnostic test is currently available for ROHHAD, diagnosis must be based on observation of the clinical presentation and therefore requires expert consultation in several specialties, including respiratory physiology, endocrinology, autonomic medicine, cardiology, oncology, nutrition, critical care, and psychiatry. When a child with rapid-onset obesity is seen by a general pediatrician or family physician, the trajectory of weight gain should signal prompt consideration of a ROHHAD diagnosis, with immediate referral to a center with expertise in this unique constellation of symptoms. Early recognition is critical for a positive outcome in children with ROHHAD. If alveolar hypoventilation is not identified and aggressively managed, cardiorespiratory arrest can occur and has proved fatal in many cases.
Initial evaluations should include overnight polysomnography to identify OSA or central hypoventilation, awake comprehensive physiologic recording in activities of daily living, cardiac evaluation to evaluate for cor pulmonale, endocrine function evaluation, screening for neural crest tumors (chest radiograph, abdominopelvic ultrasound), and a psychiatric evaluation, especially if any behavioral, psychologic, or developmental disorders are seen or suspected. Brain imaging should be performed to rule out intracranial lesions that may account for the observed hypothalamic-pituitary abnormalities. If the criteria are met, and a ROHHAD diagnosis is made, successful management requires ongoing cooperation among the various specialists, with a team leader to orchestrate all testing, to provide integrated care for the child.
Management
There is currently no cure for ROHHAD. Rather, treatment consists of early identification, meticulous monitoring, and symptomatic management of the various symptoms as they develop. Comprehensive initial evaluations should determine the nature and severity of hypoventilation, HD, and ANS dysregulation, and appropriate interventions should be implemented. Obesity is very difficult to control, but in consultation with a nutritionist and endocrinologist, the trajectory of advancing weight gain can be diminished with moderate exercise and calorie restriction, leading to improved body mass index (BMI) with advancing age. Specific signs of HD and ANS dysregulation should be evaluated by a pediatric endocrinologist and expert in pediatric autonomic medicine, respectively, and treated as necessary. Such treatments or management strategies may include hormone replacement; regimented fluid intake; ophthalmologic assessment and treatment; longitudinal monitoring of peripheral, core, and ambient temperature; and management of constipation with stool softeners. Disordered water balance to prevent dehydration should be addressed, as well as regulation of heart rate, since bradycardia is seen in some patients (usually with decreased core temperature).
Neural crest tumors should be assessed and resected by a pediatric surgeon together with a pediatric oncologist, because the sheer size of these benign tumors creates serious compromise to surrounding tissues. If no tumor is identified initially, screening should continue every 6 mo until age 7 yr and thereafter annually.
Most critical is the management of hypoventilation. Initial intervention for OSA will likely involve surgical relief of the upper airway obstruction. This will usually unveil central hypoventilation, and initiation of supported ventilation will be required. If no central hypoventilation is identified, the patient should continue to be vigilantly monitored by a respiratory physiologist because all ROHHAD patients will eventually develop central hypoventilation requiring artificial ventilation. Optimal oxygenation and ventilation can then be maintained using a mechanical ventilator with mask or tracheostomy. This should be accompanied by highly trained home nursing and continuous monitoring with oximetry and capnography during sleep, with spot checks during wakefulness. The goal should be to maintain hemoglobin saturation values of ≥95% and end-tidal CO2 values of 35-45 mm Hg, with vigilant evaluation for awake hypoventilation necessitating artificial ventilation up to 24 hr/day as necessary.
Given that the ROHHAD phenotype evolves with advancing age, ongoing care requires regularly scheduled evaluation of all the systems involved to identify and treat further symptoms as they appear. Comprehensive evaluation should ideally occur at a Center of Excellence for ROHHAD and should include respiratory physiology assessment both asleep and awake (in ADLs including varied levels of exertion, concentrational tasks, quiet play, and eating), screening of chest and abdomen for neural crest tumors in the adrenals or along the sympathetic chain, evaluation of the hypothalamic-pituitary axis with hormonal replacement as necessary, age-appropriate noninvasive evaluation of ANS dysregulation, comprehensive cardiac evaluation for evidence of recurrent hypoxemia, and neurocognitive testing. These evaluations should initially occur at 3-6 mo intervals, but this schedule may be altered with advancing age, depending on each patient's clinical condition.
Without proper management, oxygen deprivation can lead to irreversible deterioration in patients. However, with prompt diagnosis and aggressive management, including careful attention to the child's airway, breathing, and circulation, complications can be minimized and the prognosis can be quite favorable, although long-term outcome remains unknown but the focus of an international registry (https://clinicaltrials.gov/show/NCT03135730 ).
Etiology: Studies and Hypotheses
Despite advances made in the characterization of the ROHHAD phenotype and early identification, the cause of the disease is unknown. The interrelationships among the observed symptoms, as well as the mechanisms that underlie them, remain to be elucidated.
Genetic Studies
Because the related but seemingly distinct disorder CCHS has a genetic basis (PHOX2B mutation), ROHHAD may be genetic as well. ROHHAD patients do not have CCHS-related PHOX2B mutations, however, and no numerical or structural chromosomal rearrangements have been described. The sporadic occurrence of ROHHAD, without familial recurrence, is consistent with de novo mutations. Exome-sequencing analysis of 7 ROHHAD family trios did not identify any causative de novo protein-altering mutations, or any candidates under autosomal recessive or autosomal dominant inheritance models, even when a replication cohort of 28 additional ROHHAD probands was included.
Another genetic mutation model that can account for sporadic occurrence of a phenotype is a somatic mutation model, in which mutations occur postzygotically and are thus only present in a subset of an individual's cells. Consistent with a somatic mutation hypothesis, 2 pairs of monozygotic twins discordant for the ROHHAD phenotype have been reported. The exomes (from blood samples) of 1 twin pair were compared, but no discordant coding mutations were identified. The challenge is that the “correct” tissue needs to be sampled for the somatic mutation to be identified. In other phenotypes caused by somatic mutations, such as Proteus syndrome, the mutation causes overgrowth, so the affected (mutated) tissue is visible and can be sampled and sequenced. In ROHHAD, presumably the affected (mutated) cells are in the hypothalamus and/or ANS, which cannot be sampled and sequenced from living individuals. The neural crest tumors in many ROHHAD patients might represent an additional affected tissue. The exomes of neural crest tumors from 4 ROHHAD patients were compared to the exomes of blood samples from the same patients, but no tumor-specific mutations were identified.
The observation of monozygotic twins discordant for the ROHHAD phenotype could be consistent with a somatic genetic mutation, but could also suggest an alternative, nongenetic etiology. For example, epigenetic variation can account for some discordance between monozygotic twins and also can play a role in diseases involving respiratory and autonomic function, such as Prader-Willi syndrome (see later) and Rett syndrome.
Paraneoplastic/Autoimmune Hypothesis
Paraneoplastic syndromes are rare disorders caused by a neoplasm triggering an altered immune response that aberrantly attacks and destroys neurons, leading to the nervous system symptoms. An autoimmune or paraneoplastic basis for ROHHAD has been suggested based on neural crest tumors occurring in 40% of ROHHAD patients and 2 early cases with autopsies revealing low-density lesions in the basal ganglia and neuronal loss from lymphocytic infiltration of the hypothalamus, thalamus, midbrain, and pons. Autopsy of another ROHHAD patient revealed similar findings of hypothalamic inflammation with lymphocytic infiltrates and gliosis, although other autopsies have found no such pathology. Some cerebrospinal fluid (CSF) analyses have revealed pleocytosis, elevated neopterins, and oligoclonal bands consistent with intrathecal synthesis of oligoclonal immunoglobulin G. However, other studies report a lack of oligoclonal IgG and antineuronal antibodies, as well as clear CSF microscopies and cultures. Thus the evidence so far is conflicting, with some reports supporting the autoimmune hypothesis, while others do not. Further, the onset of ROHHAD symptoms often precedes the diagnosis of a neural crest tumor in ROHHAD patients, and in many cases, neural crest tumors have not been discovered with MRI or even an autopsy, although these tumors are often difficult to detect. However, this is also seen in other paraneoplastic syndromes, such as opsoclonus-myoclonus, where only some cases are associated with a neoplasm, the remainder being idiopathic.
After a patient with idiopathic HD was treated with immune globulin therapy, several other studies pursued similar treatments with immunoglobulins and corticosteroids in ROHHAD patients. After high-dose cyclophosphamide treatment, some ROHHAD patients reported symptomatic and neurophysiologic improvements while others had poor clinical results. Notably, reports indicate that immunotherapy has not consistently halted unfolding and advancing of the unique constellation of symptoms described in ROHHAD. Even with complete tumor resection and immunoablation, only partial recovery has been reported. The lack of return to baseline has been attributed to late treatment, where early rapid progressive disease left residual damage. This would be consistent with an immune-mediated hypothesis, in which an autoimmune process is initiated by a neural crest tumor but maintained in its absence, resulting in irreversible injury that prevents complete symptom resolution.
Neurocristopathy
Neurocristopathies are disorders caused by abnormal development of any of the tissues or systems that develop from the embryonic neural crest cell lineage. Given that the systems involved in the ROHHAD phenotype (hypothalamus, ANS, endocrine system) share a neural crest origin, ROHHAD fits into this class. One could then hypothesize that the observed symptoms are caused by abnormal development of neural crest cells at an early embryonic stage. This is indeed the case for the related disorder, CCHS, caused by mutations in the gene PHOX2B, which is important for the development of the ANS from neural crest cells. Under this hypothesis, the neural crest tumors seen in ROHHAD patients, rather than being the trigger for the rest of the phenotype (as proposed by the paraneoplastic theory), would be a result of the same abnormal development that caused the rest of the phenotype.
Differential Diagnosis
As noted earlier, congenital central hypoventilation syndrome is a rare pediatric disorder of the ANS and respiratory control. CCHS is caused by mutations in the PHOX2B gene, which plays an important role in the differentiation and development of the ANS from neural crest progenitor cells. The hallmark feature of CCHS is life-threatening hypoventilation while sleeping (and in some cases, also while awake). As with ROHHAD patients, CCHS patients require artificial ventilatory support, typically by tracheostomy and mechanical ventilator. Unlike ROHHAD, however, CCHS usually presents in the newborn period, although late-onset CCHS has been diagnosed in later childhood, adolescence, and even adulthood. CCHS also presents with other symptoms of ANS dysregulation, including altered heart rate regulation and altered vasomotor tone, altered temperature regulation, ophthalmologic manifestations, and reduced gastrointestinal motility. However, CCHS patients are not obese and do not typically have HD. When hypoventilation is observed, a simple blood test can confirm a CCHS diagnosis by looking for PHOX2B mutations. If PHOX2B mutations are not identified and the other features of the ROHHAD phenotype are identified, a ROHHAD diagnosis must be considered.
Prader-Willi syndrome (PWS) is similar to ROHHAD in that childhood obesity is one of the most prominent features; however, many important differences set these two conditions apart. PWS is caused by chromosomal abnormalities at chromosome 15q11-q13, specifically by a lack of the paternal contribution at this region (from genomic deletion, uniparental disomy, or imprinting error). Infants with PWS present with neonatal hypotonia and failure to thrive (malnutrition). Later, children with PWS develop extreme hyperphagia and obesity. Other major symptoms include mild intellectual impairment, maladaptive behaviors, short stature caused by GH insufficiency, hypogonadism, and SDB. In addition, many PWS patients show signs of ANS dysregulation, including altered temperature perception and regulation, strabismus, and high pain threshold. Although there are several apparently overlapping symptoms (pediatric obesity, SDB, ANS dysregulation), ROHHAD patients do not have the characteristic PWS genomic abnormality, hypogonadism, or consistent neurocognitive impairment. ROHHAD patients also are healthy in the neonatal period, showing none of the early PWS symptoms.
Bibliography
Barclay SF, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): exome sequencing of trios, monozygotic twins and tumours. Orphanet J Rare Dis . 2015;10:103.
Barclay SF, et al. Absence of mutations in HCRT , HCRTR1 and HCRTR2 in patients with ROHHAD. Respir Physiol Neurobiol . 2016;221:59–63.
Barclay SF, Rand CM, Nguyen L, et al. ROHHAD and Prader-Willi syndrome (PWS): clinical and genetic comparison. Orphanet J Rare Dis . 2018;13:124.
Bougneres P, et al. Endocrine manifestations of the rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neural tumor syndrome in childhood. J Clin Endocrinol Metab . 2008;93(10):3971–3980.
Carroll MS, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): response to ventilatory challenges. Pediatr Pulmonol . 2015;50:1336–1345.
De Pontual L, et al. Delineation of late onset hypoventilation associated with hypothalamic dysfunction syndrome. Pediatr Res . 2008;64(6):689–694.
Grudnikoff E, et al. Nocturnal anxiety in a youth with rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD). J Can Acad Child Adolesc Psychiatry . 2013;22:235–237.
Ize-Ludlow D, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics . 2007;120(1):e179–e188.
Katz ES, et al. Late-onset central hypoventilation with hypothalamic dysfunction: a distinct clinical syndrome. Pediatr Pulmonol . 2000;29(1):62–68.
Kocaay P, Siklar Z, Camtosun E, et al. ROHHAD syndrome: reasons for diagnostic difficulties in obesity. J Clin Res Pediatr Endocrinol . 2014;6(4):254–257.
Paz-Priel I, et al. Cyclophosphamide for rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome. J Pediatr . 2011;158(2):337–339.
Rand CM, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Pediatr Res . 2011;70(4):375–378.
Reppucci D, et al. ROHHAD syndrome and evolution of sleep disordered breathing. Orphanet J Rare Dis . 2016;11:106.
Sartori S, et al. Intrathecal synthesis of oligoclonal bands in rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome: new evidence supporting immunological pathogenesis. J Child Neurol . 2014;29:421–425.
Sethi K, et al. ROHHADNET syndrome presenting as major behavioral changes in a 5-year-old obese girl. Pediatrics . 2014;134:e586–e589.
Thaker VV, et al. Whole exome sequencing identifies RA/1 mutation in a morbidly obese child diagnosed with ROHHAD syndrome. J Clin Endocrinol Metab . 2015;100(5):1723–1730.