Listeria monocytogenes
Thomas S. Murray, Robert S. Baltimore
Listeriosis in humans is caused principally by Listeria monocytogenes, 1 of 6 species of the genus Listeria that are widely distributed in the environment and throughout the food chain. Human infections can usually be traced to an animal reservoir. Infection usually occurs at the extremes of age. In the pediatric population, perinatal infections predominate and usually occur secondary to maternal infection or colonization. Outside the newborn period, disease is most often encountered in immunosuppressed (usually T-cell deficiencies) children and adults and in elderly persons. For most people the major risk for infection with Listeria is food-borne transmission . In the United States, food-borne outbreaks are caused by improperly processed dairy products and contaminated vegetables and principally affect the same individuals at risk for sporadic disease.
Etiology
Members of the genus Listeria are facultatively anaerobic, non–spore-forming, motile, gram-positive bacilli that are catalase positive. In the laboratory, Listeria can be distinguished from other gram-positive bacilli by their characteristic tumbling motility and growth at cold temperature (4-10°C [39.2-50°F]). The 6 Listeria spp. are divided into 2 genomically distinct groups on the basis of DNA-DNA hybridization studies. One group contains the species Listeria grayi, considered nonpathogenic. The 2nd group contains 5 species: the nonhemolytic species Listeria innocua and L. welshimeri and the hemolytic species Listeria monocytogenes, L. seeligeri, and L. ivanovii. Listeria ivanovii is pathogenic primarily in animals, and the vast majority of both human and animal disease is caused by L. monocytogenes.
Subtyping of L. monocytogenes isolates for epidemiologic purposes has been attempted with the use of heat-stable somatic O and heat-labile flagellar H antigens, phage typing, pulsed-field gel electrophoresis, ribotyping, and multilocus enzyme electrophoresis. Electrophoretic typing demonstrates the clonal structure of populations of L. monocytogenes as well as the sharing of populations between human and animal sources. Subtyping is an important component of determining whether cases are connected or sporadic but usually requires collaboration with a specialized laboratory.
Selected biochemical tests, together with the demonstration of tumbling motility , umbrella-type formation below the surface in semisolid medium, hemolysis, and a typical cyclic adenosine monophosphate test, are usually sufficient to establish a presumptive identification of L. monocytogenes.
Epidemiology
Listeria monocytogenes is widespread in nature, has been isolated throughout the environment, and is associated with epizootic disease and asymptomatic carriage in >42 species of wild and domestic animals and 22 avian species. Epizootic disease in large animals (e.g., sheep, cattle) is associated with abortion and “circling disease,” a form of basilar meningitis. L. monocytogenes is isolated from sewage, silage, and soil, where it survives for >295 days. Human-to-human transmission rarely occurs except in maternal-fetal transmission. The annual incidence of listeriosis decreased by 36% between 1996 and 2004 and has remained level since then. However, food-borne outbreaks continue to occur. In 2011, 84 cases and 15 deaths in 19 states were traced to cantaloupes from a single source. The cases were connected by use of pulsed-field gel electrophoresis, which showed that 4 different strains traced to the same source. The rate of Listeria infections varies among states. Epidemic human listeriosis has been associated with food-borne transmission in several large outbreaks, especially in association with aged soft cheeses; improperly pasteurized milk and milk products; contaminated raw and ready-to-eat beef, pork, and poultry, and packaged meats and salads; and vegetables both fresh and frozen harvested from farms where the ground is contaminated with the feces of colonized animals. Food-borne outbreaks in 2016 included raw milk, packaged salads, and frozen vegetables. The ability of L. monocytogene s to grow at temperatures as low as 4°C (39.2°F) increases the risk for transmission from aged soft cheeses and stored contaminated food. Listeriosis is an uncommon but important recognized etiology of neonatal sepsis and meningitis. Small clusters of nosocomial person-to-person transmission have occurred in hospital nurseries and obstetric suites. Sporadic endemic listeriosis is less well characterized. Likely routes include food-borne infection and zoonotic spread. Zoonotic transmission with cutaneous infections occurs in veterinarians and farmers who handle sick animals.
Reported cases of listeriosis are clustered at the extremes of age. Some studies show higher rates in males and a seasonal predominance in the late summer and fall in the Northern hemisphere. Outside the newborn period and during pregnancy, disease is usually reported in patients with underlying immunosuppression, with a 100-300 times increased risk in HIV-infected persons and in the elderly population (Table 215.1 ). In a recent surveillance study from England, malignancies accounted for one third of cases, with special risk associated with cancer in elderly persons.
Table 215.1
Types of Listeria monocytogenes Infections
The incubation period, which is defined only for common-source food-borne disease, is 21-30 days but in some cases may be longer. Asymptomatic carriage and fecal excretion are reported in 1–5% of healthy persons and 5% of abattoir workers, but duration of excretion, when studied, is short (<1 mo.).
Pathology
One of the major concepts of Listeria pathology and pathogenesis is its ability to survive as an intracellular pathogen. Listeria incites a mononuclear response and elaboration of cytokines, producing multisystem disease, particularly pyogenic meningitis. Granulomatous reactions and microabscess formation develop in many organs, including liver, lungs, adrenals, kidneys, central nervous system (CNS), and notably the placenta. Animal models demonstrate translocation , the transfer of intraluminal organisms across intact intestinal mucosa. Histologic examination of tissues, including the placenta, shows granulomatous inflammation and microabscess formation. Intracellular organisms can often be demonstrated with special stains.
Pathogenesis
Listeria organisms usually enter the host through the gastrointestinal (GI) tract. Gastric acidity provides some protection, and drugs that raise gastric pH may promote infection. Studies of intracellular and intercellular spread of L. monocytogenes have revealed a complex pathogenesis. Four pathogenic steps are described: internalization by phagocytosis, escape from the phagocytic vacuole, nucleation of actin filaments, and cell-to-cell spread. Listeriolysin , a hemolysin and the best-characterized virulence factor, probably mediates lysis of vacuoles and is responsible for the zone of hemolysis around colonies on blood-containing solid media. In cell-to-cell spread, locomotion proceeds via cytochalasin-sensitive polymerization of actin filaments, which extrude the bacteria in pseudopods, which in turn are phagocytosed by adjacent cells, necessitating escape from a double-membrane vacuole. This mechanism protects intracellular bacteria from the humoral arm of immunity and is responsible for the well-known requirement of T-cell–mediated activation of monocytes by lymphokines for clearance of infection and establishment of immunity. It appears that secretion of cyclic di-adenosine monophosphate by the bacteria induces the host to produce interferon, which activates the immune system to fight the organism. The significant risk for listeriosis in patients with depressed T-cell immunity speaks for the role of this arm of the immune system. The role of opsonizing antibody in protecting against infection is unclear. In addition, siderophores scavenge iron from the host, enhancing growth of the organism and likely explaining the relatively high risk of listeriosis in iron overload syndromes.
Clinical Manifestations
The clinical presentation of listeriosis depends greatly on the age of the patient and the circumstances of the infection.
Listeriosis in Pregnancy
Pregnant women have increased susceptibility to Listeria infectious (approximately 20 times higher than nonpregnant women), probably because of a relative impairment in cell-mediated immunity. L. monocytogenes has been grown from placental and fetal cultures of pregnancies ending in spontaneous abortion. The usual presentation in the 2nd and 3rd trimesters is a flulike illness that may result in seeding of the uterine contents by bacteremia. Rarely is maternal listeriosis severe, but meningitis in pregnancy has been reported. Recognition and treatment at this stage are associated with normal pregnancy outcomes, but the fetus may not be infected even if listeriosis in the mother is not treated. In other instances, placental listeriosis develops with infection of the fetus that may be associated with stillbirth or premature delivery. Delivery of an infected premature fetus is associated with very high infant mortality. Disseminated disease is apparent at birth, often with a diffuse pustular rash. Infection in the mother usually resolves without specific therapy after delivery, but postpartum fever and infected lochia may occur.
Neonatal Listeriosis
Two clinical presentations are recognized for neonatal listeriosis: early-onset neonatal disease (<5 days, usually within 1-2 days of birth), which is a predominantly septicemic form, and late onset neonatal disease (>5 days, mean 14 days of life), which is a predominantly meningitic form (Table 215.2 ). The principal characteristics of the 2 presentations resemble the clinical syndromes described for group B streptococcus (see Chapter 211 ).
Table 215.2
Characteristic Features of Early- and Late-Onset Neonatal Listeriosis
| EARLY ONSET (<5 DAYS) | LATE ONSET (≥5 DAYS) |
|---|---|
| Positive result of maternal Listeria culture | Negative results of maternal Listeria culture |
| Obstetric complications | Uncomplicated pregnancy |
| Premature delivery | Term delivery |
| Low birthweight | Normal birthweight |
| Neonatal sepsis | Neonatal meningitis |
| Mean age at onset 1.5 days | Mean age at onset 14.2 days |
| Mortality rate >30% | Mortality rate <10% |
| Nosocomial outbreaks |
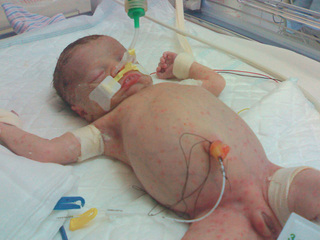
Early-onset disease occurs with milder transplacental or ascending infections from the female genital tract. There is a strong association with recovery of L. monocytogenes from the maternal genital tract, obstetric complications, prematurity, and neonatal sepsis with multiorgan involvement, including rash, but without CNS localization (Fig. 215.1 ). The mortality rate is approximately 20–30%.
The epidemiology of late-onset disease is poorly understood. Onset is usually after 5 days but before 30 days of age. Affected infants frequently are full-term, and the mothers are culture negative and asymptomatic. The presenting syndrome is usually purulent meningitis with parenchymal brain involvement, which, if adequately treated, has a mortality rate of <20%.
Postneonatal Infections
Listeriosis beyond the newborn period may rarely occur in otherwise healthy children but is most often encountered in association with underlying malignancies (especially lymphomas) or immunosuppression. When associated with food-borne outbreaks, disease may cause GI symptoms or any of the Listeria syndromes. The clinical presentation is usually meningitis, less commonly sepsis, and rarely other CNS involvement, such as cerebritis, meningoencephalitis, brain abscess, spinal cord abscess, or a focus outside the CNS, such as suppurative arthritis, osteomyelitis, endocarditis, peritonitis (associated with peritoneal dialysis), or liver abscess. It is not known whether the frequent GI signs and symptoms result from enteric infection, because the mode of acquisition is often unknown.
Diagnosis
Listeriosis should be included in the differential diagnosis of infections in pregnancy, of neonatal sepsis and meningitis, and of sepsis or meningitis in older children who have underlying malignancies (lymphomas), are receiving immunosuppressive therapy, or have undergone transplantation. The diagnosis is established by culture of L. monocytogenes from blood or cerebrospinal fluid (CSF). Cultures from the maternal cervix, vagina, lochia, and placenta, if possible, should be obtained when intrauterine infections lead to premature delivery or early-onset neonatal sepsis. Cultures from closed-space infections may also be useful. It is helpful to alert the laboratory to suspected cases so that Listeria isolates are not discarded as contaminating diphtheroids.
Histologic examination of the placenta is also useful. Molecular assays are now commercially available to detect L. monocytogenes from CNS samples. Serodiagnostic tests have not proved useful.
Differential Diagnosis
Listeriosis is indistinguishable clinically from neonatal sepsis and meningitis caused by other organisms. The presence of increased peripheral blood monocytes suggests listeriosis. Monocytosis or lymphocytosis may be modest or striking. Beyond the neonatal period, L. monocytogenes CNS infection is associated with fever, headache, seizures, and signs of meningeal irritation. The brainstem may be characteristically affected. The white blood cell concentration may vary from normal to slightly elevated, and the CSF laboratory findings are variable and less striking than in the more common causes of bacterial meningitis. Polymorphonuclear leukocytes or mononuclear cells may predominate, with shifts from polymorphonuclear to mononuclear cells in sequential lumbar puncture specimens. The CSF glucose concentration may be normal, but a low level mirrors the severity of disease. The CSF protein concentration is moderately elevated. L. monocytogenes is isolated from the blood in 40–75% of cases of meningitis caused by the organism. Deep focal infections from L. monocytogenes, such as endocarditis, osteomyelitis, and liver abscess, are also indistinguishable clinically from such infections from more common organisms. Cutaneous infections should be suspected in patients with a history of contact with animals, especially products of conception.
Treatment
The emergence of multiantibiotic resistance mandates routine susceptibility testing of all isolates. The recommended therapy is ampicillin (100-200 mg/kg/day divided every 6 hr intravenously [IV]; 200-400 mg/kg/day divided every 6 hr IV if meningitis is present) alone or in combination with an aminoglycoside (5.0-7.5 mg/kg/day divided every 8 hr IV). The aminoglycoside enhances the bactericidal activity and is generally recommended in cases of endocarditis and meningitis. The adult dose is ampicillin, 4-6 g/day divided every 6 hr, plus an aminoglycoside. The ampicillin dose is doubled if meningitis is present. Special attention to dosing is required for neonates, who require longer dosing intervals because of the longer half-lives of the antibiotics in their bodies. L. monocytogenes is not susceptible to the cephalosporins, including third-generation cephalosporins. If these agents are used for empirical therapy for neonatal sepsis or meningitis in a newborn, ampicillin must be added for possible L. monocytogenes infection. Vancomycin, vancomycin plus an aminoglycoside, trimethoprim-sulfamethoxazole, and erythromycin are alternatives to ampicillin. The duration of therapy is usually 2-3 wk, with 3 wk recommended for immunocompromised persons and patients with meningitis. A longer course is needed for endocarditis, brain abscess, and osteomyelitis. Antibiotic treatment is unnecessary for gastroenteritis without invasive disease.
Prognosis
Early gestational listeriosis may be associated with abortion or stillbirth, although maternal infection with sparing of the fetus has been reported. There is no convincing evidence that L. monocytogenes is associated with repeated spontaneous abortions in humans. The mortality rate is >50% for premature infants infected in utero, 30% for early-onset neonatal sepsis, 15% for late-onset neonatal meningitis, and <10% in older children with prompt institution of appropriate antimicrobial therapy. Mental retardation, hydrocephalus, and other CNS sequelae are reported in survivors of Listeria meningitis.
Prevention
Listeriosis can be prevented by pasteurization and thorough cooking of foods. Irradiation of meat products may also be beneficial. Consumption of unpasteurized or improperly processed dairy products should be avoided, especially aged soft cheeses, uncooked and precooked meat products that have been stored at 4°C (39.2°F) for extended periods, and unwashed vegetables (Table 215.3 ). This avoidance is particularly important during pregnancy and for immunocompromised persons. Infected domestic animals should be avoided when possible. Education regarding risk reduction is aimed particularly at pregnant women and people being treated for cancer.
Table 215.3
Prevention of Food-Borne Listeriosis
| GENERAL RECOMMENDATIONS TO PREVENT LISTERIA INFECTION |
|
FDA recommendations for washing and handling food: • Rinse raw produce, such as fruits and vegetables, thoroughly under running tap water before eating, cutting, or cooking. Even if the produce will be peeled, it should still be washed first. • Scrub firm produce, such as melons and cucumbers, with a clean produce brush. • Dry the produce with a clean cloth or paper towel. • Separate uncooked meats and poultry from vegetables, cooked foods, and ready-to-eat foods. Keep your kitchen and environment cleaner and safer. • Wash hands, knives, countertops, and cutting boards after handling and preparing uncooked foods. • Be aware that Listeria monocytogenes can grow in foods in the refrigerator. Use an appliance thermometer, such as a refrigerator thermometer, to check the temperature inside your refrigerator. The refrigerator should be 4.5°C (40°F) or lower and the freezer −17.8°C (0°F) or lower. • Clean up all spills in your refrigerator promptly, especially juices from hot dog and lunch meat packages, raw meat, and raw poultry. • Clean the inside walls and shelves of your refrigerator with hot water and liquid soap, then rinse. Cook meat and poultry thoroughly. • Thoroughly cook raw food from animal sources, such as beef, pork, or poultry to a safe internal temperature. For a list of recommended temperatures for meat and poultry, visit the safe minimum cooking temperatures chart at http://www.FoodSafety.gov . • Use precooked or ready-to-eat food as soon as you can. Do not store the product in the refrigerator beyond the use-by date; follow USDA refrigerator storage time guidelines: • Hot dogs: store opened package no longer than 1 wk and unopened package no longer than 2 wk in the refrigerator. • Luncheon and deli meat: store factory-sealed, unopened package no longer than 2 wk. Store opened packages and meat sliced at a local deli no longer than 3-5 days in the refrigerator. • Divide leftovers into shallow containers to promote rapid, even cooling. Cover with airtight lids or enclose in plastic wrap or aluminum foil. Use leftovers within 3-4 days. • Do not drink raw (unpasteurized) milk, and do not eat foods that have unpasteurized milk in them. |
| RECOMMENDATIONS FOR PERSONS AT HIGHER RISK * |
| Meats |
|
• Do not eat hot dogs, luncheon meats, cold cuts, other deli meats (e.g., bologna), or fermented or dry sausages unless they are heated to an internal temperature of 73.9°C (165°F) or until steaming hot just before serving. • Avoid getting fluid from hot dog and lunch meat packages on other foods, utensils, and food preparation surfaces, and wash hands after handling hot dogs, luncheon meats, and deli meats. • Pay attention to labels. Do not eat refrigerated pâté or meat spreads from a deli or meat counter or from the refrigerated section of a store. Foods that do not need refrigeration, such as canned or shelf-stable pâté and meat spreads, are safe to eat. Refrigerate after opening. |
| Cheeses |
| Seafood |
|
• Do not eat refrigerated smoked seafood, unless it is contained in a cooked dish, such as a casserole, or unless it is a canned or shelf-stable product. • Refrigerated smoked seafood, such as salmon, trout, whitefish, cod, tuna, and mackerel, is most often labeled as “nova-style,” “lox,” “kippered,” “smoked,” or “jerky.” • These fish are typically found in the refrigerator section or sold at seafood and deli counters of grocery stores and delicatessens. • Canned and shelf stable tuna, salmon, and other fish products are safe to eat. Follow this general FDA advice for melon safety: • Consumers and food preparers should wash their hands with warm water and soap for at least 20 sec before and after handling any whole melon, such as cantaloupe, watermelon, or honeydew. • Scrub the surface of melons, such as cantaloupes, with a clean produce brush under running water and dry them with a clean cloth or paper towel before cutting. Be sure that your scrub brush is sanitized after each use, to avoid transferring bacteria between melons. • Promptly consume cut melon or refrigerate promptly. Keep your cut melon refrigerated ≤4.5°C (40°F) (0-1.1°C [32-34°F] is best), for no more than 7 days. |
* Including pregnant women, persons with weakened immune system, and older adults.
FDA, Food and Drug Administration; USDA, U.S. Department of Agriculture.
Adapted from Centers for Disease Control and Prevention: Listeria (listeriosis): prevention. http://www.cdc.gov/listeria/prevention.html .
Careful handwashing is essential to prevent nosocomial spread within obstetric and neonatal units. Immunocompromised patients given prophylaxis with trimethoprim-sulfamethoxazole are protected from Listeria infections. Cases and especially outbreaks should be reported immediately to public health authorities so that timely investigation can be initiated in order to interrupt transmission from the contaminated source.