Pneumocystis jirovecii
Francis Gigliotti, Terry W. Wright
Pneumocystis jirovecii pneumonia (interstitial plasma cell pneumonitis) in an immunocompromised person is a life-threatening infection. Primary infection in the immunocompetent person is usually subclinical and goes unrecognized. The disease most likely results from new or repeat acquisition of the organism rather than reactivation of latent organisms. Even in the most severe cases, with rare exceptions, the organisms remain localized to the lungs.
Etiology
P. jirovecii is a common extracellular parasite found worldwide in the lungs of mammals. The taxonomic placement of this organism has not been unequivocally established, but nucleic acid homologies place it closest to fungi despite sharing morphologic features and drug susceptibility with protozoa. Detailed studies of the basic biology of the organism are not possible because of the inability to maintain P. jirovecii in culture. Phenotypic and genotypic analyses demonstrate that each mammalian species is infected by a unique strain (or possibly species) of Pneumocystis. A biologic correlate of these differences is evidenced by animal experiments that have shown organisms are not transmissible from one mammalian species to another. These observations have led to the suggestion that organisms be renamed, with those infecting humans renamed P. jirovecii .
Epidemiology
Serologic surveys show that most humans are infected with P. jirovecii before 4 yr. of age. In the immunocompetent child, these infections are usually asymptomatic. P. jirovecii DNA can occasionally be detected in nasopharyngeal aspirates of normal infants. Pneumonia caused by P. jirovecii occurs almost exclusively in severely immunocompromised hosts, including those with congenital or acquired immunodeficiency disorders, malignancies, or transplanted organs. Patients with primary immunodeficiency diseases at risk for infection include severe combined immunodeficiency disease, X-linked CD40 ligand deficiency, major histocompatibility complex class II deficiency, nuclear factor kappa B essential modulator deficiency, dedicator of cytokinesis 8 deficiency, Wiskott-Aldrich syndrome, and caspase recruitment domain 11 deficiency. Small numbers of P. jirovecii can be found in the lungs of infants who have died with the diagnosis of sudden infant death syndrome. This observation could indicate a cause-and-effect relationship or simply that there is overlap in the timing of the primary infection with P. jirovecii and sudden infant death syndrome.
Without chemoprophylaxis, approximately 40% of infants and children with AIDS, 70% of adults with AIDS, 12% of children with leukemia, and 10% of patients with organ transplants experience P. jirovecii pneumonia. Epidemics that occurred among debilitated infants in Europe during and after World War II are attributed to malnutrition. The use of new biologic immunosuppressive agents has expanded at-risk populations. The addition of tumor necrosis factor-α inhibitors to the management of patients with inflammatory bowel disease has resulted in a demonstrable increase in P. jirovecii pneumonia in this patient population, as has the use of rituximab in patients with hematologic malignancies.
The natural habitat and mode of transmission to humans are unknown, but animal studies clearly demonstrate airborne transmission. Animal-to-human transmission is unlikely because of the host specificity of P. jirovecii . Thus person-to-person transmission is likely but has not been conclusively demonstrated.
Pathogenesis
Two forms of P. jirovecii are found in the alveolar spaces: cysts, which are 5-8 µm in diameter and contain up to 8 pleomorphic intracystic sporozoites (or intracystic bodies), and extracystic trophozoites (or trophic forms), which are 2-5 µm cells derived from excysted sporozoites. The terminology of sporozoite and trophozoite is based on the morphologic similarities to protozoa, because there are no exact correlates for these forms of the organism among the fungi. P. jirovecii attaches to type I alveolar epithelial cells, possibly by adhesive proteins such as fibronectin or mannose-dependent ligands.
Control of infection depends on intact cell-mediated immunity. Studies in patients with AIDS show an increased incidence of P. jirovecii pneumonia with markedly decreased CD4+ T-lymphocyte counts. The CD4+ cell count provides a useful indicator in both older children and adults of the need for prophylaxis for P. jirovecii pneumonia. Although normally functioning CD4+ T cells are central to controlling infection by P. jirovecii, the final effector pathway for destruction of P. jirovecii is poorly understood but likely depends on alveolar macrophages. A role for CD4+ T cells could be to provide help for the production of specific antibody that is then involved in the clearance of organisms through interaction with complement, phagocytes, or T cells or through direct activation of alveolar macrophages.
In the absence of an adaptive immune response, as can be modeled in severe combined immunodeficient mice, infection with P. murina produces little alteration in lung histology or function until late in the course of the disease. If functional lymphocytes are given to severe combined immunodeficient mice infected with P. murina, there is rapid onset of an inflammatory response that results in an intense cellular infiltrate, markedly reduced lung compliance, and significant hypoxia, mimicking the characteristic changes of P. jirovecii pneumonia in humans. These inflammatory changes are also associated with marked disruption of surfactant function. T-cell subset analysis has shown that CD4+ T cells produce an inflammatory response that clears the organisms but also results in lung injury. CD8+ T cells are ineffective in the eradication of P. jirovecii. CD8+ T cells do help to modulate the inflammation produced by CD4+ T cells, but in the absence of CD4+ T cells the ineffectual inflammatory response of CD8+ T cells contributes significantly to lung injury. These various T-cell effects are likely responsible for the variations in presentation and outcome of P. jirovecii pneumonia observed in different patient populations.
Pathology
The histopathologic features of P. jirovecii pneumonia are of 2 types. The 1st type is infantile interstitial plasma cell pneumonitis, which was seen in epidemic outbreaks in debilitated infants 3-6 mo of age. Extensive infiltration with thickening of the alveolar septum occurs, and plasma cells are prominent. The 2nd type is a diffuse desquamative alveolar pneumonitis found in immunocompromised children and adults. The alveoli contain large numbers of P. jirovecii in a foamy exudate with alveolar macrophages active in the phagocytosis of organisms. The alveolar septum is not infiltrated to the extent it is in the infantile type, and plasma cells are usually absent.
Clinical Manifestations
There are at least three distinct clinical presentations of P. jirovecii pneumonia. In patients with profound congenital immunodeficiency or in AIDS patients with very few CD4+ T cells, the onset of hypoxia and symptoms is subtle, with tachypnea progressing to nasal flaring, often without fever; intercostal, suprasternal, and infrasternal retractions; and cyanosis in severe cases. In cases of P. jirovecii pneumonia occurring in children and adults with immunodeficiency resulting from immunosuppressive medications, the onset of hypoxia and symptoms is often more abrupt, with fever, tachypnea, dyspnea, and cough, progressing to severe respiratory compromise. This type accounts for the majority of cases, although the severity of clinical expression can vary. Rales are usually not detected on physical examination. The 3rd pattern of disease is seen in severely immunocompromised patients with P. jirovecii pneumonia who appear to be responding to therapy but then have an acute and seemingly paradoxical deterioration thought to be associated with return of immune function. This condition is referred to as immune reconstitution inflammatory syndrome and is most commonly seen in patients with newly diagnosed AIDS who present with P. jirovecii pneumonia and who have a rapid response to antiretroviral therapy that is instituted at the same time as anti-Pneumocystis therapy. It can also occur in stem cell transplant recipients who engraft while infected with P. jirovecii.
Laboratory Findings
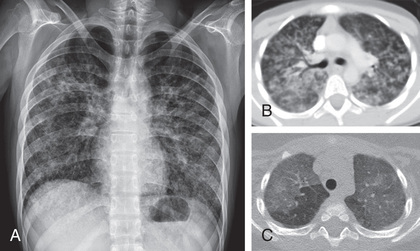
The chest radiograph reveals bilateral diffuse interstitial or alveolar ground glass infiltrates (Fig. 271.1 ). The earliest densities are perihilar, and progression proceeds peripherally, sparing the apical areas until last. The arterial oxygen tension (PaO 2 ) is invariably decreased. The major role of the laboratory in establishing a diagnosis of P. jirovecii pneumonia is in identifying organisms in lung specimens by a variety of methods. Once obtained, the specimens are typically stained with 1 of 4 commonly used stains: Grocott-Gomori silver stain and toluidine blue stain for the cyst form, polychrome stains such as Giemsa stain for the trophozoites and sporozoites, and the fluorescein-labeled monoclonal antibody stains for both trophozoites and cysts. Polymerase chain reaction analysis of respiratory specimens offers promise as a rapid diagnostic method, but a standardized system for clinical use has not been established. Serum lactate dehydrogenase levels are often elevated but are not specific.
Diagnosis
Definitive diagnosis requires demonstration of P. jirovecii in the lung in the presence of clinical signs and symptoms of the infection. Organisms can be detected in specimens collected by bronchoalveolar lavage (BAL), tracheal aspirate, transbronchial lung biopsy, bronchial brushings, percutaneous transthoracic needle aspiration, and open lung biopsy. Hypertonic saline–induced sputum samples are helpful if P. jirovecii is found, but the absence of the organisms in induced sputum does not exclude the infection and BAL should be performed. Open lung biopsy is the most reliable method, although BAL is more practical in most cases. Estimates of the diagnostic yield of the various specimens are 20–40% for induced sputum, 50–60% for tracheal aspirate, 75–95% for BAL, 75–85% for transbronchial biopsy, and 90–100% for open lung biopsy.
Treatment
The recommended therapy for P. jirovecii pneumonia is trimethoprim-sulfamethoxazole (TMP-SMX) (15-20 mg TMP and 75-100 mg SMX/kg/day in 4 divided doses) administered intravenously, or orally if there is mild disease and no malabsorption or diarrhea. The duration of treatment is 3 wk for patients with AIDS and 2 wk for other patients. Unfortunately, adverse reactions often occur with TMP-SMX, especially rash and neutropenia in patients with AIDS. For patients who cannot tolerate or who fail to respond to TMP-SMX after 5-7 days, pentamidine isethionate (4 mg/kg/day as a single dose IV) may be used. Adverse reactions are frequent and include renal and hepatic dysfunction, hyperglycemia or hypoglycemia, rash, and thrombocytopenia. Atovaquone (750 mg twice daily with food, for patients >13 yr of age) is an alternative treatment that has been used primarily in adults with mild to moderate disease. Limited experience is available for younger children. Pharmacokinetic studies of atovaquone show that a dose of 30 mg/kg/day PO in 2 divided doses for children 0-3 mo of age and older than 2 yr of age is adequate and safe; a dose of 45 mg/kg/day PO in 2 divided doses is needed for children between 4 mo and 2 yr of age. Other effective therapies include trimetrexate glucuronate or combinations of trimethoprim plus dapsone or clindamycin plus primaquine.
Some studies in adults suggest that administration of corticosteroids as adjunctive therapy to suppress the inflammatory response increases the chances for survival in moderate and severe cases of P. jirovecii pneumonia. The recommended regimen of corticosteroids for adolescents older than 13 yr of age and for adults is oral prednisone, 80 mg/day PO in 2 divided doses on days 1-5, 40 mg/day PO once daily on days 6-10, and 20 mg/day PO once daily on days 11-21. A reasonable regimen for children is oral prednisone, 2 mg/kg/day for the 1st 7-10 days, followed by a tapering regimen for the next 10-14 days.
Supportive Care
Basic supportive care is dictated by the condition of the patient, with careful attention to maintain appropriate hydration and oxygenation. Only 5–10% of AIDS patients require mechanical ventilation compared with 50–60% of patients without AIDS, consistent with the hypothesis that the patient's ability to mount an inflammatory response correlates with severity and outcome. There are anecdotal reports of giving surfactant to children with severe P. jirovecii pneumonia, although the use of surfactant to treat adult-type respiratory distress syndrome is controversial.
Complications
Most complications occur as adverse events associated with the drugs used or the mechanical ventilation used for treatment. The most severe pulmonary complication of P. jirovecii pneumonia is adult-type respiratory distress syndrome. Rarely, P. jirovecii infection affects extrapulmonary sites (e.g., retina, spleen, and bone marrow), but such infections are usually not symptomatic and also respond to treatment.
Prognosis
Without treatment, P. jirovecii pneumonitis is fatal in almost all immunocompromised hosts within 3-4 wk of onset. The mortality rate varies with patient population and is related to inflammatory response rather than organism burden. AIDS patients have a mortality rate of 5–10%, and patients with other diseases such as malignancies have mortality rates as high as 20–25%. Patients who require mechanical ventilation have mortality rates of 60–90%. Patients remain at risk for P. jirovecii pneumonia as long as they are immunocompromised. Continuous prophylaxis should be initiated or reinstituted at the end of therapy for patients with AIDS (see Chapter 302 ).
Prevention
Patients at high risk for P. jirovecii pneumonia should be placed on chemoprophylaxis. Prophylaxis in infants born to HIV-infected mothers and for HIV-infected infants and children is based on age and CD4 cell counts (see Chapter 302 ). Because CD4 counts fluctuate rapidly during the first year of life, infants born to HIV-infected mothers should be placed on prophylaxis during the 1st year of life until HIV infection is ruled out. Patients with severe combined immunodeficiency syndrome, patients receiving intensive immunosuppressive therapy for cancer or other diseases, and organ transplant recipients are also candidates for prophylaxis. TMP-SMX (5 mg/kg TMP and 25 mg SMX/kg PO once daily or divided into 2 doses daily) is the drug of choice and may be given for 3 consecutive days each wk, or, alternatively, each day. Alternatives for prophylaxis include dapsone (2 mg/kg/day PO, maximum: 100 mg/dose; or 4 mg/kg PO once weekly, maximum: 200 mg/dose), atovaquone (30 mg/kg/day PO for infants 1-3 mo. and ≥24 mo of age; 45 mg/kg/day for infants and toddlers 4-23 mo of age), and aerosolized pentamidine (300 mg monthly by Respirgard II nebulizer), but all of these agents are inferior to TMP-SMX. Finally, limited clinical experience suggests that pentamidine can be given intravenously once monthly to prevent P. jirovecii pneumonia. Prophylaxis must be continued as long as the patient remains immunocompromised. Some AIDS patients who reconstitute adequate immune response during highly active antiretroviral therapy may have prophylaxis withdrawn.