Pseudomonas, Burkholderia, and Stenotrophomonas
Pseudomonas aeruginosa
Thomas S. Murray, Robert S. Baltimore
Etiology
Pseudomonas aeruginosa is a gram-negative rod and is a strict aerobe. It can multiply in a great variety of environments that contain minimal amounts of organic compounds. Strains from clinical specimens do not ferment lactose, are oxidase positive, and may produce β-hemolysis on blood agar. Many strains produce pigments, including pyocyanin, pyoverdine, and pyorubrin, that diffuse into and color the surrounding medium. Strains of P. aeruginosa are differentiated for epidemiologic purposes by a variety of genotyping methods, including restriction fragment length polymorphisms using pulsed-field gel electrophoresis, multilocus sequence typing, and more recently, whole genome sequencing.
Epidemiology
P. aeruginosa is a classic “opportunist.” It rarely causes disease in people who do not have a predisposing risk factor. Compromised host defense mechanisms resulting from trauma, neutropenia, mucositis, immunosuppression, or impaired mucociliary transport explain the predominant role of this organism in producing opportunistic infections. In pediatric settings, it is most frequently seen in the respiratory secretions of children with cystic fibrosis (CF). P. aeruginosa was found in 1% of neonates with fever and bacteremia in a review of 6 U.S. centers. One series of neonatal intensive care unit (NICU) infections reported that 3.8%episodes of neonatal bacteremia from 1989–2003 were caused by P. aeruginosa . Another children's hospital reported 232 episodes of P. aeruginosa bacteremia over a 10 yr period, with half the infected children diagnosed with an underlying malignancy.
P. aeruginosa and other pseudomonads frequently enter the hospital environment on the clothes, skin, or shoes of patients or hospital personnel, with plants or vegetables brought into the hospital, and in the gastrointestinal (GI) tract of patients. Colonization of any moist or liquid substance may ensue; the organisms may be found growing in any water reservoir, including distilled water, and in hospital kitchen sinks and laundries, some antiseptic solutions, and equipment used for respiratory therapy and urinary procedures. Colonization of skin, throat, stool, and nasal mucosa of patients is low at admission to the hospital but increases to as high as 50–70% with prolonged hospitalization and with the use of broad-spectrum antibiotics, chemotherapy, mechanical ventilation, and urinary catheters. Patients’ intestinal microbial flora may be altered by the broad-spectrum antibiotics, reducing resistance to colonization and permitting P. aeruginosa in the environment to populate the GI tract. Intestinal mucosal breakdown associated with medications, especially cytotoxic agents, and nosocomial enteritis may provide a pathway by which P. aeruginosa spreads to the lymphatics or bloodstream.
Pathology
The pathologic manifestations of P. aeruginosa infections depend on the site and type of infection. Because of its elaboration of toxins and invasive factors, the organism can often be seen invading blood vessels and causing vascular necrosis. In some infections there is spread through tissues with necrosis and microabscess formation. In patients with CF, focal and diffuse bronchitis/bronchiolitis leading to bronchiolitis obliterans has been reported.
Pathogenesis
Invasiveness of P. aeruginosa is mediated by a host of virulence factors. Bacterial attachment is facilitated by pili that adhere to epithelium damaged by prior injury or infection. Extracellular proteins, proteases, elastases, and cytotoxins disrupt cell membranes, and in response, host-produced cytokines cause capillary vascular permeability and induce an inflammatory response. Dissemination and bloodstream invasion follow extension of local tissue damage and are facilitated by the antiphagocytic properties of endotoxin, the exopolysaccharide, and protease cleavage of immunoglobulin G. P. aeruginosa also produces numerous exotoxins, including exotoxin A, which causes local necrosis and facilitates systemic bacterial invasion. P. aeruginosa possesses a type III secretion system composed of a needle structure that inserts into host cell membranes and allows secretion of exotoxins directly into host cells. P. aeruginosa strains with the gene encoding the type III secretion system–dependent phospholipase ExoU are associated with increased mortality compared with ExoU-negative strains, in retrospective studies of patients with P. aeruginosa ventilator-associated pneumonia . The host responds to infection with a robust inflammatory response, recruiting neutrophils to the infection site and producing antibodies to P. aeruginosa proteins such as exotoxin A and endotoxin. There is a lack of convincing data that these antibodies are protective against the establishment of infection.
In addition to acute infection, P. aeruginosa is also capable of chronic persistence thought to be partly a result of the formation of biofilms , organized communities of bacteria encased in an extracellular matrix that protects the organisms from the host immune response and the effects of antibiotics. Biofilm formation requires pilus-mediated attachment to a surface, proliferation of the organism, and production of exopolysaccharide as the main bacterial component of the extracellular matrix. A mature biofilm can persist despite an intense host immune response, is resistant to many antimicrobials, and is difficult to eradicate with current therapies.
Clinical Manifestations
Most clinical patterns are related to opportunistic infections in immunocompromised hosts (see Chapter 205 ) or are associated with shunts and indwelling catheters (Chapter 206 ). P. aeruginosa may be introduced into a minor wound of a healthy person as a secondary invader, and cellulitis and a localized abscess that exudes green or blue pus may follow. The characteristic skin lesions of P. aeruginosa, ecthyma gangrenosum , whether caused by direct inoculation or a metastatic focus secondary to septicemia, begin as pink macules and progress to hemorrhagic nodules and eventually to ulcers with ecchymotic and gangrenous centers with eschar formation, surrounded by an intense red areola (Table 232.1 and Fig. 232.1 ).
Table 232.1
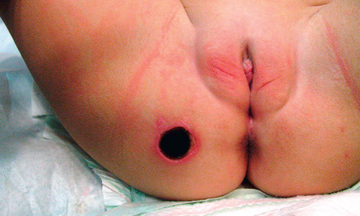
Outbreaks of dermatitis and urinary tract infections (UTIs) caused by P. aeruginosa have been reported in healthy persons after use of pools or hot tubs. Skin lesions of folliculitis develop several hours to 2 days after contact with these water sources. Skin lesions may be erythematous, macular, papular, or pustular. Illness may vary from a few scattered lesions to extensive truncal involvement. In some children, malaise, fever, vomiting, sore throat, conjunctivitis, rhinitis, and swollen breasts may be associated with dermal lesions. UTIs caused by P. aeruginosa are most often nosocomial and are often associated with the presence of an indwelling urinary catheter, urinary tract malformations, and previous antibiotic use. UTIs may be minimized or prevented by prompt removal of the catheter and by early identification and corrective surgery of obstructive lesions when present.
Burns and Wound Infection
The surfaces of burns or wounds are frequently populated by P. aeruginosa and other gram-negative organisms; this initial colonization with a low number of adherent organisms is a prerequisite to invasive disease. P. aeruginosa colonization of a burn site may develop into burn wound sepsis , which has a high mortality rate when the density of organisms reaches a critical concentration. Administration of antibiotics may diminish the susceptible microbiologic flora, permitting strains of relatively resistant P. aeruginosa to flourish. Multiplication of organisms in devitalized tissues or associated with prolonged use of intravenous or urinary catheters increases the risk for septicemia with P. aeruginosa, a major problem in burned patients (see Chapter 92 ).
Cystic Fibrosis
P. aeruginosa is common in children with CF, with a prevalence that increases with increasing age and severity of pulmonary disease (see Chapter 432 ). Initial infection is caused by nonmucoid environmental strains of P. aeruginosa, but after a variable period, mucoid strains of P. aeruginosa that produce the antiphagocytic exopolysaccharide alginate, which are rarely encountered in other conditions, predominate. Repeated isolation of mucoid P. aeruginosa from the sputum is associated with increased morbidity and mortality. The infection begins insidiously or even asymptomatically, and the progression has a highly variable pace. In children with CF, antibody does not eradicate the organism, and antibiotics are only partially effective; thus, after infection becomes chronic, it cannot be completely eradicated. Repeated courses of antibiotics select for P. aeruginosa strains that are resistant to multiple antibiotics.
Immunocompromised Persons
Children with leukemia or other malignancies, particularly those who are receiving immunosuppressive therapy and who are neutropenic, typically with intravascular catheters, are extremely susceptible to septicemia caused by invasion of the bloodstream by P. aeruginosa that is colonizing the respiratory or GI tract. Signs of sepsis are often accompanied by a generalized vasculitis, and hemorrhagic necrotic lesions may be found in all organs, including the skin (ecthyma gangrenosum) (see Fig. 232.1 ). Hemorrhagic or gangrenous perirectal cellulitis or abscesses may occur, associated with ileus and profound hypotension.
Nosocomial Pneumonia
Although not a frequent cause of community-acquired pneumonia in children, P. aeruginosa does cause nosocomial pneumonia, especially ventilator-associated pneumonia, in patients of all ages. P. aeruginosa has historically been found to contaminate ventilators, tubing, and humidifiers. Such contamination is uncommon now because of disinfection practices and routine changing of equipment. Nevertheless, colonization of the upper respiratory tract and the GI tract may be followed by aspiration of P. aeruginosa –contaminated secretions, resulting in severe pneumonia. Prior use of broad-spectrum antibiotics is a risk factor for colonization with antibiotic-resistant strains of P. aeruginosa. One of the most challenging situations is distinguishing between colonization and pneumonia in intubated patients. This distinction can often only be resolved by using invasive culture techniques such as quantitative bronchoalveolar lavage.
Infants
P. aeruginosa is an occasional cause of nosocomial bacteremia in newborns and accounts for 2–5% of positive blood culture results in NICUs. A frequent focus preceding bacteremia is conjunctivitis . Older infants rarely present with community-acquired sepsis caused by P. aeruginosa. In the few reports describing community-acquired sepsis, preceding conditions included ecthyma-like skin lesions, virus-associated transient neutropenia, and prolonged contact with contaminated bath water or a hot tub.
Diagnosis
P. aeruginosa infection is rarely clinically distinctive. Diagnosis depends on recovery of the organism from the blood, cerebrospinal fluid (CSF), urine, or needle aspirate of the lung, or from purulent material obtained by aspiration of subcutaneous abscesses or areas of cellulitis. In the appropriate clinical setting, recovery of P. aeruginosa from a coughed or suctioned sputum may represent infection; but it also may only represent colonization, and clinical judgment is required. Rarely, skin lesions that resemble P. aeruginosa infection may follow septicemia caused by Aeromonas hydrophila, other gram-negative bacilli, and Aspergillus. When P. aeruginosa is recovered from nonsterile sites such as skin, mucous membranes, or voided urine, quantitative cultures may be useful to differentiate colonization from invasive infection. In general, ≥100,000 colony-forming units/mL of fluid or gram of tissue is evidence suggestive of invasive infection. Quantitative cultures of tissue and skin are not routine and require consultation with the clinical microbiology laboratory.
Treatment
Systemic infections with P. aeruginosa should be treated promptly with an antibiotic to which the organism is susceptible in vitro. Response to treatment may be limited, and prolonged treatment may be necessary for systemic infection in immunocompromised hosts.
Septicemia and other aggressive infections should be treated with either 1 or 2 bactericidal agents. Although the number of agents required is controversial, the evidence continues to suggest that the benefit of adding a 2nd agent is questionable, even when studies have included immunosuppressed patients. Whether the use of 2 agents delays the development of resistance is also controversial, with evidence both for and against. Appropriate antibiotics for single-agent therapy include ceftazidime, cefepime, ticarcillin-clavulanate, and piperacillin-tazobactam. Gentamicin or another aminoglycoside may be used concomitantly for synergistic effect.
Ceftazidime has proved to be extremely effective in patients with CF, at 150-250 mg/kg/day divided every 6-8 hr intravenously (IV) to a maximum of 6 g/day. Piperacillin or piperacillin-tazobactam, 300-450 mg/kg/day divided every 6-8 hr IV to a maximum of 12 g/day, also has proved to be effective therapy for susceptible strains of P. aeruginosa when combined with an aminoglycoside. Studies of acute Pseudomonas infection in ICUs show that continuous infusions of piperacillin-tazobactam are more effective than the same daily dose given as pulse infusions.
Additional effective antibiotics include imipenem-cilastatin, meropenem, and aztreonam. Ciprofloxacin is an effective outpatient therapy, and while commonly used in children with CF, it is not approved in the United States for persons <18 yr old, except for oral treatment of UTIs or when there are no other agents to which the organism is susceptible. Inhaled therapy with either tobramycin or aztreonam is also used for chronic pulmonary infection, with inhaled colistin reserved for the treatment of resistant pseudomonads. It is important to base continued treatment on the results of susceptibility tests because antibiotic resistance of P. aeruginosa to 1 or more antibiotics is increasing. Macrolide therapy decreases pulmonary exacerbations in patients with chronic lung disease and P. aeruginosa infection. The mechanism likely relates to altering the virulence properties of P. aeruginosa rather than direct bacterial killing.
P. aeruginosa displays intrinsic and acquired resistance to antibiotics. It has many mechanisms for resistance to multiple classes of antibiotics, including but not limited to genetic mutation, production of β-lactamases, and drug efflux pumps. Throughout the United States there has been an alarming increase in multidrug-resistant (MDR) P. aeruginosa isolates recovered from children, with resistance to at least 3 classes of antibiotics. The rate of MDR P. aeruginosa increased to 26% in 2012 from 15.9% in 1999. Also, the rate of carbapenem-resistant P. aeruginosa increased from 12% to 20% during the same period. A newer agent with efficacy against many MDR P. aeruginosa isolates is ceftazidime/avibactam, a drug that combines ceftazidime with a β-lactamase inhibitor.
Meningitis can occur by spread from a contiguous focus, as a secondary focus when there is bacteremia, or after invasive procedures. P. aeruginosa meningitis is best treated with ceftazidime in combination with an aminoglycoside such as gentamicin, both given IV. Concomitant intraventricular or intrathecal treatment with gentamicin may be required when IV therapy fails but is not recommended for routine use.
Supportive Care
P. aeruginosa infections vary in severity from superficial to intense septic presentations. With severe infections there is often multisystem involvement and a systemic inflammatory response. Supportive care is similar to care for severe sepsis caused by other gram-negative bacilli and requires support of blood pressure, oxygenation, and appropriate fluid management.
Prognosis
The prognosis is dependent primarily on the nature of the underlying factors that predisposed the patient to P. aeruginosa infection. In severely immunocompromised patients, the prognosis for patients with P. aeruginosa sepsis is poor unless susceptibility factors such as neutropenia or hypogammaglobulinemia can be reversed. The overall mortality rate was 12.3% in one series of 232 children with P. aeruginosa bacteremia, with 3% dying within 48 hr of admission. Resistance of the organism to first-line antibiotics also decreases the chance of survival. The outcome may be improved when there is a urinary tract portal of entry, absence of neutropenia or recovery from neutropenia, and drainage of local sites of infection.
P. aeruginosa is recovered from the lungs of most children who die of CF and adds to the slow deterioration of these patients. The prognosis for normal development is poor in the few infants who survive P. aeruginosa meningitis.
Prevention
Prevention of infections is dependent on limiting contamination of the healthcare environment and preventing transmission to patients. Effective hospital infection control programs are necessary to identify and eradicate sources of the organism as quickly as possible. In hospitals, infection can be transmitted to children by the hands of personnel, from washbasin surfaces, from catheters and other hospital equipment, and from solutions used to rinse suction catheters.
Strict attention to hand hygiene before and between contacts with patients may prevent or interdict epidemic disease. Meticulous care and sterile procedures in suctioning of endotracheal tubes, insertion and maintenance of indwelling catheters, and removal of catheters as soon as medically reasonable greatly reduce the hazard of extrinsic contamination by P. aeruginosa and other gram-negative organisms. Prevention of follicular dermatitis caused by P. aeruginosa contamination of whirlpools or hot tubs is possible by maintaining pool water at a pH of 7.2-7.8. Antimicrobial stewardship programs that promote the appropriate use of antibiotics in the hospital setting are critical for reducing the rates of MDR P. aeruginosa by limiting unnecessary antibiotic use.
Infections in burned patients may be minimized by protective isolation, debridement of devitalized tissue, and topical applications of bactericidal cream. Administration of intravenous immunoglobulin may be used. Approaches under investigation to prevent infection include development of a P. aeruginosa vaccine. No vaccine is currently licensed in the United States.
Bibliography
Bizzarro MJ, Shabanova V, Baltimore RS, et al. Neonatal sepsis 2004–2013: the rise and fall of coagulase-negative staphylococci. J Pediatr . 2015;166(5):1193–1199.
Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomized pathogen directed phase III study. Lancet Infect Dis . 2016;16:661–673.
Centers for Disease Control and Prevention. Melioidosis treatment . http://www.cdc.gov/melioidosis/treatment/index.html .
Logan LK, Gandra S, Mandal S, et al. Multidrug- and carbapenem-resistant Pseudomonas aeruginosa in children, United States, 1999–2012. J Pediatr Infect Dis . 2017;6:352–359.
Planquette B, Timsit JF, Misset BY, et al. Pseudomonas aeruginosa ventilator-associated pneumonia. Am J Respir Crit Care Med . 2013;188:69–76.
Saiman L, Siegel JD, LiPuma JJ, et al. Infection prevention and control guideline: update for cystic fibrosis 2013. Infect Control Hosp Epidemiol . 2014;35:S1–S67.
Sawa T, Shimizu M, Moriyama K, et al. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care . 2014;18:668–679.
Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non–cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA . 2013;309(12):1260–1267.
Burkholderia cepacia Complex
Thomas S. Murray, Robert S. Baltimore
Burkholderia cepacia is a filamentous gram-negative rod now recognized to be a group of related species or genomovars (B. cepacia, B. cenocepacia , B. multivorans ). It is ubiquitous in the environment but may be difficult to isolate from respiratory specimens in the laboratory, requiring an enriched, selective media oxidation-fermentation base supplemented with polymyxin B–bacitracin-lactose agar (OFPBL) and as long as 3 days of incubation.
B. cepacia is a classic opportunist that rarely infects normal tissue but can be a pathogen for individuals with preexisting damage to respiratory epithelium, especially persons with CF or with immune dysfunction such as chronic granulomatous disease. B. cepacia has multiple virulence factors, including lipopolysaccharide, flagella, and a type III secretion system that promotes invasion of respiratory epithelial cells. Resistance to many antibiotics and disinfectants appears to be a factor in the emergence of B. cepacia as a nosocomial pathogen. In critical care units it may colonize the tubing used to ventilate patients with respiratory failure. In some patients this colonization may lead to invasive pneumonia and septic shock. Although B. cepacia is found throughout the environment, human-to-human spread among CF patients occurs either directly by inhalation of aerosols or indirectly from contaminated equipment or surfaces, accounting for the strict infection control measures for children with CF who are colonized with B. cepacia . For example, CF patients colonized with B. cepacia are asked not to attend events where other persons with CF will be present. B. cepacia infections in persons with CF may represent chronic infection in some patients, but others, especially those with Burkholderia cenocepacia , genomovar III, can develop an acute respiratory syndrome of fever, leukocytosis, and progressive respiratory failure, with more rapid decline in pulmonary function and lower survival rate.
Treatment in hospitals should include standard precautions and avoidance of placing colonized and uncolonized patients in the same room. The use of antibiotics is guided by susceptibility studies of a patient's isolates, because the susceptibility pattern of this species is quite variable, and multiply resistant strains are common. Trimethoprim-sulfamethoxazole (TMP-SMX) and doxycycline or minocycline are potential oral therapies for B. cepacia complex . For IV therapy, meropenem with a 2nd agent such as TMP-SMX, doxycycline, minocycline, ceftazidime, or amikacin are potential options. Even though there is primary resistance to aminoglycosides, these agents may be useful in combination with other antibiotics. Treatment with 2 or more agents may be necessary to control the infection and avoid the development of resistance. No vaccine is currently available.
Burkholderia mallei (Glanders)
Glanders is a severe infectious disease of horses and other domestic and farm animals that is caused by Burkholderia mallei, a nonmotile gram-negative bacillus that is occasionally transmitted to humans. It is acquired by inoculation into the skin, usually at the site of a previous abrasion, or by inhalation of aerosols. Laboratory workers may acquire it from clinical specimens. The disease is relatively common in Asia, Africa, and the Middle East. The clinical manifestations include septicemia, acute or chronic pneumonitis, and hemorrhagic necrotic lesions of the skin, nasal mucous membranes, and lymph nodes. The diagnosis is usually made by recovery of the organism in cultures of affected tissue. Glanders is treated with sulfadiazine, tetracyclines, or chloramphenicol and streptomycin over many months. The disease has been eliminated from the United States, but interest in this organism has increased because of the possibility of its use as a bioterrorism agent (see Chapter 741 ). Although standard precautions are appropriate when caring for hospitalized infected patients, biosafety level 3 precautions are required for laboratory staff working with B. mallei. No vaccine is available.
Burkholderia pseudomallei (Melioidosis)
Melioidosis is an important disease of Southeast Asia and northern Australia and occurs in the United States mainly in persons returning from endemic areas. The causative agent is Burkholderia pseudomallei, an inhabitant of soil and water in the tropics. It is ubiquitous in endemic areas, and infection follows inhalation of dust, ingestion, or direct contamination of abrasions or wounds. Human-to-human transmission has only rarely been reported. Serologic surveys demonstrate that asymptomatic infection occurs in endemic areas. The disease may remain latent and appear when host resistance is reduced, sometimes years after the initial exposure. Diabetes mellitus is a risk factor for severe melioidosis.
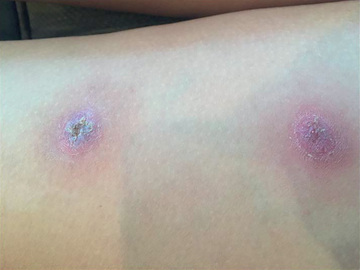
Melioidosis may present as a primary skin lesion (vesicle, bulla, or urticaria) (Fig. 232.2 ). Pulmonary infection may be subacute and mimic tuberculosis or may present as an acute necrotizing pneumonia. Occasionally, septicemia occurs and numerous abscesses are noted in various organs of the body. Myocarditis, pericarditis, endocarditis, intestinal abscess, cholecystitis, acute gastroenteritis, UTIs, septic arthritis, paraspinal abscess, osteomyelitis, mycotic aneurysm, and generalized lymphadenopathy all have been observed. Melioidosis may also present as an encephalitic illness with fever and seizures. It is also an agent of severe wound infections after contact with contaminated water following a tsunami. Diagnosis is based on visualization of characteristic small, gram-negative rods in exudates or growth on laboratory media such as eosin–methylene blue or MacConkey agar. Serologic tests are available, and diagnosis can be established by a 4-fold or greater increase in antibody titer in an individual with an appropriate syndrome. It has been recognized as a possible agent of bioterrorism (see Chapter 741 ).
B. pseudomallei is susceptible to many antimicrobial agents, and the U.S. Centers for Disease Control and Prevention (CDC) recommends meropenem or ceftazidime as IV therapies and TMP-SMX or doxycycline as oral therapy. Other choices include aminoglycosides, tetracycline, chloramphenicol, and amoxicillin-clavulanate. Therapy should be guided by antimicrobial susceptibility tests; 2 or 3 agents such as ceftazidime or meropenem plus either TMP-SMX, sulfisoxazole, or an aminoglycoside are usually chosen for severe or septicemic disease. For severe disease, prolonged treatment for 2-6 mo is recommended to prevent relapses. Appropriate antibiotic therapy generally results in recovery.
Bibliography
Burkholderia cepacia Complex
David J, Bell RE, Clark GC. Mechanisms of disease: host-pathogen interactions between Burkholderia species and lung epithelial cells. Front Cell Infect Microbiol . 2015;5:80.
Mahenthiralingam E, Vandamme P. Taxonomy and pathogenesis of the Burkholderia cepacia complex. Chron Respir Dis . 2005;2:209–217.
Saiman L, Siegel JD, LiPuma JJ, et al. Infection prevention and control guideline: update for cystic fibrosis 2013. Infect Control Hosp Epidemiol . 2014;35:S1–S67.
Burkholderia mallei and B. pseudomallei
Anderson EW, Mackay MT, Ryan MM. Neurologic melioidosis: case report of a rare cause of acute flaccid paralysis. J Pediatr . 2016;170:319–321.
Centers for Disease Control and Prevention. Melioidosis: treatment . http://www.cdc.gov/melioidosis/treatment/index.html .
Currie BJ, Haslam A, Pearson T, et al. Identification of melioidosis outbreak by multilocus variable number tandem repeat analysis. Emerg Infect Dis . 2009;15:169–174.
McLeod C, Morris PS, Bauert PA, et al. Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature. Clin Infect Dis . 2015;60:21–26.
Mitchell PK, Campbell C, Montgomery MP, et al. Travel-associated melioidosis and resulting laboratory exposures—United States, 2016. MMWR Morb Mortal Wkly Rep . 2017;66(37):1001–1002.
Stenotrophomonas
Thomas S. Murray, Robert S. Baltimore
Stenotrophomonas maltophilia (formerly Xanthomonas maltophilia or Pseudomonas maltophilia ) is a short to medium-sized, straight gram-negative bacillus. It is ubiquitous in nature and can be found in the hospital environment, especially in tap water or standing water, and may contaminate sinks and hospital equipment such as nebulizers. Strains isolated in the laboratory may be contaminants, may be a commensal from the colonized surface of a patient, or may represent an invasive pathogen. The species is an opportunist and is often recovered from immunosuppressed patients and patients with CF after multiple courses of antimicrobial therapy. Serious infections usually occur among those requiring intensive care, including neonatal intensive care, typically patients with ventilator-associated pneumonia or catheter-associated infections. Prolonged antibiotic exposure appears to be a frequent factor in nosocomial S. maltophilia infections, probably because of its endogenous antibiotic resistance pattern. Common types of infection include pneumonia following airway colonization and aspiration, bacteremia, soft tissue infections, endocarditis, and osteomyelitis. S. maltophilia bacteremia is a nosocomial infection associated with the presence of a central venous catheter.
Strains vary as to antibiotic susceptibility, and the treatment of S. maltophilia can be difficult because of inherent antimicrobial resistance. Data are lacking on whether there is clinical benefit to treat S. maltophilia recovered from the respiratory tract of a patient with CF. For invasive infections, TMP-SMX is the treatment of choice and is the only antimicrobial for which susceptibility is routinely reported. Minocycline monotherapy has recently been shown to be a viable alternative to TMP-SMX with fewer adverse effects and similar clinical outcomes. Mean inhibitory concentration testing is available for other antibiotics, such as ticarcillin-clavulanate, and reserved for TMP-SMX–resistant isolates. For resistant organisms or for patients who cannot tolerate sulfa drugs, other options based on clinical outcome include ciprofloxacin, as well as ceftazidime alone, or in combination with other agents such as aminoglycosides. Tigecycline is a newer agent reported to have efficacy for treating a highly resistant isolate.
Bibliography
Amin R, Waters AR. Antibiotic treatment for Stenotrophomonas maltophilia in people with cystic fibrosis. Cochrane Database Syst Rev . 2016;(14):1–14.
Hand E, Davis H, Kim T, et al. Monotherapy with minocycline or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother . 2016;71:1071–1075.