Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Ericka V. Hayes
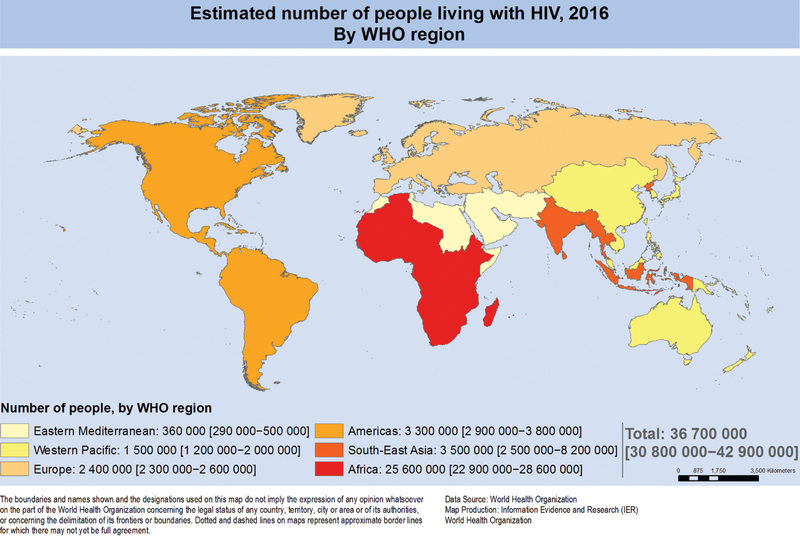
Advances in research and major improvements in the treatment and management of HIV infection have brought about a substantial decrease in the incidence of new HIV infections and AIDS in children. Globally, from 2000 to 2015, there has been an estimated 70% decline in new infections in children aged 0-14 yr, largely the result of antiretroviral treatment (ART) of HIV-infected pregnant women for the prevention of mother-to-child transmission. Seventy percent of adults and children with HIV infection live in sub-Saharan Africa, where the disease continues to have a devastating impact (Fig. 302.1 ). Children experience more rapid disease progression than adults, with up to half of untreated children dying within the first 2 yr of life. This rapid progression is correlated with a higher viral burden and faster depletion of infected CD4 lymphocytes in infants and children than in adults. Accurate diagnostic tests and the early initiation of potent drugs to inhibit HIV replication have dramatically increased the ability to prevent and control this disease.
Etiology
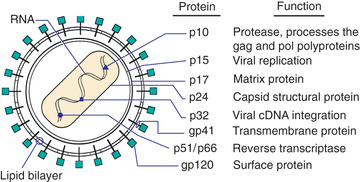
HIV-1 and HIV-2 are members of the Retroviridae family and belong to the Lentivirus genus, which includes cytopathic viruses causing diverse diseases in several animal species. The HIV-1 genome contains two copies of single-stranded RNA that is 9.2 kb in size. At both ends of the genome there are identical regions, called long terminal repeats, which contain the regulation and expression genes of HIV. The remainder of the genome includes three major sections: the GAG region, which encodes the viral core proteins (p24 [capsid protein: CA], p17 [matrix protein: MA], p9, and p6, which are derived from the precursor p55); the POL region, which encodes the viral enzymes (i.e., reverse transcriptase [p51], protease [p10], and integrase [p32]); and the ENV region, which encodes the viral envelope proteins (gp120 and gp41, which are derived from the precursor gp160). Other regulatory proteins, such as transactivator of transcription (tat: p14), regulator of virion (rev: p19), negative regulatory factor (nef: p27), viral protein r (vpr: p15), viral infectivity factor (vif: p23), viral protein u (vpu in HIV-1: P16), and viral protein x (vpx in HIV-2: P15), are involved in transactivation, viral messenger RNA expression, viral replication, induction of cell cycle arrest, promotion of nuclear import of viral reverse transcription complexes, downregulation of the CD4 receptors and class I major histocompatibility complex, proviral DNA synthesis, and virus release and infectivity (Fig. 302.2 ).
The HIV tropism to the target cell is determined by its envelope glycoprotein (Env). Env consists of two components, namely, the surface, heavily glycosylated subunit, gp120 protein and the associated transmembrane subunit glycoprotein gp41. Both gp120 and gp41 are produced from the precursor protein gp160. The glycoprotein gp41 is very immunogenic and is used to detect HIV-1 antibodies in diagnostic assays; gp120 is a complex molecule that includes the highly variable V3 loop. This region is immunodominant for neutralizing antibodies. The heterogeneity of gp120 presents major obstacles in establishing an effective HIV vaccine. The gp120 glycoprotein also carries the binding site for the CD4 molecule, the most common host cell surface receptor of T lymphocytes. This tropism for CD4+ T cells is beneficial to the virus because of the resulting reduction in the effectiveness of the host immune system. Other CD4-bearing cells include macrophages and microglial cells. The observations that CD4− cells are also infected by HIV and that some CD4+ T cells are resistant to such infections suggests that other cellular attachment sites are needed for the interaction between HIV and human cells. Several chemokines serve as coreceptors for the envelope glycoproteins, permitting membrane fusion and entry into the cell. Most HIV strains have a specific tropism for one of the chemokines, including the fusion-inducing molecule CXCR-4, which acts as a coreceptor for HIV attachment to lymphocytes, and CCR-5, a β chemokine receptor that facilitates HIV entry into macrophages. Several other chemokine receptors (CCR-3) have also been shown in vitro to serve as virus coreceptors. Other mechanisms of attachment of HIV to cells use nonneutralizing antiviral antibodies and complement receptors. The Fab portion of these antibodies attaches to the virus surface, and the Fc portion binds to cells that express Fc receptors (macrophages, fibroblasts), thus facilitating virus transfer into the cell. Other cell-surface receptors, such as the mannose-binding protein on macrophages or the DC-specific, C-type lectin (DC-SIGN) on dendritic cells, also bind to the HIV-1 envelope glycoprotein and increase the efficiency of viral infectivity. Cell-to-cell transfer of HIV without formation of fully formed particles is a more rapid mechanism of spreading the infection to new cells than is direct infection by the virus.
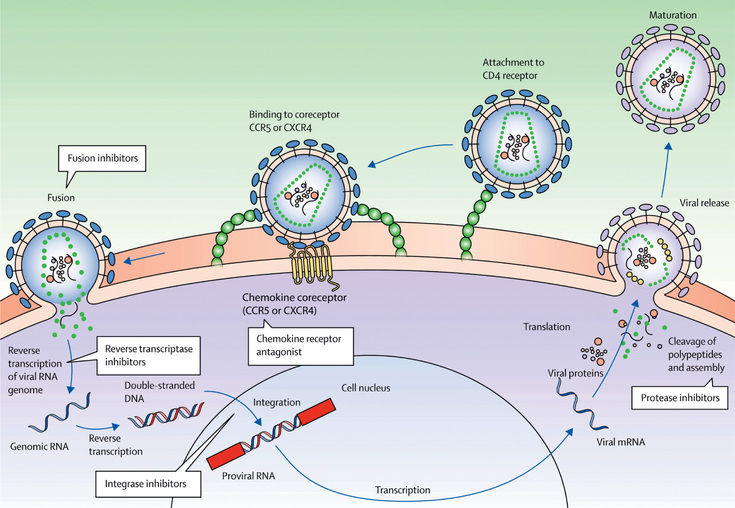
Following viral attachment, gp120 and the CD4 molecule undergo conformational changes, and gp41 interacts with the fusion receptor on the cell surface (Fig. 302.3 ). Viral fusion with the cell membrane allows entry of viral RNA into the cell cytoplasm. This process involves accessory viral proteins (nef, vif) and binding of cyclophilin A (a host cellular protein) to the capsid protein (p24). The p24 protein is involved in virus uncoating, recognition by restriction factors, and nuclear importation and integration of the newly created viral DNA. Viral DNA copies are then transcribed from the virion RNA through viral reverse transcriptase enzyme activity, which builds the first DNA strand from the viral RNA and then destroys the viral RNA and builds a second DNA strand to produce double-stranded circular DNA. The HIV-1 reverse transcriptase is error prone and lacks error-correcting mechanisms. Thus, many mutations arise, creating a wide genetic variation in HIV-1 isolates even within an individual patient. Many of the drugs used to fight HIV infection were designed to block the reverse transcriptase action. The circular DNA is transported into the cell nucleus, using viral accessory proteins such as vpr, where it is integrated (with the help of the virus integrase) into the host chromosomal DNA and referred to as the provirus. The provirus has the advantage of latency, because it can remain dormant for extended periods, making it extremely difficult to eradicate. The infected CD4+ T cells that survive long enough to revert to resting memory state become the HIV latent reservoir where the virus persists indefinitely even in patients who respond favorably to potent antiretroviral therapy. The molecular mechanisms of this latency are complex and involve unique biologic properties of the latent provirus (e.g., absence of tat, epigenetic changes inhibiting HIV gene expression) and the nature of the cellular host (e.g., absence of transcription factors such as nuclear factor κB). Integration usually occurs near active genes, which allow a high level of viral production in response to various external factors such as an increase in inflammatory cytokines (by infection with other pathogens) and cellular activation. Anti-HIV drugs that block the integrase enzyme activity have been developed. Depending on the relative expression of the viral regulatory genes (tat, rev, nef), the proviral DNA may encode production of the viral RNA genome, which, in turn, leads to production of viral proteins necessary for viral assembly.
HIV-1 transcription is followed by translation. A capsid polyprotein is cleaved to produce the virus-specific protease (p10), among other products. This enzyme is critical for HIV-1 assembly because it cleaves the long polyproteins into the proper functional pieces. Several HIV-1 antiprotease drugs have been developed, targeting the increased sensitivity of the viral protease, which differs from the cellular proteases. The regulatory protein vif is active in virus assembly and Gag processing. The RNA genome is then incorporated into the newly formed viral capsid that requires zinc finger domains (p7) and the matrix protein (MA: p17). The matrix protein forms a coat on the inner surface of the viral membrane, which is essential for the budding of the new virus from the host cell's surface. As new virus is formed, it buds through specialized membrane areas, known as lipid rafts, and is released. The virus release is facilitated by the viroporin vpu, which induces rapid degradation of newly synthesized CD4 molecules that impede viral budding. In addition, vpu counteracts host innate immunity (e.g., hampering natural killer T-cell activity).
Full-length sequencing of the HIV-1 genome demonstrated three different groups (M [main], O [outlier], and N [non-M, non-O]), probably occurring from multiple zoonotic infections from primates in different geographic regions. The same technique identified eight groups of HIV-2 isolates. Group M diversified to nine subtypes (or clades A to D, F to H, J, and K). In each region of the world, certain clades predominate, for example, clade A in Central Africa, clade B in the United States and South America, clade C in South Africa, clade E in Thailand, and clade F in Brazil. Although some subtypes were identified within group O, none was found in any of the HIV-2 groups. Clades are mixed in some patients as a result of HIV recombination, and some crossing between groups (i.e., M and O) has been reported.
HIV-2 has a similar life cycle to HIV-1 and is known to cause infection in several monkey species. Subtypes A and B are the major causes of infection in humans, but rarely cause infection in children. HIV-2 differs from HIV-1 in its accessory genes (e.g., it has no vpu gene but contains the vpx gene, which is not found in HIV-1). It is most prevalent in western Africa, but increasing numbers of cases are reported from Europe and southern Asia. The diagnosis of HIV-2 infection is more difficult because of major differences in the genetic sequences between HIV-1 and HIV-2. Thus, several of the standard confirmatory assays (immunoblot), which are HIV-1 specific, may give indeterminate results with HIV-2 infection. If HIV-2 infection is suspected, a combination screening test that detects antibody to HIV-1 and HIV-2 peptides should be used. In addition, the rapid HIV detection tests have been less reliable in patients suspected to be dually infected with HIV-1 and HIV-2, because of lower antibody concentrations against HIV-2. HIV-2 viral loads also have limited availability. Notably, HIV-2 infection demonstrates a longer asymptomatic stage of infection and slower declines of CD4+ T-cell counts than HIV-1, as well as is less efficiently transmitted from mother to child, likely related to lower levels of viremia with HIV-2.
Epidemiology
In 2015, the World Health Organization (WHO) estimated that 1.8 million children younger than 15 yr of age worldwide were living with HIV-1 infection; the 150,000 new infections annually in children was a 70% reduction since 2000. Approximately 80% of new infections in this age-group occur in sub-Saharan Africa. These trends reflect the slow but steady expansion of services to prevent perinatal transmission of HIV to infants. Notably, there are still 110,000 deaths worldwide of children < 15 yr of age with HIV. Unfortunately, through 2016, an estimated 16.5 million children have been orphaned by AIDS, defined as having one or both parents die from AIDS.
Globally, the vast majority of HIV infections in childhood are the result of vertical transmission from an HIV-infected mother. In the United States, approximately 11,700 children, adolescents, or young adults were reported to be living with perinatally acquired HIV infection in 2014. The number of U.S. children with AIDS diagnosed each year increased from 1984 to 1992 but then declined by more than 95% to < 100 cases annually by 2003, largely from the success of prenatal screening and perinatal antiretroviral treatment of HIV-infected mothers and infants. From 2009 to 2013, there were 497 infants born with perinatally acquired HIV in the United States and Puerto Rico. Children of racial and ethnic minority groups are disproportionately overrepresented, particularly non-Hispanic African-Americans and Hispanics. Race and ethnicity are not risk factors for HIV infection but more likely reflect other social factors that may be predictive of an increased risk for HIV infection, such as lack of educational and economic opportunities. As of 2014, New York, Florida, Texas, Georgia, Illinois, and California are the states with the highest numbers of perinatally acquired cases of HIV in the United States.
Adolescents (13-24 yr of age) constitute an important growing population of newly infected individuals; in 2015, 22% of all new HIV infections occurred in this age-group, with 81% of youth cases occurring in young males who have sex with males (MSM); 8% of cases of AIDS also occurred in this age-group. Targeted efforts have decreased new cases by 18% among youth MSM from 2008 to 2014. It is estimated than 50% of HIV-positive youth are unaware of their diagnosis, the highest of any age-group. Considering the long latency period between the time of infection and the development of clinical symptoms, reliance on AIDS case definition surveillance data significantly underrepresents the impact of the disease in adolescents. Based on a median incubation period of 8-12 yr, it is estimated that 15–20% of all AIDS cases were acquired between 13 and 19 yr of age.
Risk factors for HIV infection vary by gender in adolescents. For example, 91–93% of males between the ages of 13 and 24 yr with HIV acquire infection through sex with males. In contrast, 91–93% of adolescent females with HIV are infected through heterosexual contact. Adolescent racial and ethnic minority populations are overrepresented, especially among females.
Transmission
Transmission of HIV-1 occurs via sexual contact, parenteral exposure to blood, or vertical transmission from mother to child via exposure to vaginal secretions during birth or via breast milk. The primary route of infection in the pediatric population (<15 yr) is vertical transmission. Rates of transmission of HIV from mother to child have varied in high- and low-resource countries; the United States and Europe have documented transmission rates in untreated women of between 12% and 30%, whereas transmission rates in Africa and Haiti have been higher (25–52%), likely because of more advanced maternal disease and the presence of coinfections. Perinatal treatment of HIV-infected pregnant women with antiretroviral drugs has dramatically decreased the rate to < 2%.
Vertical transmission of HIV can occur before delivery (intrauterine), during delivery (intrapartum), or after delivery (postpartum through breastfeeding). Although intrauterine transmission has been suggested by identification of HIV by culture or polymerase chain reaction (PCR) in fetal tissue as early as 10 wk, statistical modeling data suggest that the majority of in utero transmissions likely occur in late gestation, when the vascular integrity of the placenta weakens and microtransfusions across the maternal–fetal circulation occur. It is generally accepted that 20–30% of infected newborns are infected in utero, because this percentage of infants has laboratory evidence of infection (positive viral culture or PCR) within the first week of life. Some studies have found that viral detection soon after birth is also correlated with an early onset of symptoms and rapid progression to AIDS, consistent with more long-standing infection during gestation.
A higher percentage of HIV-infected children acquire the virus intrapartum, evidenced by the fact that 70–80% of infected infants do not demonstrate detectable virus until after 1 wk of age. The mechanism of transmission appears to be mucosal exposure to infected blood and cervicovaginal secretions in the birth canal, and intrauterine contractions during active labor/delivery could also increase the risk of late microtransfusions. Breastfeeding is the least-common route of vertical transmission in high resource nations, but is responsible for as much as 40% of perinatal infections in resource-limited countries. Both free and cell-associated viruses have been detected in breast milk from HIV-infected mothers. The risk for transmission through breastfeeding is approximately 9–16% in women with established infection, but is 29–53% in women who acquire HIV postnatally, suggesting that the viremia experienced by the mother during primary infection at least triples the risk for transmission. Where replacement feeding is readily available and safe, it seems reasonable for women to substitute infant formula for breast milk if they are known to be HIV infected or are at risk for ongoing sexual or parenteral exposure to HIV. However, the WHO recommends that in low-resource countries where other diseases (diarrhea, pneumonia, malnutrition) substantially contribute to a high infant mortality rate, the benefit of breastfeeding outweighs the risk for HIV transmission, and HIV-infected women in developing countries should exclusively breastfeed their infants for at least the first 6 mo of life (see Prevention later in this chapter).
Several risk factors influence the rate of vertical transmission: maternal viral load at delivery, preterm delivery (<34 wk gestation), and low maternal antenatal CD4 count. The most important variable appears to be the level of maternal viremia; the odds of transmission may be increased more than two-fold for every log10 increase in viral load at delivery. Elective cesarean delivery was shown to decrease transmission by 87% if used in conjunction with zidovudine therapy in the mother and infant. However, because these data predated the advent of combined antiretroviral therapy (cART, also called HAART), the additional benefit of cesarean section appears to be negligible if the mother's viral load is < 1,000 copies/mL. It should be noted that rarely (≤0.1%), transmission may occur with maternal viral loads < 50 copies/mL.
Transfusions of infected blood or blood products have accounted for 3–6% of all pediatric AIDS cases. The period of highest risk was between 1978 and 1985, before the availability of HIV antibody–screened blood products. Whereas the prevalence of HIV infection in individuals with hemophilia treated before 1985 was as high as 70%, heat treatment of factor VIII concentrate and HIV antibody screening of donors has virtually eliminated HIV transmission in this population. Donor screening has dramatically reduced, but not eliminated, the risk for blood transfusion–associated HIV infection: nucleic acid amplification testing of minipools (pools of 16-24 donations) performed on antibody-nonreactive blood donations (to identify donations made during the window period before seroconversion) reduced the residual risk of transfusion-transmitted HIV-1 to approximately 1 in 2 million blood units. However, in many resource-limited countries, screening of blood is not uniform, and the risk for transmitting HIV infection via transfusion remains in these settings.
Although HIV can be isolated rarely from saliva, it is in very low titers (<1 infectious particle/mL) and has not been implicated as a transmission vehicle. Studies of hundreds of household contacts of HIV-infected individuals have found that the risk for household HIV transmission is essentially nonexistent. Only a few cases have been reported in which urine or feces (possibly devoid of visible blood) have been proposed as a possible vehicle of HIV transmission, though these cases have not been fully verified.
In the pediatric population, sexual transmission is infrequent, but a small number of cases resulting from sexual abuse have been reported. Sexual contact is a major route of transmission in the adolescent population, accounting for most of the cases.
Pathogenesis
HIV infection affects most of the immune system and disrupts its homeostasis (see Fig. 302.3 ). In most cases, the initial infection is caused by low amounts of a single virus. Therefore, disease may be prevented by prophylactic drug(s) or vaccine. When the mucosa serves as the portal of entry for HIV, the first cells to be affected are the dendritic cells. These cells collect and process antigens introduced from the periphery and transport them to the lymphoid tissue. HIV does not infect the dendritic cell but binds to its DC-SIGN surface molecule, allowing the virus to survive until it reaches the lymphatic tissue. In the lymphatic tissue (e.g., lamina propria, lymph nodes), the virus selectively binds to cells expressing CD4 molecules on their surface, primarily helper T lymphocytes (CD4+ T cells) and cells of the monocyte-macrophage lineage. Other cells bearing CD4, such as microglia, astrocytes, oligodendroglia, and placental tissue containing villous Hofbauer cells, may also be infected by HIV. Additional factors (coreceptors) are necessary for HIV fusion and entry into cells. These factors include the chemokines CXCR4 (fusion) and CCR5. Other chemokines (CCR1, CCR3) may be necessary for the fusion of certain HIV strains. Several host genetic determinants affect the susceptibility to HIV infection, the progression of disease, and the response to treatment. These genetic variants vary in different populations. A deletion in the CCR5 gene that is protective against HIV infection (CCR5Δ32) is relatively common in whites but is rare in individuals of African descent. Several other genes that regulate chemokine receptors, ligands, the histocompatibility complex, and cytokines also influence the outcome of HIV infection. Usually, CD4+ lymphocytes migrate to the lymphatic tissue in response to viral antigens and then become activated and proliferate, making them highly susceptible to HIV infection. This antigen-driven migration and accumulation of CD4 cells within the lymphoid tissue may contribute to the generalized lymphadenopathy characteristic of the acute retroviral syndrome in adults and adolescents. HIV preferentially infects the very cells that respond to it (HIV-specific memory CD4 cells), accounting for the progressive loss of these cells and the subsequent loss of control of HIV replication. The continued destruction of memory CD4+ cells in the gastrointestinal tract (in the gut-associated lymphoid tissue or GALT) leads to reduced integrity of the gastrointestinal epithelium followed by leakage of bacterial particles into the blood and increased inflammatory response, which cause further CD4+ cell loss. When HIV replication reaches a threshold (usually within 3-6 wk from the time of infection), a burst of plasma viremia occurs. This intense viremia causes acute HIV infection, formerly known as acute retroviral syndrome which can present similar to the flu or mononucleosis (fever, rash, pharyngitis, lymphadenopathy, malaise, arthralgia, fatigue, elevated liver enzymes) in 50–70% of infected adults. With establishment of a cellular and humoral immune response within 2-4 mo, the viral load in the blood declines substantially, and patients enter a phase characterized by a lack of symptoms and a return of CD4 cells to only moderately decreased levels. Typically, adult patients who are not treated eventually progress to achieve a virologic set point (steady state), usually ranging from 10,000-100,000 during this clinical latency. This is in contrast to untreated infants with vertically acquired HIV who can achieve viral loads that are much higher, resulting in faster CD4 count declines and earlier onset of significant immunodeficiency. HIV rapidly responds to the immune system pressure by developing a genetically complex population (quasispecies) that successfully evades it. In addition, inappropriate use of antiretroviral treatment increases the ability of the virus to diverge even further by selecting for mutants with fitness or resistance advantages in the presence of subtherapeutic drug levels. Early HIV-1 replication in children has no apparent clinical manifestations. Whether tested by virus isolation or by PCR for viral nucleic acid sequences, fewer than 40% of HIV-1–infected infants demonstrate evidence of the virus at birth. The viral load increases by 1-4 mo, and essentially all perinatally HIV-infected infants have detectable HIV-1 in peripheral blood by 4 mo of age, except for those who may acquire infection via ongoing breast feeding.
In adults, the long period of clinical latency (8-12 yr) is not indicative of viral latency. In fact, there is a very high turnover of virus and CD4 lymphocytes (more than a billion cells per day), gradually causing deterioration of the immune system, marked by depletion of CD4 cells. Several mechanisms for the depletion of CD4 cells in adults and children have been suggested, including HIV-mediated single cell killing, formation of multinucleated giant cells of infected and uninfected CD4 cells (syncytia formation), virus-specific immune responses (natural killer cells, antibody-dependent cellular cytotoxicity), superantigen-mediated activation of T cells (rendering them more susceptible to infection with HIV), autoimmunity, and programmed cell death (apoptosis). The viral burden is greater in the lymphoid organs than in the peripheral blood during the asymptomatic period. As HIV virions and their immune complexes migrate through the lymph nodes, they are trapped in the network of dendritic follicular cells. Because the ability of HIV to replicate in T cells depends on the state of activation of the cells, the immune activation that takes place within the microenvironment of the lymph nodes in HIV disease serves to promote infection of new CD4 cells, as well as subsequent viral replication within these cells. Monocytes and macrophages can be productively infected by HIV yet resist the cytopathic effect of the virus and, with their long lifespan, explain their role as reservoirs of HIV and as effectors of tissue damage in organs such as the brain. In addition, they reside in anatomic viral sanctuaries where current treatment agents are less effective.
The innate immune system responds almost immediately following HIV infection by recognizing the viral nucleic acids, once the virus fuses to the infected cell, by the toll-like receptor 7. This engagement leads to activation of proinflammatory cytokines and interferon (IFN-α), which blocks virus replication and spread. The virus uses its Nef protein to downregulate the expression of major histocompatibility complex (MHC) and non-MHC ligands to reduce the natural killer (NK) cell–mediated anti-HIV activity. It also modulates NK cell differentiation and maturation, dysregulates cytokine production, and increases apoptosis. Although the mechanism by which the innate system triggers the adaptive immune responses is not yet fully understood, cell-mediated and humoral responses occur early in the infection. CD8 T cells play an important role in containing the infection. These cells produce various ligands (macrophage inflammatory proteins 1α and 1β, RANTES), which suppress HIV replication by blocking the binding of the virus to the coreceptors (CCR5). HIV-specific cytotoxic T lymphocytes (CTLs) develop against both the structural (ENV, POL, GAG) and regulatory (tat) viral proteins. The CTLs appear at the end of the acute infection, as viral replication is controlled by killing HIV-infected cells before new viruses are produced and by secreting potent antiviral factors that compete with the virus for its receptors (CCR5). Neutralizing antibodies appear later in the infection and seem to help in the continued suppression of viral replication during clinical latency. There are at least two possible mechanisms that control the steady-state viral load level during the chronic clinical latency. One mechanism may be the limited availability of activated CD4 cells, which prevent a further increase in the viral load. The other mechanism is the development of an active immune response, which is influenced by the amount of viral antigen and limits viral replication at a steady state. There is no general consensus about which of these two mechanisms is more important. The CD4 cell limitation mechanism accounts for the effect of antiretroviral therapy, whereas the immune response mechanism emphasizes the importance of immune modulation treatment (cytokines, vaccines) to increase the efficiency of immune-mediated control. A group of cytokines that includes tumor necrosis factor TNF-α, TNF-β, interleukin IL-1, IL-2, IL-3, IL-6, IL-8, IL-12, IL-15, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor plays an integral role in upregulating HIV expression from a state of quiescent infection to active viral replication. Other cytokines such as IFN-γ, IFN-β, and IL-13 exert a suppressive effect on HIV replication. Certain cytokines (IL-4, IL-10, IFN-γ, transforming growth factor-β) reduce or enhance viral replication depending on the infected cell type. The interactions among these cytokines influence the concentration of viral particles in the tissues. Plasma concentrations of cytokines need not be elevated for them to exert their effect, because they are produced and act locally in the tissues. The activation of virtually all the cellular components of the immune system (i.e., T and B cells, NK cells, and monocytes) plays a significant role in the pathologic aspects of HIV infection. Further understanding of their interactions during the infection will expand our treatment options. Commonly, HIV isolated during the clinical latency period grows slowly in culture and produces low titers of reverse transcriptase. These isolates from earlier in clinical latency use CCR5 as their coreceptor. By the late stages of clinical latency, the isolated virus is phenotypically different. It grows rapidly and to high titers in culture and uses CXCR4 as its coreceptor. The switch from CCR5 receptor to CXCR4 receptor increases the capacity of the virus to replicate, to infect a broader range of target cells (CXCR4 is more widely expressed on resting and activated immune cells), and to kill T cells more rapidly and efficiently. As a result, the clinical latency phase is over and progression toward AIDS is noted. The progression of disease is related temporally to the gradual disruption of lymph node architecture and degeneration of the follicular dendritic cell network with loss of its ability to trap HIV particles. The virus is freed to recirculate, producing high levels of viremia and an increased disappearance of CD4 T cells during the later stages of disease.
The clinical course of HIV infection shows substantial heterogeneity. This variation is determined by both viral and host factors. HIV viruses that use coreceptor CXCR4 in the course of the infection are associated with an accelerated deterioration of the immune system and more rapid progression to AIDS. In addition, several known host genetic determinants (e.g., variants in the human leukocyte antigen region, polymorphisms in the CCR5 region such as CCR5Δ32) were already identified as affecting the disease course. There are likely additional host and viral factors yet to be identified that contribute to the variable course of HIV infection in individuals, as well. Three distinct patterns of disease are described in children. Approximately 15–25% of HIV-infected newborns in developed countries present with a rapid progression course, with onset of AIDS and symptoms during the first few months of life and a median survival time of 6-9 mo if untreated. In resource-limited countries, the majority of HIV-infected newborns will have this rapidly progressing disease course. It has been suggested that if intrauterine infection coincides with the period of rapid expansion of CD4 cells in the fetus, the virus could effectively infect the majority of the body's immunocompetent cells. The normal migration of these cells to the marrow, spleen, and thymus would result in efficient systemic delivery of HIV, unchecked by the immature immune system of the fetus. Thus, infection would be established before the normal ontogenic development of the immune system, causing more-severe impairment of immunity. Most children in this group have detectable virus in the plasma (median level: 11,000 copies/mL) in the first 48 hr of life. This early evidence of viral presence suggests that the newborn was infected in utero. The viral load rapidly increases, peaking by 2-3 mo of age (median: 750,000 copies/mL) and staying high for at least the first 2 yr of life.
Sixty percent to 80% of perinatally infected newborns in high resource countries present with a much slower progression of disease, with a median survival time of 6 yr representing the second pattern of disease. Many patients in this group have a negative PCR in the first week of life and are therefore considered to be infected intrapartum. In a typical patient, the viral load rapidly increases, peaking by 2-3 mo of age (median: 100,000 copies/mL) and then slowly declines over a period of 24 mo. The slow decline in viral load is in sharp contrast to the rapid decline after primary infection seen in adults. This observation can be explained only partially by the immaturity of the immune system in newborns and infants.
The third pattern of disease occurs in < 5% of perinatally infected children, referred to as long-term survivors or long-term nonprogressors, who have minimal or no progression of disease with relatively normal CD4 counts and very low viral loads for longer than 8 yr. Mechanisms for the delay in disease progression include effective humoral immunity and/or CTL responses, host genetic factors (e.g., human leukocyte antigen profile), and infection with an attenuated (defective-gene) virus. A subgroup of the long-term survivors called elite survivors or elite suppressors has no detectable virus in the blood and may reflect different or greater mechanisms of protection from disease progression. Note that both groups warrant long-term close follow-up because later in their course they may begin to progress with their disease.
HIV-infected children have changes in the immune system that are similar to those in HIV-infected adults. Absolute CD4 cell depletion may be less dramatic because infants normally have a relative lymphocytosis. A value of 750 CD4 cells/µL in children younger than 1 yr of age is indicative of severe CD4 depletion and is comparable to < 200 CD4 cells/µL in adults. Lymphopenia is relatively rare in perinatally infected children and is usually only seen in older children or those with end-stage disease. Although cutaneous anergy is common during HIV infection, it is also frequent in healthy children younger than 1 yr of age, and thus its interpretation is difficult in infected infants. The depletion of CD4 cells also decreases the response to soluble antigens such as the in vitro mitogens phytohemagglutinin and concanavalin A.
Polyclonal activation of B cells occurs in most children early in the infection, as evidenced by elevation of immunoglobulins IgA, IgM, IgE, and, particularly, IgG (hypergammaglobulinemia), with high levels of anti–HIV-1 antibody. This response may reflect both dysregulation of the T-cell suppression of B-cell antibody synthesis and active CD4 enhancement of the B-lymphocyte humoral response. As a result, the antibody response to routine childhood vaccinations may be abnormal. The B-cell dysregulation precedes the CD4 depletion in many children and may serve as a surrogate marker of HIV infection in symptomatic children in whom specific diagnostic tests (PCR, culture) are not available or are too expensive. Despite the increased levels of immunoglobulins, some children lack specific antibodies or protective antibodies. Hypogammaglobulinemia is very rare (<1%).
Central nervous system (CNS) involvement is more common in pediatric patients than in adults. Macrophages and microglia play an important role in HIV neuropathogenesis, and data suggest that astrocytes may also be involved. Although the specific mechanisms for encephalopathy in children are not yet clear, the developing brain in young infants is affected by at least two mechanisms. The virus itself may directly infect various brain cells or cause indirect damage to the nervous system by the release of cytokines (IL-1α, IL-1β, TNF-α, IL-2) or reactive oxygen damage from HIV-infected lymphocytes or macrophages.
Clinical Manifestations
The clinical manifestations of HIV infection vary widely among infants, children, and adolescents. In most infants, physical examination at birth is normal. Initial symptoms may be subtle, such as lymphadenopathy and hepatosplenomegaly, or nonspecific, such as failure to thrive, chronic or recurrent diarrhea, respiratory symptoms, or oral thrush and may be distinguishable only by their persistence. Whereas systemic and pulmonary findings are common in the United States and Europe, chronic diarrhea, pneumonia, wasting, and severe malnutrition predominate in Africa. Clinical manifestations found more commonly in children than adults with HIV infection include recurrent bacterial infections, chronic parotid swelling, lymphocytic interstitial pneumonitis (LIP), and early onset of progressive neurologic deterioration; note that chronic parotid swelling and LIP are associated with a slower progression of disease.
The CDC Surveillance Case Definition for HIV infection is based on the age-specific CD4+ T-lymphocyte count or the CD4+ T-lymphocyte percentage of total lymphocytes (Table 302.1 ), except when a stage 3–defining opportunistic illness (Table 302.2 ) supersedes the CD4 data. Age adjustment of the absolute CD4 count is necessary because counts that are relatively high in normal infants decline steadily until age 6 yr, when they reach adult norms. The CD4 count takes precedence over the CD4 T-lymphocyte percentage, and the percentage is considered only if the count is unavailable.
Table 302.1
HIV Infection Stage* Based on Age-Specific CD4+ T-Lymphocyte Count or CD4+ T-Lymphocyte Percentage of Total Lymphocytes
| STAGE | AGE ON DATE OF CD4+ T-LYMPHOCYTE TEST | |||||
|---|---|---|---|---|---|---|
| <1 Yr | 1-5 Yr | ≥6 Yr | ||||
| CELLS/µL | % | CELLS/µL | % | CELLS/µL | % | |
| 1 | ≥1,500 | ≥34 | ≥1,000 | ≥30 | ≥500 | ≥26 |
| 2 | 750-1,499 | 26-33 | 500-999 | 22-29 | 200-499 | 14-25 |
| 3 | <750 | <26 | <500 | <22 | <200 | <14 |
* Stage is based primarily on the CD4+ T-lymphocyte count. The CD4+ T-lymphocyte count takes precedence over the CD4+ T-lymphocyte percentage, and the percentage is considered only if the count is missing.
From Centers for Disease Control and Prevention: Revised surveillance case definition for HIV infection—United States, 2014, MMWR 63(No RR-3):1-10, 2014.
Table 302.2
Stage 3–Defining Opportunistic Illnesses in HIV Infection
|
Bacterial infections, multiple or recurrent* Candidiasis of bronchi, trachea, or lungs Cervical cancer, invasive † Coccidioidomycosis, disseminated or extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis, chronic intestinal (>1 mo duration) Cytomegalovirus disease (other than liver, spleen, or nodes), onset at age > 1 mo Cytomegalovirus retinitis (with loss of vision) Encephalopathy attributed to HIV ‡ Herpes simplex: chronic ulcers (>1 mo duration) or bronchitis, pneumonitis, or esophagitis (onset at age > 1 mo) Histoplasmosis, disseminated or extrapulmonary Isosporiasis, chronic intestinal (>1 mo duration) Lymphoma, Burkitt (or equivalent term) Lymphoma, immunoblastic (or equivalent term) Mycobacterium avium complex or Mycobacterium kansasii, disseminated or extrapulmonary Mycobacterium tuberculosis of any site, pulmonary, † disseminated, or extrapulmonary Mycobacterium, other species or unidentified species, disseminated or extrapulmonary Pneumocystis jiroveci (previously known as Pneumocystis carinii ) pneumonia Pneumonia, recurrent † Progressive multifocal leukoencephalopathy Salmonella septicemia, recurrent Toxoplasmosis of brain, onset at age > 1 mo Wasting syndrome attributed to HIV ‡ |
* Only among children aged < 6 yr.
† Only among adults, adolescents, and children aged ≥ 6 yr.
‡ Suggested diagnostic criteria for these illnesses, which might be particularly important for HIV encephalopathy and HIV wasting syndrome, are described in the following references: Centers for Disease Control and Prevention: 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age, MMWR 43(No. RR-12), 1994; Centers for Disease Control and Prevention: 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults, MMWR 41(No. RR-17), 1992.
From Centers for Disease Control and Prevention: Revised surveillance case definition for HIV infection—United States, 2014, MMWR 63(No RR-3):1-10, 2014.
Infections
Approximately 20% of AIDS-defining illnesses in children are recurrent bacterial infections caused primarily by encapsulated organisms such as Streptococcus pneumoniae and Salmonella as a result of disturbances in humoral immunity. Other pathogens, including Staphylococcus, Enterococcus, Pseudomonas aeruginosa, and Haemophilus influenzae, and other Gram-positive and Gram-negative organisms may also be seen. The most common serious infections in HIV-infected children are bacteremia, sepsis, and bacterial pneumonia, accounting for more than 50% of infections in these patients. Meningitis, urinary tract infections, deep-seated abscesses, and bone/joint infections occur less frequently. Milder recurrent infections, such as otitis media, sinusitis, and skin and soft tissue infections, are very common and may be chronic with atypical presentations.
Opportunistic infections are generally seen in children with severe depression of the CD4 count. In adults, these infections often represent reactivation of a latent infection acquired early in life. In contrast, young children generally have primary infection and often have a more fulminant course of disease reflecting the lack of prior immunity. In addition, infants < 1 yr of age have a higher incidence of developing stage 3–defining opportunistic infections and mortality rates compared with older children and adults even at higher CD4 counts, reflecting that the CD4 count may overpredict the immune competence in young infants. This principle is best illustrated by Pneumocystis jiroveci (formerly Pneumocystis carinii ) pneumonia, the most common opportunistic infection in the pediatric population (see Chapter 271 ). The peak incidence of Pneumocystis pneumonia occurs at age 3-6 mo in the setting of undiagnosed perinatally acquired disease, with the highest mortality rate in children younger than 1 yr of age. Aggressive approaches to treatment have improved the outcome substantially. Although the overall incidence of opportunistic infections has markedly declined since the era of combination antiretroviral therapy, opportunistic infections still occur in patients with severe immunodepletion as the result of unchecked viral replication, which often accompanies poor antiretroviral therapy adherence.
The classic clinical presentation of Pneumocystis pneumonia includes an acute onset of fever, tachypnea, dyspnea, and marked hypoxemia; in some children, more indolent development of hypoxemia may precede other clinical or x-ray manifestations. In some cases, fever may be absent or low grade, particularly in more indolent cases. Chest x-ray findings most commonly consist of interstitial infiltrates or diffuse alveolar disease, which rapidly progresses. Chest x-ray in some cases can have very subtle findings and can mimic the radiologic appearance of viral bronchiolitis. Nodular lesions, streaky or lobar infiltrates, or pleural effusions may occasionally be seen. The diagnosis is established by demonstration of P. jiroveci with appropriate staining of induced sputum or bronchoalveolar fluid lavage; rarely, an open lung biopsy is necessary. Bronchoalveolar lavage and open lung biopsy have significantly improved sensitivity (75–95%) for Pneumocystis testing than induced sputum (20–40%), such that if an induced sputum is negative it does not exclude the diagnosis. PCR testing on respiratory specimens is also available and is more sensitive than microscopy but also has less specificity; it is also not widely available.
The first-line therapy for Pneumocystis pneumonia is trimethoprim-sulfamethoxazole (TMP-SMX) (15-20 mg/kg/day of the TMP component divided every 6 hr intravenously) with adjunctive corticosteroids for moderate to severe disease, usually defined as if the PaO 2 is < 70 mm Hg while breathing room air. After improvement, therapy with oral TMP-SMX should continue for a total of 21 days while the corticosteroids are weaned. An alternative therapy for Pneumocystis pneumonia includes intravenous administration of pentamidine (4 mg/kg/day). Other regimens such as TMP plus dapsone, clindamycin plus primaquine, or atovaquone are used as alternatives in adults but have not been widely used in children to date.
Nontuberculous mycobacteria (NTM), with Mycobacterium avium-intracellulare complex (MAC) being most common, may cause disseminated disease in HIV-infected children who are severely immunosuppressed. The incidence of MAC infection in antiretroviral therapy–naïve children >6 yr with < 100 CD4 cells/µL is estimated to be as high as 10%, but effective cART that results in viral suppression makes MAC infections rare. Disseminated MAC infection is characterized by fever, malaise, weight loss, and night sweats; diarrhea, abdominal pain, and, rarely, intestinal perforation or jaundice (a result of biliary tract obstruction by lymphadenopathy) may also be present. Labs may be notable for significant anemia. The diagnosis is made by the isolation of MAC from blood, bone marrow, or tissue; the isolated presence of MAC in the stool does not confirm a diagnosis of disseminated MAC. Treatment can reduce symptoms and prolong life but is at best only capable of suppressing the infection if severe CD4 depletion persists. Therapy should include at least two drugs: clarithromycin or azithromycin and ethambutol. A third drug (rifabutin, rifampin, ciprofloxacin, levofloxacin, or amikacin) is generally added to decrease the emergence of drug-resistant isolates. Careful consideration of possible drug interactions with antiretroviral agents is necessary before initiation of disseminated MAC therapy. Drug susceptibilities should be ascertained, and the treatment regimen should be adjusted accordingly in the event of an inadequate clinical response to therapy. Because of the great potential for toxicity with most of these medications, surveillance for adverse effects should be ongoing. Less commonly, NTM infections can also be focal in these patients, including lymphadenitis, osteomyelitis, tenosynovitis, and pulmonary disease.
Oral candidiasis is the most common fungal infection seen in HIV-infected children. Oral nystatin suspension (2-5 mL qid) is often effective. Clotrimazole troches or fluconazole (3-6 mg/kg orally qd) are effective alternatives. Oral thrush progresses to involve the esophagus in as many as 20% of children with severe CD4 depletion, presenting with symptoms such as anorexia, dysphagia, vomiting, and fever. Treatment with oral fluconazole for 7-14 days generally results in rapid improvement in symptoms. Fungemia rarely occurs, usually in the setting of indwelling venous catheters, and up to 50% of cases may be caused by non–albicans species. Disseminated histoplasmosis, coccidioidomycosis, and cryptococcosis are rare in pediatric patients but may occur in endemic areas.
Parasitic infections such as intestinal cryptosporidiosis and microsporidiosis and rarely isosporiasis or giardiasis are other opportunistic infections that cause significant morbidity. Although these intestinal infections are usually self-limiting in healthy hosts, they cause severe chronic diarrhea in HIV-infected children with low CD4 counts, often leading to malnutrition. Nitazoxanide therapy is partially effective at improving cryptosporidia diarrhea, but immune reconstitution with cART is the most important factor for clearance of the infection. Albendazole has been reported to be effective against most microsporidia (excluding Enterocytozoon bieneusi ), and TMP-SMX appears to be effective for isosporiasis.
Viral infections, especially with the herpesvirus group, pose significant problems for HIV-infected children. HSV causes recurrent gingivostomatitis, which may be complicated by local and distant cutaneous dissemination. Primary varicella-zoster virus infection (chickenpox) may be prolonged and complicated by bacterial superinfections or visceral dissemination, including pneumonitis. Recurrent, atypical, or chronic episodes of herpes zoster are often debilitating and require prolonged therapy with acyclovir; in rare instances, varicella-zoster virus has developed a resistance to acyclovir, requiring the use of foscarnet. Disseminated cytomegalovirus infection occurs in the setting of severe CD4 depletion (<50 CD4 cells/µL for >6 yr) and may involve single or multiple organs. Retinitis, pneumonitis, esophagitis, gastritis with pyloric obstruction, hepatitis, colitis, and encephalitis have been reported, but these complications are rarely seen if cART is given. Ganciclovir and foscarnet are the drugs of choice and are often given together in children with sight-threatening cytomegalovirus retinitis. Intraocular injections of foscarnet or intraocular ganciclovir implants plus oral valganciclovir have also been efficacious in adults and older children with cytomegalovirus retinitis. Measles may occur despite immunization and may present without the typical rash. It often disseminates to the lung or brain with a high mortality rate in these patients. HIV-infected children with low CD4 counts can also develop extensive cutaneous molluscum contagiosum infection. Respiratory viruses such as respiratory syncytial virus and adenovirus may present with prolonged symptoms and persistent viral shedding. In parallel with the increased prevalence of genital tract human papillomavirus infection, cervical intraepithelial neoplasia and anal intraepithelial neoplasia also occur with increased frequency among HIV-1–infected adult women compared with HIV-seronegative women. The relative risk for cervical intraepithelial neoplasia is 5-10 times higher for HIV-1 seropositive women. Multiple modalities are used to treat human papillomavirus infection (see Chapter 293 ), although none is uniformly effective and the recurrence rate is high among HIV-1–infected persons.
Appropriate therapy with antiretroviral agents may result in immune reconstitution inflammatory syndrome (IRIS), which is characterized by an increased inflammatory response from the recovered immune system to subclinical opportunistic infections (e.g., Mycobacterium infection, HSV infection, toxoplasmosis, CMV infection, Pneumocystis infection, cryptococcal infection). This condition is more commonly observed in patients with progressive disease and severe CD4+ T-lymphocyte depletion. Patients with IRIS develop fever and worsening of the clinical manifestations of the opportunistic infection or new manifestations (e.g., enlargement of lymph nodes, pulmonary infiltrates), typically within the first few weeks after initiation of antiretroviral therapy. Determining whether the symptoms represent IRIS, worsening of a current infection, a new opportunistic infection, or drug toxicity is often very difficult. If the syndrome does represent IRIS, adding nonsteroidal antiinflammatory agents or corticosteroids may alleviate the inflammatory reaction, although the use of corticosteroids is controversial. The inflammation may take weeks or months to subside. In most cases, continuation of cART while treating the opportunistic infection (with or without antiinflammatory agents) is sufficient. If opportunistic infection is suspected prior to the initiation of antiretroviral therapy, appropriate antimicrobial treatment should be started first.
Central Nervous System
The incidence of CNS involvement in perinatally infected children is as high as 50–90% in resource-limited countries but significantly lower in high income countries, with a median onset at 19 mo of age. Manifestations may range from subtle developmental delay to progressive encephalopathy with loss or plateau of developmental milestones, cognitive deterioration, impaired brain growth resulting in acquired microcephaly, and symmetric motor dysfunction. Encephalopathy may be the initial manifestation of the disease or may present much later when severe immune suppression occurs. With progression, marked apathy, spasticity, hyperreflexia, and gait disturbance may occur, as well as loss of language and oral, fine, and/or gross motor skills. The encephalopathy may progress intermittently, with periods of deterioration followed by transiently stable plateaus. Older children may exhibit behavioral problems and learning disabilities. Associated abnormalities identified by neuroimaging techniques include cerebral atrophy in up to 85% of children with neurologic symptoms, increased ventricular size, basal ganglia calcifications, and, less frequently, leukomalacia.
Fortunately, since the advent of cART, the incident rate of encephalopathy has dramatically declined to as low as 0.08% in 2006. However, as HIV-infected children progress through adolescence and young adulthood, other subtle manifestations of CNS disease are evident, such as cognitive deficits, attention problems, and psychiatric disorders. Living with a chronic, often stigmatizing, disease; parental loss; and the requirement for lifelong pristine medication adherence compounds these issues, making it challenging for these youth as they inherit responsibility for managing their disease as adults.
Focal neurologic signs and seizures are unusual and may imply a comorbid pathologic process such as a CNS tumor, opportunistic infection, or stroke. CNS lymphoma may present with new-onset focal neurologic findings, headache, seizures, and mental status changes. Characteristic findings on neuroimaging studies include a hyperdense or isodense mass with variable contrast enhancement or a diffusely infiltrating contrast-enhancing mass. CNS toxoplasmosis is exceedingly rare in young infants but may occur in vertically HIV-infected adolescents and is typically associated with serum antitoxoplasma IgG as a marker of infection. Other opportunistic infections of the CNS are rare and include infection with CMV, JC virus (progressive multifocal leukoencephalopathy), HSV, Cryptococcus neoformans, and Coccidioides immitis. Although the true incidence of cerebrovascular disorders (both hemorrhagic and nonhemorrhagic strokes) is unclear, 6–10% of children from large clinical series have been affected.
Respiratory Tract
Recurrent upper respiratory tract infections such as otitis media and sinusitis are very common. Although the typical pathogens (S. pneumoniae, H. influenzae, Moraxella catarrhalis) are most common, unusual pathogens such as P. aeruginosa, yeast, and anaerobes may be present in chronic infections and result in complications such as invasive sinusitis and mastoiditis.
LIP (lymphocytic interstitial pneumonia) is the most common chronic lower respiratory tract abnormality reported to the Centers for Disease Control and Prevention (CDC) for HIV-infected children; historically this occurred in approximately 25% of HIV-infected children, although the incidence has declined in the cART era. LIP is a chronic process with nodular lymphoid hyperplasia in the bronchial and bronchiolar epithelium, often leading to progressive alveolar capillary block over months to years. It has a characteristic chronic diffuse reticulonodular pattern on chest radiography rarely accompanied by hilar lymphadenopathy, allowing a presumptive diagnosis to be made radiographically before the onset of symptoms. There is an insidious onset of tachypnea, cough, and mild to moderate hypoxemia with normal auscultatory findings or minimal rales. Progressive disease presents with symptomatic hypoxemia, which usually resolves with oral corticosteroid therapy, accompanied by digital clubbing. Several studies suggest that LIP is a lymphoproliferative response to a primary Epstein-Barr virus infection in the setting of HIV infection. It is also associated with a slower immunologic decline.
Most symptomatic HIV-infected children experience at least one episode of pneumonia during their disease. S. pneumoniae is the most common bacterial pathogen, but P. aeruginosa and other Gram-negative bacterial pneumonias may occur in end-stage disease and are often associated with acute respiratory failure and death. Rarely, severe recurrent bacterial pneumonia results in bronchiectasis. Pneumocystis pneumonia is the most common opportunistic infection, but other pathogens, including CMV, Aspergillus, Histoplasma, and Cryptococcus can cause pulmonary disease. Infection with common respiratory viruses, including respiratory syncytial virus, parainfluenza, influenza, and adenovirus, may occur simultaneously and have a protracted course and period of viral shedding from the respiratory tract. Pulmonary and extrapulmonary tuberculosis (TB) has been reported with increasing frequency in HIV-infected children in low-resource countries, although it is considerably more common in HIV-infected adults. Because of drug interactions between rifampin and ritonavir-based antiretroviral therapy and poor tolerability of the combination of multiple drugs required, treatment of TB/HIV coinfection is particularly challenging in children.
Cardiovascular System
Cardiac dysfunction, including left ventricular hypertrophy, left ventricular dilation, reduced left ventricular fractional shortening, and/or heart failure occurred in 18–39% of HIV-infected children in the pre-cART era; among those affected, a lower nadir CD4 percentage and a higher viral load were associated with lower cardiac function. However, a more current evaluation of HIV-infected children taking long-term cART found that echocardiographic findings were closer to normal and none had symptomatic heart disease, suggesting that cART has a cardioprotective effect. What is still unclear is whether an increased rate of premature cardiovascular disease that has been seen in adults will be seen in children who have disease- or treatment-related hyperlipidemia, and prospective studies will be needed to assess this risk. Because of this risk, regular monitoring of cholesterol and lipids, as well as education regarding a heart-healthy lifestyle, is an important part of pediatric HIV care.
Gastrointestinal and Hepatobiliary Tract
Oral manifestations of HIV disease include erythematous or pseudomembranous candidiasis, periodontal disease (e.g., ulcerative gingivitis or periodontitis), salivary gland disease (i.e., swelling, xerostomia), and, rarely, ulcerations or oral hairy leukoplakia. Gastrointestinal tract involvement is common in HIV-infected children. A variety of pathogens can cause gastrointestinal disease, including bacteria (Salmonella, Campylobacter, Shigella, MAC), protozoa (Giardia, Cryptosporidium, Isospora, microsporidia), viruses (CMV, HSV, rotavirus), and fungi (Candida). MAC and the protozoal infections are most severe and protracted in patients with severe CD4 cell depletion. Infections may be localized or disseminated and affect any part of the gastrointestinal tract from the oropharynx to the rectum. Oral or esophageal ulcerations, either viral in origin or idiopathic, are painful and often interfere with eating. AIDS enteropathy, a syndrome of malabsorption with partial villous atrophy not associated with a specific pathogen, has been postulated to be a result of direct HIV infection of the gut. Disaccharide intolerance is common in HIV-infected children with chronic diarrhea.
The most common symptoms of gastrointestinal disease are chronic or recurrent diarrhea with malabsorption, abdominal pain, dysphagia, and failure to thrive. Prompt recognition of weight loss or poor growth velocity in the absence of diarrhea is critical. Linear growth impairment is often correlated with the level of HIV viremia. Supplemental enteral feedings should be instituted, either by mouth or with nighttime nasogastric tube feedings in cases associated with more severe chronic growth problems; placement of a gastrostomy tube for nutritional supplementation may be necessary in severe cases. The wasting syndrome, defined as a loss of > 10% of body weight, is not as common as failure to thrive in pediatric patients, but the resulting malnutrition is associated with a grave prognosis. Chronic liver inflammation evidenced by fluctuating serum levels of transaminases with or without cholestasis is relatively common, often without identification of an etiologic agent. Cryptosporidial cholecystitis is associated with abdominal pain, jaundice, and elevated γ-glutamyltransferase. In some patients, chronic hepatitis caused by CMV, hepatitis B, hepatitis C, or MAC may lead to portal hypertension and liver failure. Several of the antiretroviral drugs or other drugs such as didanosine, protease inhibitors, nevirapine, and dapsone may also cause reversible elevation of transaminases.
Pancreatitis with increased pancreatic enzymes with or without abdominal pain, vomiting, and fever may be the result of drug therapy (e.g., with pentamidine, didanosine, or stavudine) or, rarely, opportunistic infections such as MAC or CMV.
Renal Disease
Nephropathy is an unusual presenting symptom of HIV infection, more commonly occurring in older symptomatic children. A direct effect of HIV on renal epithelial cells has been suggested as the cause, but immune complexes, hyperviscosity of the blood (secondary to hyperglobulinemia), and nephrotoxic drugs are other possible factors. A wide range of histologic abnormalities has been reported, including focal glomerulosclerosis, mesangial hyperplasia, segmental necrotizing glomerulonephritis, and minimal change disease. Focal glomerulosclerosis generally progresses to renal failure within 6-12 mo, but other histologic abnormalities in children may remain stable without significant renal insufficiency for prolonged periods. Nephrotic syndrome is the most common manifestation of pediatric renal disease, with edema, hypoalbuminemia, proteinuria, and azotemia with normal blood pressure. Cases resistant to steroid therapy may benefit from cyclosporine therapy. Polyuria, oliguria, and hematuria have also been observed in some patients.
Skin Manifestations
Many cutaneous manifestations seen in HIV-infected children are inflammatory or infectious disorders that are not unique to HIV infection. These disorders tend to be more disseminated and respond less consistently to conventional therapy than in the uninfected child. Seborrheic dermatitis or eczema that is severe and unresponsive to treatment may be an early nonspecific sign of HIV infection. Recurrent or chronic episodes of HSV, herpes zoster, molluscum contagiosum, flat warts, anogenital warts, and candidal infections are common and may be difficult to control.
Allergic drug eruptions are also common, in particular related to nonnucleoside reverse transcription inhibitors; they generally respond to withdrawal of the drug but also may resolve spontaneously without drug interruption; rarely, progression to Stevens-Johnson syndrome has been reported. Epidermal hyperkeratosis with dry, scaling skin is frequently observed, and sparse hair or hair loss may be seen in the later stages of the disease.
Hematologic and Malignant Diseases
Anemia occurs in 20–70% of HIV-infected children, more commonly in children with AIDS. The anemia may be a result of chronic infection, poor nutrition, autoimmune factors, virus-associated conditions (hemophagocytic syndrome, parvovirus B19 red cell aplasia), or the adverse effect of drugs (zidovudine).
Leukopenia occurs in almost 30% of untreated HIV-infected children, and neutropenia often occurs. Multiple drugs used for treatment or prophylaxis for opportunistic infections, such as Pneumocystis pneumonia (TMP-SMX), MAC, and CMV (ganciclovir), or antiretroviral drugs (zidovudine) may also cause leukopenia and/or neutropenia. In cases in which therapy cannot be changed, treatment with subcutaneous granulocyte colony-stimulating factor may be necessary.
Thrombocytopenia has been reported in 10–20% of patients. The etiology may be immunologic (i.e., circulating immune complexes or antiplatelet antibodies) or, less commonly, from drug toxicity, or idiopathic. Antiretroviral therapy (cART) may also reverse thrombocytopenia in ART-naïve patients. In the event of sustained severe thrombocytopenia (<10,000 platelets/µL), treatment with intravenous immunoglobulin or anti-D immune globulin offers temporary improvement in most patients already taking cART. If ineffective, a course of steroids may be an alternative, but consultation with a hematologist should be sought. Deficiency of clotting factors (factors II, VII, IX) is not rare in children with advanced HIV disease and is often easy to correct with vitamin K. A novel disease of the thymus has been observed in a few HIV-infected children. These patients were found to have characteristic anterior mediastinal multilocular thymic cysts without clinical symptoms. Histologic examination shows focal cystic changes, follicular hyperplasia, and diffuse plasmacytosis and multinucleated giant cells. Treatment with cART may result in resolution, or spontaneous involution occurs in some cases.
Malignant diseases have been reported infrequently in HIV-infected children, representing only 2% of AIDS-defining illnesses. Non-Hodgkin lymphoma (including Burkitt lymphoma), primary CNS lymphoma, and leiomyosarcoma are the most commonly reported neoplasms among HIV-infected children. Epstein-Barr virus is associated with most lymphomas and with all leiomyosarcomas (see Chapter 281 ). Kaposi sarcoma, which is caused by human herpesvirus 8, occurs frequently among HIV-infected adults but is exceedingly uncommon among HIV-infected children in resource-rich countries (see Chapter 284 ).
Diagnosis
All infants born to HIV-infected mothers test antibody-positive at birth because of passive transfer of maternal HIV antibody across the placenta during gestation; therefore, antibody should not be used to establish the diagnosis of HIV in an infant. Most uninfected infants without ongoing exposure (i.e., who are not breastfed) lose maternal antibody between 6 and 18 mo of age and are known as seroreverters. Because a small proportion of uninfected infants continue to test HIV antibody-positive for up to 24 mo of age, positive IgG antibody tests, including the rapid tests, cannot be used to make a definitive diagnosis of HIV infection in infants younger than 24 mo. The presence of IgA or IgM anti-HIV in the infant's circulation can indicate HIV infection, because these immunoglobulin classes do not cross the placenta; however, IgA and IgM anti-HIV assays have been both insensitive and nonspecific and therefore are not valuable for clinical use. In any child older than 24 mo of age, demonstration of IgG antibody to HIV by a repeatedly reactive enzyme immunoassay and confirmatory HIV PCR establishes the diagnosis of HIV infection. Breastfed infants should have antibody testing performed 12 wk following cessation of breastfeeding to identify those who became infected at the end of lactation by the HIV-infected mother. Certain diseases (e.g., syphilis, autoimmune diseases) may cause false-positive or indeterminate results. In such cases, specific viral diagnostic tests (see later) have to be done.
Several rapid HIV tests are currently available with sensitivity and specificity better than those of the standard enzyme immunoassay. Many of these tests require only a single step that allows test results to be reported within less than 30 min. Performing rapid HIV testing during delivery or immediately after birth is crucial for the care of HIV-exposed newborns whose mother's HIV status was unknown during pregnancy. A positive rapid test in the mother has to be confirmed by a second different rapid test (testing different HIV-associated antibodies) or by HIV RNA PCR (viral load). Given the earlier detection of fourth-generation HIV ELISA testing (p24 antigen + HIV-1, HIV-2 IgG and IgM antibodies), Western blots are not appropriate to confirm testing, because the fourth generation assays can be positive before the Western blot becomes positive (i.e., in acute infection). In infants who are at risk of exposure to HIV-2 infection (e.g., born to an HIV-infected woman from West Africa or who has an HIV+ partner from West Africa), a rapid test that can detect both HIV-1 and HIV-2 should be used. However, if the HIV testing is negative or the Western blot test reveals an unusual pattern, further diagnostic tests should be considered. In addition, they should be tested with an HIV-2–specific DNA PCR assay; this assay has very limited availability.
Viral diagnostic assays, such as HIV DNA or RNA PCR, are considerably more useful in young infants, allowing a definitive diagnosis in most infected infants by 1-4 mo of age (Table 302.3 ). By 4 mo of age, HIV PCR testing identifies all infected nonbreastfed infants. Historically, HIV DNA PCR testing was the preferred virologic assay over HIV RNA PCR testing in developed countries for young infants due to what was thought to be a modest advantage in detecting intrapartum acquired infection for DNA PCR in the first month of life. The perinatal use of ART prophylaxis (either single drug or combination) to prevent vertical transmission has not affected the predictive value of viral diagnostic testing. The FDA-approved HIV DNA PCR test is no longer commercially available in the United States, but other assays exist; however, the sensitivity and specificity of noncommercial HIV-1 DNA tests (using individual laboratory reagents) may differ from the sensitivity and specificity of the FDA-approved commercial test. HIV RNA PCR also has increased sensitivity for non-subtype B HIV (rare in the United States). Almost 40% of infected newborns have positive test results in the first 2 days of life, with > 90% testing positive by 2 wk of age. Plasma HIV RNA PCR assays, which detect viral replication, are as sensitive as the DNA PCR for early diagnosis. Either the DNA or RNA PCR is considered acceptable for infant testing. The commercially available HIV-1 assays are not designed for quantification of HIV-2 RNA and thus should not be used to monitor patients with this infection.
Table 302.3
PCR, polymerase chain reaction.
Data from American Academy of Pediatrics, Committee of Pediatric AIDS: Diagnosis of HIV-1 infection in children younger than 18 months in the United States, Pediatrics 120:e1547-e1562, 2007.
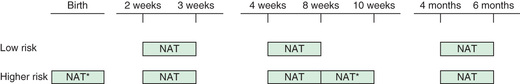
Viral diagnostic testing should be performed within the first 12-24 hr of life, particularly for high-risk infants (i.e., those of mothers without sustained virologic suppression, a late cART start, or a diagnosis with acute HIV during the pregnancy); the tests can identify almost 40% of HIV-infected children. It seems that many of these children have a more rapid progression of their disease and deserve more aggressive therapy. Data suggest that if cART treatment starts at this point, the outcome will be much better. In exposed children with negative virologic testing at 1-2 days of life, additional testing should be done at 2-3 wk of age, 4-8 wk of age, and 4-6 mo of age. For higher-risk infants, additional virologic diagnostic testing should be considered at 2 to 4 wk after cessation of ARV prophylaxis (i.e., at 8-10 wk of life) (Fig. 302.4 ). A positive virologic assay (i.e., detection of HIV by PCR) suggests HIV infection and should be confirmed by a repeat test on a second specimen as soon as possible because false-positive tests can occur. A confirmed diagnosis of HIV infection can be made with two positive virologic test results obtained from different blood samples. HIV infection can be presumptively excluded in nonbreastfed infants with two or more negative virologic tests (one at age ≥ 14 days and one at age ≥ 4 wk) or one negative virologic test (i.e., negative NAT [RNA or DNA]) at age ≥ 8 wk or one negative HIV antibody test at age ≥ 6 mo. Definitive exclusion of HIV infection in nonbreastfed infants is based on two or more negative virologic tests, with one obtained at age ≥ 1 mo and one at age ≥ 4 mo, or two negative HIV antibody tests from separate specimens obtained at age ≥ 6 mo. Some experts recommend documentation of seroreversion by testing for antibody at 12-18 mo of age; in low-risk infants with subtype B virus, this is likely not necessary, but antibody testing should be strongly considered in high-risk infants or infants infected with non–subtype B viruses.
Treatment
The currently available therapies do not eradicate the virus and cure the patient; instead they suppress the virus for extended periods of time and changes the course of the disease to a chronic process. Decisions about ART for pediatric HIV-infected patients are based on the magnitude of viral replication (viral load), CD4 lymphocyte count or percentage, and clinical condition. Because cART therapy changes as new drugs become available, decisions regarding therapy should be made in consultation with an expert in pediatric HIV infection. Plasma viral load monitoring and measurement of CD4 values have made it possible to implement rational treatment strategies for viral suppression, as well as to assess the efficacy of a particular drug combination. The following principles form the basis for cART:
- 1. Uninterrupted HIV replication causes destruction of the immune system and progression to AIDS.
- 2. The magnitude of the viral load predicts the rate of disease progression, and the CD4 cell count reflects the risk of opportunistic infections and HIV infection complications.
- 3. cART, which includes at least three drugs with at least two different mechanisms of action, should be the initial treatment. Potent combination therapy that suppresses HIV replication to an undetectable level restricts the selection of ART-resistant mutants; drug-resistant strains are the major factor limiting successful viral suppression and delay of disease progression.
- 4. The goal of sustainable suppression of HIV replication is best achieved by the simultaneous initiation of combinations of ART to which the patient has not been exposed previously and that are not cross resistant to drugs with which the patient has been treated previously.
- 5. Drug-related interactions and toxicities should be minimal.
- 6. Adherence to the complex drug regimens is crucial for a successful outcome.
Increasing data have shown a benefit in adult studies to starting treatment earlier, which has led to recommendations to treat earlier in children, as well. There are strong data to support the treatment of all infants < 12 mo of age, regardless of the clinical symptoms, viral load, or CD4 count from the Children with HIV Early Antiretroviral (CHER) study. Urgent treatment is recommended for older children with stage 3 opportunistic infections or immunologic suppression. Treatment is recommended for all other children, as well. Rarely, treatment may need to be deferred on a case-by-case basis based on clinical or psychosocial factors that may affect adherence with the caregivers and child.
Combination Therapy
As of January 2019, 20 individual ART drugs, with 21 coformulated combination tablets as well as two pharmacokinetic boosters, were approved by the FDA for use in HIV-infected adults and adolescents. Of these, 19 were approved for at least some portion of the pediatric population (0-12 yr of age), with many but not all of them available as a liquid, powder, or small tablet/capsule (Table 302.4 ). ART drugs are categorized by their mechanism of action, such as preventing viral entrance into CD4+ T cells, inhibiting the HIV reverse transcriptase or protease enzymes, or inhibiting integration of the virus into the human DNA. Within the reverse transcriptase inhibitors, a further subdivision can be made: nucleoside (or nucleotide) reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) (see Fig. 302.3 ). The NRTIs have a structure similar to that of the building blocks of DNA (e.g., thymidine, cytosine). When incorporated into DNA, they act like chain terminators and block further incorporation of nucleosides, preventing viral DNA synthesis. Among the NRTIs, thymidine analogs (e.g., stavudine, zidovudine) are found in higher concentrations in activated or dividing cells, producing > 99% of the HIV virion population, and nonthymidine analogs (e.g., didanosine, lamivudine) have more activity in resting cells, which account for < 1% of the HIV virions but may serve as a reservoir for HIV. Suppression of replication in both populations is thought to be an important component of long-term viral control. NNRTIs (i.e., nevirapine, efavirenz, etravirine, rilpivirine) act differently than the NRTIs. They attach to the reverse transcriptase and cause a conformational change, reducing the activity of the enzyme. The protease inhibitors (PIs) are potent agents that act farther along the viral replicative cycle. They bind to the site where the viral long polypeptides are cut into individual, mature, and functional core proteins that produce the infectious virions before they leave the cell. The virus entry into the cell is a complex process that involves several cellular receptors and fusion. Several drugs have been developed to prevent this process. The fusion inhibitor enfuvirtide (T-20), which binds to viral gp41, causes conformational changes that prevent fusion of the virus with the CD4+ cell and entry into the cell. Maraviroc is an example of a selective CCR5 coreceptor antagonist that blocks the attachment of the virus to this chemokine (an essential process in the viral binding and fusion to the CD4+ cells). Integrase inhibitors (INSTIs) (i.e., raltegravir, dolutegravir, elvitegravir, bictegravir) block the enzyme that catalyzes the incorporation of the viral genome into the host's DNA.
Table 302.4
Summary of Antiretroviral Therapies Available in 2019
| DRUG (TRADE NAMES, FORMULATIONS) | DOSING | SIDE EFFECTS | COMMENTS |
|---|---|---|---|
| NUCLEOSIDE/NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS | Class adverse effects: Lactic acidosis with hepatic steatosis, particularly for older members of the class | ||
|
Abacavir (Ziagen, ABC): tablet: 300 mg; oral solution: 20 mg/mL Trizivir: combination of zidovudine (ZDV), lamivudine, ABC (300, 150, 300 mg) Epzicom: combination of lamivudine, ABC (300, 600 mg) Triumeq: combination of ABC, lamivudine, dolutegravir (600, 300, 50 mg) |
Children: ≥3 mo to 13 yr: 8 mg/kg/dose bid (maximum dose: 300 mg bid) >25 kg: 300 mg bid Children with stable CD4 counts and undetectable viral load > 6 mo while taking ABC can transition to 16 mg/kg once daily (max: 600 mg) Adolescents and adults: 600 mg once daily Trizivir (>40 kg): 1 tablet bid Epzicom (>25 kg): 1 tablet qd Triumeq: 1 tablet qd |
Common: nausea, vomiting, anorexia, fever, headache, diarrhea, rash Less common: hypersensitivity, which can be fatal, Rare: lactic acidosis with hepatic steatosis, pancreatitis, elevated triglycerides, myocardial infarction |
Can be given with food Genetic screening for HLAB*5701 must be done prior to initiation of ABC-containing treatment. If test is positive, avoid ABC. Do not restart ABC in patients who had hypersensitivity-like symptoms (e.g., flu-like symptoms) |
|
Didanosine (Videx, ddl): powder for oral solution (prepared with solution containing antacid): 10 mg/mL |
2 wk to < 3 mo: 50 mg/m2 /dose bid 3-8 mo: 100 mg/m2 /dose bid >8 mo: 120 mg/m2 /dose (max: 200 mg/dose) bid Adolescents (>13 yr) and adults < 60 kg: 250 mg once daily >60 kg: 400 mg once daily (to increase adherence) |
Common: diarrhea, abdominal pain, nausea, vomiting Less common: pancreatitis, peripheral neuropathy, electrolyte abnormalities, lactic acidosis with hepatic steatosis, hepatomegaly, retinal depigmentation |
Food decreases bioavailability by up to 50%. Take 30 min before or 2 hr after meal. Tablets dissolved in water are stable for 1 hr (4 hr in buffered solution) Drug interactions: antacids/gastric acid antagonists may increase bioavailability; possible decreased absorption of fluoroquinolones, ganciclovir, ketoconazole, itraconazole, dapsone, and some protease inhibitors. Combination with d4T enhances toxicity; also common if combined with tenofovir. Note: Due to increased side effects compared with other NRTIs, ddI is no longer recommended for treatment of HIV in children in the US. |
| Enteric-coated didanosine (Videx EC): capsule, delayed release: 125, 200, 250, 400 mg; generic: 200, 250, 400 mg |
20-25 kg: 200 mg once daily 25-60 kg: 250 mg once daily ≥60 kg: 400 mg once daily |
Same as for ddl | Same as for ddl |
|
Emtricitabine (Emtriva, FTC): capsule: 200 mg; oral solution: 10 mg/mL Truvada: combination of FTC, tenofovir disoproxil fumarate (TDF) (200, 300 mg) Truvada Low Strength: combinations of FTC/TDF (100, 150 mg); (133, 200 mg); (167, 250 mg) Atripla: combination of FTC, TDF, efavirenz (EFV) (200, 300, 600 mg) Descovy: combination of FTC, tenofovir disoproxil alafenamide (TAF) (200, 25 mg) Complera: combination of FTC, TDF, rilpivirine (RPV) (200, 300, 25 mg) Odefsey: combination of FTC, TAF, RPV (25, 200, 25 mg) Stribild: combination of FTC, TDF, elvitegravir (EVG), cobicistat (COBI) (200, 300, 150, 150 mg) Genvoya: combination of FTC, TAF, EVG, COBI (200, 10, 150, 150 mg) Biktarvy: combination of bictegravir (BIC), FTC, TAF (50, 200, 25 mg) |
Infants: 0-3 mo: 3 mg/kg once daily Children ≥ 3 mo to 17 yr, oral solution: 6 mg/kg (max: 240 mg) once daily >33 kg, adolescents and adults: 200 mg capsule or 240 mg solution once daily Truvada, Descovy, Atripla, Complera, Descovy, Stribild, Genvoya or Biktarvy: adult dose: 1 tablet once daily |
Common: headache, insomnia, diarrhea, nausea, skin discoloration Less common: lactic acidosis with hepatic steatosis, neutropenia |
Patient should be tested for hepatitis B virus (HBV) because HBV exacerbation can occur when emtricitabine is discontinued. Can be given without regard to food. Oral solution should be refrigerated if temperature above 25°C (77°F) COBI is a pharmacokinetc enhancer (boosting agent) used to optimize drug levels; it is not interchangeable with ritonavir. It can alter renal tubular secretion of Cr, resulting in elevated Cr with normal GFR. Note oral solution is less bioavailable and has a max dose of 240 mg, while the max dose for capsules is 200 mg. |
|
Lamivudine (Epivir, Epivir HBV, 3TC): tablet: 150 (scored), 300 mg (Epivir, generic), 100 mg (Epivir HBV); Solution: 5 mg/mL (Epivir HBV), 10 mg/mL (Epivir) Combivir: combination of ZDV, lamivudine (300, 150 mg) Trizivir, Epzicom, and Triumeq combination (see abacavir) Symfi Lo combination of 3TC, TDF, EFV (300, 300, 400 mg) |
Neonates (≥32 wk gestational age through 4 wk of age for term infants): 2 mg/kg/dose bid ≥4 wk to <3 mo: 4 mg/kg/dose bid ≥3 mo to <3 yr: 5 mg/kg/dose bid (max 150 mg) ≥3 yr: 5 mg/kg/dose bid (max 150 mg) or 10 mg/kg/dose qd (max 300 mg) For ≥14 kg with scored tablet (150 mg) 14 to <20 kg: 75 mg bid or 150 mg qd (if >3 yr) ≥20 to <25 kg: 75 mg qAM and 150 mg qPM or 225 mg qd (if >3 yr) ≥25 kg: 150 mg bid or 300 mg qd Children should be switched to once-daily dosing of lamivudine (oral solution or tablets) from twice-daily dosing at ≥3 yr if clinically stable for 36 wk with an undetectable viral load and stable CD4 T lymphocyte count Adolescents and adults: Combivir (>30 kg), Trizivir (>40 kg): 1 tablet bid Epzicom (>25 kg): 1 tablet qd Triumeq (>40 kg): 1 tablet qd Symfi Lo (>35 kg): 1 tablet qd |
Common: headache, nausea Less common: pancreatitis, peripheral neuropathy, lactic acidosis with hepatic steatosis, lipodystrophy |
No food restrictions Patient should be tested for hepatitis B virus (HBV) because HBV exacerbation can occur when lamivudine is discontinued. M184V mutation for this drug decreases viral fitness and can be advantageous to maintain including inducing AZT hypersusceptibility. |
|
Stavudine (Zerit, d4T): capsule: 15, 20, 30, 40 mg; solution: 1 mg/mL |
≥14 days and < 30 kg: 1 mg/kg/dose bid >30 kg: 30 mg bid |
Common: headache, nausea, hyperlipidemia, fat maldistribution Less common: peripheral neuropathy, pancreatitis, lactic acidosis, hepatic steatosis |
No food restrictions. Should not be administered with ZDV because of virologic antagonism. Higher incidence of lactic acidosis. Increased toxicity if combined with ddl. Note: Due to increased side effects compared with other NRTIs, d4T is no longer recommended for treatment of HIV in children in the US. |
|
Tenofovir disoproxil fumarate (Viread, TDF): tablet: 150, 200, 250, 300 mg; powder: 40 mg/1 g powder Truvada: combination of FTC, TDF (200, 300 mg) Truvada Low Strength: combinations of FTC/TDF (100, 150 mg); (133, 200 mg); (167, 250 mg) Atripla: Combination of FTC, TDF, EFV (200, 300, 600 mg) Complera: combination of FTC, TDF, RPV (200, 300, 25 mg) Stribild: combination of FTC, TDF, EVG, COBI (200, 300, 150, 150 mg)Symfi Lo combination of 3TC, TDF, EFV (300, 300, 400 mg) |
2 to <12 yr: 8 mg/kg/dose qd >12 yr and 35 kg, adolescents >12 yr and 35 kg and adults: 300 mg once daily Truvada, Atripla, Complera, Symfi Lo, and Stribild: 1 tablet qd Weight probands for ≥2 yr and ≥17 kg: 17 to <22 kg: 150 mg qd 22 to <28 kg: 200 mg qd 28 to <35 kg: 250 mg qd ≥35 kg: 300 mg qd |
Common: nausea, vomiting, diarrhea Less common: lactic acidosis with hepatic steatosis, hepatomegaly, reduced bone density, renal toxicity |
High-fat meal increases absorption; coadministration with ddl increased ddl toxicity, decreases atazanavir (ATV) levels (therefore, boosting ATV with ritonavir is required). ATV and lopinavir (LPV) increase TDF levels and potential toxicity. Screen for HBV before TDF is given, because exacerbation of hepatitis may occur when TDF is discontinued |
|
Tenofovir alafenamide (Vemlidy, TAF) Descovy: combination of TAF, FTC (25, 200 mg) Genvoya: combination of TAF, FTC, EVG, COBI (10, 200, 150, 150 mg) Odefsey: combination of FTC, TAF, RPV (25, 200, 25 mg) Biktarvy: combination of BIC, FTC, TAF (50, 200, 25 mg) |
Adolescents (≥13 yr, ≥35 kg): Descovy, Genvoya, or Odefsey: 1 tablet qd Biktarvy: ≥18 yr 1 tablet qd; >12 yr to 18 yr and >35 kg investigational dose 1 tablet qd based on limited data |
Common: headache, diarrhea, nausea, increased serum lipids |
Newer version of TDF that has less renal and bone toxicity. Screen for HBV before TAF is given, because exacerbation of hepatitis may occur when TAF is discontinued. Concentrates in cells more so than TDF, so is not approved for pregnant women given lack of data. |
|
Zidovudine (Retrovir, AZT, ZDV): capsule: 100 mg; tablet: 300 mg; syrup: 10 mg/mL; intravenous injection: 10 mg/mL (all available generic) Combivir: combination of ZDV, lamivudine (300, 150 mg) Trizivir: Combination of ZDV, lamivudine, ABC (300, 150, 300 mg) |
Low Risk Prophylaxis: ≥35 wk gestation at birth: Birth to age 4-6 wk : 4 mg/kg/dose PO bid (or 3 mg/kg/dose IV q12h) ≥30 to <35 wk gestation at birth: Birth to age 2 wk : 2 mg/kg/dose PO bid (or 1.5 mg/kg/dose IV q12h) THEN Age 2 wk to 4-6 wk : 3 mg/kg/dose PO bid (or 2.3 mg/kg/dose IV q12h) <30 wk gestation at birth Birth to age 4-6 wk : 2 mg/kg/dose PO bid (or 1.5 mg/kg/dose IV q12h) |
Common: bone marrow suppression (e.g., macrocytic anemia, neutropenia), headache, nausea, vomiting, anorexia Less common: liver toxicity, lactic acidosis with hepatic steatosis, myopathy, fat redistribution |
No food restrictions Drug interactions: should not be given with d4T or doxorubicin. Rifampin may increase metabolism. Cimetidine, fluconazole, valproic acid may decrease metabolism. Ganciclovir, IFN-α, ribavirin increase ZDV toxicity. Only antiretroviral with an IV formulation currently. |
|
High Risk Prophylaxis and Treatment: ≥35 wk gestation at birth: Birth to age 4 wk: 4 mg/kg/dose PO bid THEN Age >4 wk: 12 mg/kg/dose PO bid ≥30 to <35 wk gestation at birth: Birth to age 2 wk: 2 mg/kg/dose PO bid THEN Age 2 wk to 6-8 wk: 3 mg/kg/dose PO bid THEN Age >6-8 wk: 12 mg/kg/dose PO bid <30 wk gestation at birth: Birth to age 4 wk: 2 mg/kg/dose PO bid THEN Age 4 wk to 8-10 wk: 3 mg/kg/dose PO bid THEN Age >8-10 wk: 12 mg/kg/dose PO bid Infants >4 kg and ≥4 wk post delivery and children: 4 kg to <9 kg: 12 mg/kg/dose PO bid 9 kg to <30 kg: 9 mg/kg/dose PO bid >30 kg, adolescents and adults: 300 mg bid Alternative body surface area dosing: 180-240 mg/m2 /dose PO bid Combivir or Trizivir: 1 tablet bid |
|||
| NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS | Class adverse effects: Rash is mild to severe, usually within first 6 wk. Discontinue the drug if severe rash (with blistering, desquamation, muscle involvement, or fever) | ||
|
Efavirenz (Sustiva, EFV): capsule: 50, 200 mg; tablet: 600 mg Atripla: combination of EFV, FTC, TDF (600, 200, 300 mg) Symfi Lo combination of 3TC, TDF, EFV (300, 300, 400 mg) |
Children < 3 yr: consult with expert Children ≥ 3 yr: 10 to < 15 kg: 200 mg qd 15 to < 20 kg: 250 mg qd 20 to < 25 kg: 300 mg qd 25 to < 32.5 kg: 350 mg qd 32.5 to < 40 kg: 400 mg qd ≥40 kg: 600 mg qd or 367 mg/m2 body surface area Atripla (>40 kg, adult dose): 1 tablet qd Symfi Lo (>35 kg, adult dose): 1 tablet qd |
Common: skin rashes, CNS abnormalities (e.g., vivid dreams, impaired concentration, insomnia, depression, hallucination) Less common: increased liver enzymes; potentially teratogenic, QTc prolongation (be careful with other QT-prolonging medications), false positives on some cannabinoid and benzodiazepine tests |
Capsules can be opened for mixing in food. Administer at bedtime on empty stomach to minimize CNS side effects. Taking with food, especially fatty meal, can increase absorption and CNS side effects. Drug interactions: Efavirenz induces/inhibits CYP3A4 enzymes. Increase clearance of drugs metabolized by this pathway (e.g., antihistamines, sedatives and hypnotics, cisapride, ergot derivatives, warfarin, ethinyl estradiol) and several other ARVs (i.e., protease inhibitors). Drugs that induce CYP3A4 (e.g., phenobarbital, rifampin, rifabutin) decrease efavirenz levels. Clarithromycin levels decrease with EFV, and azithromycin should be considered. Use with caution in female adolescents with reproductive potential because of potential teratogenicity. Avoid using in individuals with a history of past or active psychiatric issues and use with caution in adolescents and young adults owing to possible affective side effects, including increased suicidality. |
| Etravirine (ETR, Intelence): tablet: 25, 100, 200 mg |
Children <6 yr: consult with expert 16 to < 20 kg: 100 mg bid 20 to < 25 kg: 125 mg bid 25 to < 30 kg: 150 mg bid >30 kg, adolescents and adults: 200 mg bid |
Common: nausea, rash, diarrhea Less common: hypersensitivity reactions |
Always administer following a meal for absorption; taking on empty stomach decreases absorption by 50%. Tablets can be dispersed in water Inducer of CYP3A4 enzymes and inhibitor of CYP2C9 and CYP2C19, causing multiple interactions that should be checked before initiating ETR. Should not be given in combination with TPV, FPV, ATV, or other nonnucleoside reverse transcriptase inhibitors |
|
Nevirapine (Viramune, NVP): tablet: 200 mg; extended-release (XR) tablet: 100, 400 mg; suspension: 10 mg/mL |
High risk Prophylaxis: 3-dose series for high-risk infants >32 wk gestation at birth (including those born to mothers not taking HAART) NOTE: DOSES ARE A FLAT DOSE, NOT PER KG Dosing intervals: Within 48 hr of birth, 48 hr after first dose, 96 hr after second dose Birth weight 1.5-2 kg: 8 mg/dose PO Birth weight >2 kg: 12 mg/dose PO Treatment (including higher risk prophylaxis with empiric therapy): ≥37 wk gestation at birth: Birth to age 4 wk: 6 mg/kg/dose bid THEN Age >4 wk: 200 mg/m2 /dose bid 34 to <37 wk gestation at birth: Birth to age 1 wk: 4 mg/kg/dose bid Age 1 to 4 wk: 6 mg/kg/dose bid Age >4 wk: 200 mg/m2 /dose bid Note dose adjustment is optional at 4 wk for empiric HIV therapy for high risk infants with negative testing ≥1 mo to < 8 yr: 200 mg/m2 once daily for 14 days; then same dose bid (max: 200 mg/dose) ≥8 yr: 120-150 mg/m2 once daily for 14 days; then bid (max: 200 mg/dose) Adolescents and adults: 200 mg once daily for 14 days; then 200 mg bid or XR 400 mg qd (after 14 day lead in) |
Common: skin rash, headache, fever, nausea, abnormal liver function tests Less common: hepatotoxicity (rarely life-threatening), hypersensitivity reactions |
No food restrictions Drug interactions: induces hepatic CYP450A enzymes (including CYP3A and CYP2B6) activity and decreases protease inhibitor concentrations (e.g., IND, SQV, LPV). Should not be given with ATV. Reduces ketoconazole concentrations (fluconazole should be used as an alternative). Rifampin decreases nevirapine serum levels. Anticonvulsants and psychotropic drugs using same metabolic pathways as NVP should be monitored. Oral contraceptives may also be affected. XR formulation must be swallowed whole. For children ≤2 yr, some experts start with bid dosing without the 14 day lead-in of qd dosing. Lead-in dosing decreases occurrence of rash by allowing induction of cytochrome p450 metabolizing enzymes. |
|
Rilpivirine (Edurant, RPV): tablet: 25 mg Complera: combination of RPV, FTC, TDF (25, 200, 300 mg) Odefsey: combination of FTC, TAF, RPV (25, 200, 25 mg) Juluca: combination of RPV, Dolutegravir (DTG) (25, 50 mg) |
Pediatric patients: consult with expert Adolescents (>12 yr and 35 kg) and adults: 25 mg PO qd Complera or Odefsey: 1 tablet qd Juluca (>18 yr): 1 tablet qd; only for use in adults with ≥6 mo virologic suppression with no resistance to replace current regimen |
Headache, insomnia, rash, depression, mood changes |
Given with food only, 500 kcal meal. Do not use with proton pump inhibitors; antacids have to be spaced from dose by 2 h before or 4 h after. Should not be used if viral load > 100,000 copies/µL or drugs that induce CYP3A or with proton pump inhibitors |
| PROTEASE INHIBITORS | Class adverse effects: Gi side effects, hyperglycemia, hyperlipidemia (except atazanavir and darunavir), lipodystrophy, increased transaminases, increased bleeding disorders in hemophiliacs. Can induce metabolism of ethinyl estradiol; use alternate contraception (other than estrogen-containing oral contraceptives). All of these drugs undergo hepatic metabolism, mostly by CYP3A4, with many drug interactions. Treatment note: except in rare instances, always administer with boosting agent (ritonavir [RTV] or cobicistat [COBI]). | ||
|
Atazanavir (Reyataz, ATV): powder packet: 50 mg/packet; capsule: 150, 200, 300 mg (Note: capsules and packets are not interchangeable) Evotaz: combination of ATV, COBI (300, 150 mg) |
Infants and children ≥ 3 mo and ≥ 5 kg: 5 to < 15 kg: ATV 200 mg (4 packets) + RTV 80 mg qd 15 to < 25 kg: ATV 250 mg (5 packets) + RTV 80 mg qd Note: Capsules are not approved for < 6 yr or < 15 kg Children ≥6 yr and ≥15 kg capsule dosing: 15 to <35 kg: 200 mg + RTV 100 mg ≥35 kg: 300 mg + RTV 100 mg Adolescents and adults: 300 mg + RTV 100 mg Adults (>18 yr): Evotaz: 1 tablet qd |
Common: elevation of indirect bilirubin; headache, arthralgia, depression, insomnia, nausea, vomiting, diarrhea, paresthesias Less common: prolongation of PR interval on electrocardiogram (ECG); rash, rarely Stevens-Johnson syndrome, diabetes mellitus, nephrolithiasis |
Administer with food to increase absorption. Review drug interactions before initiating because ATV inhibits CYP3A4, CYP1A2, CYP2C9, and UGT1A1 enzymes. Use with caution with cardiac conduction disease or liver impairment. Combination with EFV should not be used in treatment-experienced patients because it decreases ATV levels. TDF, antacids, H2 -receptor antagonists, and proton-pump inhibitors decrease ATV concentrations. Patients taking buffered ddl should take it at least 2 hr before ATV COBI is a pharmacokinetc enhancer (boosting agent) used to optimize drug levels; it is not interchangeable with ritonavir. It can alter renal tubular secretion of Cr, resulting in elevated Cr with normal GFR. |
|
Darunavir (Prezista, DRV): tablets: 75, 150, 600, 800 mg; suspension: 100 mg/mL Prezcobix: combination DRV, COBI (800, 150 mg) |
<3 yr or < 10 kg: do not use 3 to < 12 yr: 10 to < 11 kg: DRV 200 mg + RTV 32 mg bid 11 to < 12 kg: DRV 220 mg + RTV 32 mg bid 12 to < 13 kg: DRV 240 mg + RTV 40 mg bid 13 to < 14 kg: DRV 260 mg + RTV 40 mg bid 14 to < 15 kg: DRV 280 mg + RTV 48 mg bid 15 to < 30 kg: DRB 375 mg + RTV 48 mg bid 30 to < 40 kg: DRV 450 mg + RTV 100 mg bid ≥40 kg: DRV 600 mg + RTV 100 mg bid Adolescents ≥ 40 kg and adults with no DRV mutations: DRV 800 mg + RTV 100 mg qd Adults (>18 yr) with no DRV mutations: Prezcobix: 1 tablet qd Adolescents ≥ 40 kg and adults with DRV mutation(s): DRV 600 mg + RTV 100 mg bid |
Common: diarrhea, nausea, vomiting, abdominal pain, fatigue, headache Less common: skin rashes (including Stevens-Johnson syndrome), lipid and liver enzyme elevations, hyperglycemia, fat maldistribution |
DRV should be given with food. Contraindicated for concurrent therapy with cisapride, ergot alkaloids, benzodiazepines, pimozide, or any major CYP3A4 substrates. Use with caution in patients taking strong CYP3A4 inhibitors, or moderate/strong CYP3A4 inducers. Adjust dose with concurrent rifamycin therapy. Contains sulfa moiety: potential for cross-sensitivity with sulfonamide class |
|
Fosamprenavir (Lexiva, FPV): tablet: 700 mg; suspension: 50 mg/mL |
6 mo to 18 yr: <11 kg: FPV 45 mg/kg/dose + RTV 7 mg/kg/dose bid 11 to < 15 kg: FPV 30 mg/kg/dose + RTV 3 mg/kg/dose bid 15 to < 20 kg: FPV 23 mg/kg/dose + RTV 3 mg/kg/dose bid >20 kg: FPV 18 mg/kg/dose (max: 700 mg) + RTV 3 mg/kg/dose (max: 100 mg) bid Adolescents > 18 yr and adults: FPV 700 mg + RTV 100 mg bid or FPV 1,400 mg + RTV 200 mg qd For protease inhibitor (PI)–experienced, the once-daily dose is not recommended |
Common: nausea, vomiting, perioral paresthesias, headache, rash, lipid abnormalities Less common: Stevens-Johnson syndrome, fat redistribution, neutropenia, elevated creatine kinase, hyperglycemia, diabetes mellitus, elevated liver enzymes, angioedema, nephrolithiasis |
Should be given with food. FPV is an inhibitor of the CYP450 system and an inducer, inhibitor, and substrate of CYP3A4, which can cause multiple drug interactions. Use with caution in sulfa-allergic individuals. |
|
Indinavir (Crixivan, IDV): capsule: 100, 200, 400 mg |
Not approved for use in infants or children Adolescents and adults: IDV 800 mg IDV + RTV (100 mg to 200 mg) bid |
Common: nausea, abdominal pain, hyperbilirubinemia, headache, dizziness, lipid abnormalities, nephrolithiasis, metallic taste Less common: fat redistribution, hyperglycemia, diabetes mellitus, hepatitis, acute hemolytic anemia |
Reduce dose (600 mg IDV every 8 hr) with mild to moderate liver dysfunction. Adequate hydration (at least 48 oz fluid/day in adults) necessary to minimize risk of nephrolithiasis. IDV is cytochrome P450 3A4 inhibitor and substrate, which can cause multiple drug interactions: rifampin reduces levels; ketoconazole, ritonavir, and other protease inhibitors increase IDV levels. Do not coadminister with EFV, astemizole, cisapride, terfenadine |
|
Lopinavir/Ritonavir (Kaletra, LPV/r): tablet: 100/25 mg, 200/50 mg; solution: 80/20 mg per/mL (contains 42% alcohol, 15% propylene glycol) |
14 days to 18 yr: LPV 300 mg/m2 /dose + RTV 75 mg/m2 /dose bid In treatment naive children >1 yr a dose of 230 mg/m2 /dose bid can be used. Adolescents (>18 yr) and adults: LPV 400 mg + RTV 100 mg bid or 800 mg LPV + 200 mg RTV qd If taken with NVP, EFV, FPV, or NFV: LPV 600 mg + RTV 150 mg bid |
Common: diarrhea, headache, nausea and vomiting, lipid elevation Less common: fat redistribution, hyperglycemia, diabetes mellitus, pancreatitis, hepatitis, PR interval prolongation |
Do not administer before postmenstrual age of 42 wk and postnatal age of 14 days owing to potential severe toxicities. No food restrictions but has better GI tolerability when given with or after a meal. Pills must be swallowed whole. Oral solution should be given with high-fat meal to increase absorption. Poor palatability of oral solution is difficult to mask with flavorings or foods. Once-daily dosing is poorly tolerated in most children, and plasma concentration variability makes qd dosing contraindicated in children. Interacts with drugs using CYP3A4, which can cause multiple drug interactions |
|
Nelfinavir (Viracept, NFV): tablet: 250, 625 mg |
<2 yr: not recommended Children 2-13 yr: 45-55 mg/kg/dose bid (max: 1,250 mg/dose) Adolescents and adults: 1,250 mg bid |
Common: diarrhea, asthenia, abdominal pain, skin rashes, lipid abnormalities Less common: exacerbation of liver disease, fat redistribution, hyperglycemia, diabetes mellitus, elevation of liver enzymes |
Administer with a meal to optimize absorption; avoid acidic food or drink (e.g., orange juice). Tablet can be crushed or dissolved in water to administer as a solution. Nelfinavir inhibits CYP3A4 activity, which may cause multiple drug interactions. Rifampin, phenobarbital, and carbamazepine reduce levels. Ketoconazole, ritonavir, indinavir, and other protease inhibitors increase levels. Do not coadminister astemizole, cisapride, terfenadine. NFV is no longer recommended for treatment for HIV due to inferior potency compared to newer agents and unpredictable pharmacokinetics particularly in adolescents. |
|
Ritonavir (Norvir, RTV): capsule: 100 mg; tablet: 100 mg; solution: 80 mg/mL (contains 43% alcohol) |
Only use is to enhance other PIs; dose varies (see information for specific PI) |
Common: nausea, headache, vomiting, abdominal pain, diarrhea, taste aversion, lipid abnormalities, perioral paresthesias Less common: fat redistribution, hyperglycemia, diabetes mellitus, pancreatitis, hepatitis, PR interval prolongation, allergic reactions |
Administration with food enhances bioavailability and reduces gastrointestinal symptoms. RTV solution should not be refrigerated (store at 20-25°C) RTV is potent inhibitor of CYP3A4 and CYP2D6 and inducer of CYP3A4 and CYP1A2 that leads to many drug interactions (e.g., protease inhibitors, antiarrhythmics, antidepressants, cisapride). Use cautiously with inhaled steroids (Cushing syndrome has been reported) |
|
Saquinavir (Invirase, SQV): capsule: 200 mg; tablet: 500 mg |
Infants and children < 16 yr: not approved for use Adolescents and adults: SQV 1,000 mg + RTV 100 mg bid |
Common: diarrhea, abdominal pain, headache, nausea, skin rashes, lipid abnormalities Less common: exacerbation of chronic liver disease, diabetes mellitus, pancreatitis, elevated liver transaminases, fat maldistribution, increase in both QT and PR in ECG |
Administer with a high-fat meal to enhance bioavailability. SQV is metabolized by CYP3A4, which may cause many drug interactions: rifampin, phenobarbital, and carbamazepine decrease serum levels. Saquinavir may decrease metabolism of calcium channel antagonists, azoles (e.g., ketoconazole), macrolides. Pretherapy EKG recommended and contraindicated in patients with prolonged QT interval. |
|
Tipranavir (Aptivus, TPV): capsule: 250 mg; solution: 100 mg/mL (contains 116 IU vitamin E/mL) |
<2 yr: not approved 2-18 yr (treatment-experienced only): TPV 375 mg/m2 /dose + RTV 150 mg/m2 (max: TPV 500 mg + RTV 200 mg) bid or TPV 14 mg/kg/dose + RTV 6 mg/kg/dose (max: same) bid Adolescents (>18 yr) and adult: TPV 500 mg + RTV 200 mg bid |
Common: diarrhea, nausea, vomiting, fatigue, headache, skin rashes, elevated liver enzymes, lipid abnormalities Less common: fat redistribution, hepatitis, hyperglycemia, diabetes mellitus, intracranial hemorrhage |
No food restrictions. Better tolerated with meal. Can inhibit human platelet aggregation: use with caution in patients at risk for increased bleeding (trauma, surgery, etc.) or in patients receiving concurrent medications that may increase the risk of bleeding. TPV is metabolized by CYP3A4, which may cause many drug interactions. Contraindicated in patients with hepatic insufficiency or in those receiving concurrent therapy with amiodarone, cisapride, ergot alkaloids, benzodiazepines, pimozide. TPV contains sulfonamide moiety, and caution should be taken in patients with sulfonamide allergy |
| FUSION INHIBITORS | |||
|
Enfuvirtide (Fuzeon, ENF): injection: lyophilized powder of 108 mg reconstituted in 1.1 mL of sterile water delivers 90 mg/mL |
<6 yr: not approved Children ≥6 yr to 16 yr: 2 mg/kg/dose SQ (max: 90 mg) bid Adolescents (>16 yr) and adults: 90 mg SQ bid |
Common: Local injection site reactions in 98% (e.g., erythema, induration nodules, cysts, ecchymoses) Less common: increased incidence of bacterial pneumonia, hypersensitivity, fever, nausea, vomiting, chills, elevated liver enzymes, hypotension, immune-mediated reactions (e.g., glomerulonephritis, Guillain-Barré syndrome, respiratory distress) |
Must be given subcutaneously. Severity of reactions increased if given intramuscularly. Apply ice after injection and massage the area to reduce local reactions. Injection sites should be rotated; recommended sites are upper arm, anterior thigh, or abdomen. |
| ENTRY INHIBITORS | |||
|
Maraviroc (Selzentry, MVC): oral solution: 20 mg/mL; tablet: 25, 75, 150, 300 mg |
Neonates/infants: not approved ≥2 yr and ≥ 10 kg: Given with CYP3A inhibitors (EVG, RTV, PIs except TPV/r): 10 to < 20 kg: 50 mg bid 20 to < 30 kg: 75 mg bid 30 to < 40 kg: 100 mg bid >40 kg: 150 mg bid Given with NRTIs, T-20, TPV/r, NVP, RAL, or other drugs not affecting CYP3A: 10 to < 30 kg: not recommended 30 to < 40 kg: 300 mg bid >40 kg: 300 mg bid Given with EFV, ETR: not recommended Adolescents > 16 yr and adults: 150 mg bid if given with potent CYP3A inhibitor (e.g., protease inhibitor except TPV) 300 mg bid if given with not potent CYP3A4 inhibitors (e.g., NRTI, TPV, NVP, ENF, RAL) 600 mg bid if given with potent CYP3A4 inducer (e.g., EFV, ETR, rifampin, phenobarbital) |
Common: fever, upper respiratory infection–like symptoms, rash, abdominal pain, musculoskeletal symptoms, dizziness Less common: cardiovascular abnormalities, cholestatic jaundice, rhabdomyolysis, myositis, osteonecrosis |
Testing for CCR5-tropic virus required; virus must not have mixed tropism (i.e., CCR5/CXC4) to have efficacy. No food restrictions. MVC is a CYP3A4 and P-glycoprotein (Pgp) substrate, which may cause many drug interactions. Tropism assay to exclude the presence of CXCR4 HIV is required before using MVC. Caution should be used when given to patients with hepatic impairment or cardiac disease or receiving CYP3A4 or P-glycoprotein-modulating drugs. |
| INTEGRASE INHIBITORS (INSTI) | |||
|
Bictegravir (BIC) Only available as Biktarvy: combination of BIC, TAF, FTC (50, 25, 200 mg) Dolutegravir (Tivicay, DTG): tablet: 10, 25, 50 mg Triumeq: combination of ABC, 3TC, DTG (600, 300, 50 mg) Juluca: combination of RPV, Dolutegravir (DTG) (25, 50 mg) |
Biktarvy: ≥18 yr 1 tablet qd; >12 yr to 18 yr and >35 kg: investigational dose 1 tablet qd based on limited data Neonates and infants: not approved ≥30 to < 40 kg: 35 mg qd (ARV-naïve or INSTI-naïve) >12 yr and ≥ 40 kg adolescents and adults: 50 mg qd (INSTI-naïve) If taken with EFV, FPV/RTV, TPV/RTV, or rifampin: 50 mg bid If INSTI-experienced with associated resistance or suspected resistance: 50 mg bid Triumeq: 1 tablet qd (INSTI-naïve, ≥ 40 kg) Juluca (>18 yr): 1 tablet qd; only for use in adults with ≥6 mo virologic suppression with no resistance to replace current regimen |
Diarrhea, nausea, headache Insomnia, headache, neuropsychiatric illness Rare: rash, hepatotoxicity, hypersensitivity reactions |
No food restrictions Metabolized by UGT1A1 and CYP450 (CYP) 3A No food restrictions. UGT1A1 and CYP450 (CYP) 3A substrate. Should be taken 2 hr before or 6 hr after taking laxatives, sucralfate, iron or calcium supplements, or buffered medications. DTG decreases tubular secretion of Cr and slightly increases measured Cr but does not affect GFR. |
|
Elvitegravir (EVG): only found in 2 coformulated fixed-dose combination (FDC) tablets Stribild: combination of EVG, FTC, TDF, COBI (150, 200, 300, 150 mg) Genvoya: combination of FTC, TAF, EVG, COBI (200, 10, 150, 150 mg) |
Genvoya: Not approved for <25 kg. Child and Adolescent (Weighing ≥25 kg; Any Sexual Maturity Rating [SMR]) and Adult Dose: 1 tablet qd Stribild: Not approved for <35 kg. Adolescent (Weighing ≥35 kg and SMR 4 or 5) and Adult Dose: 1 tablet qd |
Nausea, diarrhea, headache |
Administer with food. EVG is metabolized by CYP3A4 and modestly induces CYP2D6 that can cause multiple drug interactions. Cautiously use with nephrotoxic drugs. Do not use Stribild or Genvoya with ritonavir. COBI is a pharmacokinetc enhancer (boosting agent) used to optimize drug levels; it is not interchangeable with ritonavir. It can alter renal tubular secretion of Cr, resulting in elevated Cr with normal GFR. |
|
Raltegravir (Isentress, RAL): film-coated tablet: 400 mg; HD tablet: 600 mg chewable tablet: 25, 100 mg (scored); granules for oral suspension: 100 mg suspended in 10 mL of water for final concentration of 10 mg/mL |
Treatment and high-risk prophylaxis (empiric therapy) for neonates: >37 wk gestation at birth and >2 kg (oral suspension): Birth to Age 1 wk: approximately 1.5 mg/kg/dose qd 2 to <3 kg: 4 mg qd 3 to <4 kg: 5 mg qd 4 to <5 kg: 7 mg qd If mother on raltegravir in 2-24 h prior to delivery, delay first dose 24 to 48 hr after birth. Start other ART ASAP. Age 1-4 wk: approximately 3 mg/kg/dose bid 2 to <3 kg: 8 mg bid 3 to <4 kg: 10 mg bid 4 to <5 kg: 15 mg bid THEN Infant and Pediatrics dosing (Oral suspension) Children aged ≥4 wk and ≥3 kg to <20 kg: approximately 6 mg/kg/dose bid 3 to <4 kg: 25 mg bid 4 to <6 kg: 30 mg bid 6 to <8 kg: 40 mg bid 8 to <11 kg: 60 mg bid 11 to <14 kg: 80 mg bid 14 to <20 kg: 100 mg bid Chewable tablet: 11 to <14 kg: 75 mg bid 14 to <20 kg: 100 mg bid 20 to <28 kg: 150 mg bid 28 to <40 kg: 200 mg bid ≥40 kg: 300 mg bid Child or adolescent ≥ 25 kg and adults: 400 mg film-coated tablet bid Child and Adolescent Weighing ≥50 kg (HD tablet): 1200 mg qd (2 tablets) For treatment-naive or virologically suppressed patients on an initial regimen of 400 mg twice daily (HD tablet): 1200 mg qd (2 tablets) |
Common: nausea, headache, dizziness, diarrhea, fatigue Less common: itching, creatine phosphokinase elevation, myopathy, rhabdomyolysis, depression, hypersensitivity Rare: rash including Stevens-Johnson, TEN, hypersensitivity reaction |
No food restrictions Oral suspension, film-coated tablet and chewable tablet are not interchangeable; chewable tablets and suspension have better oral bioavailability than film-coated tablet; hence, higher-dose film-coated tablet can be taken at 25 kg. RAL is metabolized by UGT1A1 glucuronidation, and inducers of this system (e.g., rifampin, TPV) will reduce RGV levels, whereas inhibitors of this system (e.g., ATV) will increase RGV levels. Do not administer rifampin with once daily raltegravir (HD). Aluminum and magnesium containing antacids should not be co-administered. UGT1A1 metabolism is low at birth and increases rapidly over first 4-6 wk of life. No data for preterm infants. |
Antiretroviral drugs often have significant drug–drug interactions, with each other and with other classes of medicines, which should be reviewed before initiating any new medication.
The information in this table is not all-inclusive. Updated and additional information on dosages, drug–drug interactions, and toxicities is available on the AIDSinfo website at http://www.aidsinfo.nih.gov .
Modified from the Guidelines for use of antiretroviral agents in pediatric HIV infection. http://aidsinfo.nih.gov/contentfiles/pediatricguidelines.pdf .
By targeting different points in the viral life cycle and stages of cell activation and by delivering drug to all tissue sites, maximal viral suppression is feasible. Combinations of three drugs consisting of a two-NRTI backbone of (1) a thymidine analog NRTI (abacavir or zidovudine) or tenofovir and (2) a nonthymidine analog NRTI (lamivudine or emtricitabine) to suppress replication in both active and resting cells added to (3) a ritonavir-boosted PI (lopinavir/ritonavir, atazanavir, or darunavir), an NNRTI (efavirenz or nevirapine), or an INSTI (raltegravir or dolutegravir) can produce prolonged suppression of the virus. The use of three drugs from three different classes generally should be avoided but may be necessary in children with highly resistant viruses; the drugs in these regimens should only be chosen by an HIV specialist with pharmacist input. Combination treatment increases the rate of toxicities (see Table 302.4 ), and complex drug–drug interactions occur among many of the antiretroviral drugs. Many PIs are inducers or inhibitors of the cytochrome P450 system and are therefore likely to have serious interactions with multiple drug classes, including nonsedating antihistamines and psychotropic, vasoconstrictor, antimycobacterial, cardiovascular, anesthetic, analgesic, and gastrointestinal drugs (cisapride). Whenever new medications are added to an antiretroviral treatment regimen, especially a protease inhibitor or cobicistat containing regimen, a pharmacist and/or HIV specialist should be consulted to address possible drug interactions. The inhibitory effect of ritonavir (a PI) on the cytochrome P450 system has been exploited, and small doses of the drug are added to several other protease inhibitors (e.g., lopinavir, atazanavir, darunavir) to slow their metabolism by the P450 system and to improve their pharmacokinetic profile. This strategy provides more effective drug levels with less toxicity and less-frequent dosing. Recently, the development of cobicistat provides an alternative to ritonavir. Although cobicistat is a potent inhibitor of cytochrome P450 3A, it is a weak inhibitor of CYP2D6 and other CYP isoforms (e.g., CYP1A2), making pharmacologic interactions with many drugs more predictable than for ritonavir, which is also active against these isoforms. Preliminary studies with cobicistat suggest that it has a good tolerability profile and less effect on adipocytes (resulting in lesser accumulations of lipid and a milder response to insulin). The better solubility of cobicistat compared with ritonavir has helped the development of more single-tablet combination regimens with cobicistat. However, cobicistat is currently only approved for adolescents and adults; it is not approved for use in pregnancy.
Adherence
Adherence to the medication schedules and dosages is fundamental to cART success. Therefore, assessment of the likelihood of adherence to treatment is an important factor in deciding when to initiate therapy as well as choice of regimen. Numerous studies show that compliance of < 90% results in less-successful suppression of the viral load. In addition, several studies document that almost half of the pediatric patients surveyed were nonadherent to their regimen. Poor adherence to prescribed medication regimens results in subtherapeutic drug concentrations and enhances the development of resistant viruses. Several barriers to adherence are unique to children with HIV infection. Combination antiretroviral regimens are often unpalatable and require extreme dedication on the part of the caregiver and child; a reluctance to disclose the child's disease to others reduces social support; there may be a tendency to skip doses if the caregiver is not around or when the child is in school. Adolescents have other issues that reduce adherence. Denial and/or fear of their infection, an unstructured lifestyle, conduct or emotional disorders, wishing to be the same as their peers, depression, fatigue from taking a lifelong regimen, anxiety, and alcohol and substance abuse are just a few of the barriers to long-term adherence in this growing population. These and other barriers make participation of the family in the decision to initiate therapy essential. Intensive education on the relationship of drug adherence to viral suppression, training on drug administration, frequent follow-up visits, peer support, text messaging, and commitment of the caregiver and the patient (despite the inconvenience of adverse effects or the dosing schedule) are critical for successful antiviral treatment. Multiple methods such as the viral load response, self-reporting of missed doses during the last 3-7 days, and pharmacy/pill counting should be used to assess adherence. Assessing for emergence of resistant virus on sequencing (genotype) can also be a helpful tool.
Initiation of Therapy
The decision on when to initiate cART is evolving. When cART was first introduced, medication regimens had significant side effects. This led to decisions to delay therapy until it would be most beneficial, usually after advanced immunologic suppression had developed. In a large adult cohort, the Strategic Timing of Antiretroviral Treatment (START) trial demonstrated a strong benefit in starting therapy earlier in adults, even before CD4 counts fell into an immunosuppressed range; this became more feasible with the development of safer, better-tolerated medications. In adults, it has also been found that receiving suppressive cART eliminates the risk of the sexual transmission of HIV to others. Current adult guidelines recommend the initiation of cART in all adults with HIV. As with adult guidelines, the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV also recommends treatment for all children with HIV. However, the urgency of when to start treatment and the strength of the recommendations vary by age and pretreatment CD4 count. This is due to limited pediatric-specific data, as well as knowing that once pediatric patients are started on medications, treatment will need to continue for life; this means that potential concerns about adherence and toxicities will go on for an extended period. For children < 1 yr of age, the CHER trial has clearly demonstrated the benefit of early immediate ART. Data in older children suggest that mortality rates are lower and growth is more normal in children < 10 yr of age who are started on immediate cART. More studies are needed for confirmation, however.
Children younger than 1 yr of age are at high risk for disease progression, and immunologic and virologic tests to identify those likely to develop rapidly progressive disease are less predictive than in older children. Therefore, HIV-infected infants younger than 1 yr of age should be treated with cART as soon as the diagnosis of HIV infection has been confirmed, regardless of their clinical or immunologic status or viral load. Data suggest that HIV-infected infants who are treated before the age of 3 mo control their HIV infection better than infants whose cART started later than 3 mo of age.
For children 1 yr to under 6 yr of age, urgent treatment is recommended if the children have stage 3–defining opportunistic infections or stage 3 immunodeficiency (CD4 < 500 cells/µL). For children with moderate HIV-related symptoms or CD4 counts of 500-999 cells/µL, treatment is strongly recommended. Treatment is also recommended for asymptomatic or mildly symptomatic children with CD4 counts ≥ 1,000 cells/µL.
For children ≥ 6 yr, urgent treatment is recommended if the children have stage 3–defining opportunistic infections or stage 3 immunodeficiency (CD4 < 200 cells/µL). For children with moderate HIV-related symptoms or CD4 counts of 200-499 cells/µL, treatment is again strongly recommended. Treatment is also recommended for asymptomatic or mildly symptomatic children with CD4 counts ≥ 500 cells/µL. These guidelines are reviewed yearly, and care providers should check for revisions regularly at http://aidsinfo.nih.gov .
Dosages
Children are usually treated with higher doses (per kg weight) than adults because of reduced absorption or increased drug metabolism. Data on ART drug dosages for neonates, especially premature infants, are often limited. Because of the immaturity of the neonatal liver, there must often be an increase in the dosing interval of drugs primarily cleared through hepatic glucuronidation.
Adolescents should have ART dosages prescribed on the basis of the Tanner staging of puberty rather than on the basis of age. Pediatric dosing ranges should be used during early puberty (Tanner stages I, II, and III), whereas adult dosing schedules should be followed in adolescents in late puberty (Tanner stages IV and V). Dolutegravir and Efavirenz should be avoided in females who may become pregnant and do not use effective contraception because of potential teratogenicity; however, if an HIV-positive female becomes pregnant while taking a dolutegravir or efavirenz-containing regimen, the regimen can be continued, assuming that virologic suppression is maintained, because by the time the pregnancy is typically determined, the period of teratogenesis has past, specifically for neural tube defects. Because some ART agents may alter the metabolism of some hormonal contraceptives and decrease their effectiveness, interactions should be considered when choosing contraceptive agents. A comprehensive table of interactions of HIV medications with hormonal contraceptives can be found here: https://aidsinfo.nih.gov/guidelines/htmltables/3/5803 . Medroxyprogesterone (DMPA) is a reasonable choice outside of regimens containing cobicistat. Alternative contraception options, such as use of an intrauterine device, should also be considered.
Changing Antiretroviral Therapy
Therapy should be changed when the current regimen is judged ineffective as evidenced by an increase in viral load, deterioration of the CD4 cell count, or clinical progression. Development of toxicity or intolerance to drugs is another reason to consider a change in therapy. When a change is considered, the patient and family should be reassessed for adherence concerns. Because adherence is a major issue in this population, resistance testing (while the patient is taking antiretroviral medications) is important in identifying adherence issues (e.g., detectable virus sensitive to current drugs suggests a lack of adherence) or the development of resistance (e.g., evidence of resistance mutations to given drugs). In both situations, other contributing factors, such as poor absorption, an incorrect dose, or drug–drug interactions, should be carefully reviewed. While considering possible new drug choices, the potential for cross-resistance should be addressed. In starting a new regimen in a patient with virologic failure, the new regimen should include at least two, but preferably three, fully active antiretroviral medications, with assessment of the anticipated activity based on the treatment history and resistance testing (genotype or phenotype). The goal is to achieve and maintain virologic suppression. If virologic suppression cannot be achieved, the goals of therapy should focus on preserving the immunologic function and preventing further disease progression, as well as preventing the emergence of additional drug resistance (which could limit future treatment options).
Monitoring Antiretroviral Therapy
To ensure proper monitoring, the CD4 cell count, viral load, complete blood count, chemistries, urinalysis, and serum lipids should be obtained before an initiation of or change in cART to have a baseline for comparisons during treatment. At entry into care genotypic resistance testing should be done as well. Children need to be seen within 1-2 wk after initiation of new cART to reinforce and counsel regarding adherence and to screen for potential side effects. Virologic and immunologic surveillance (using the quantitative HIV RNA PCR and CD4 lymphocyte count), as well as clinical assessment, should be performed regularly while on cART. The initial virologic response (i.e., at least a five-fold [0.7 log10 ] reduction in viral load) should be achieved within 4-8 wk of initiating antiretroviral therapy. The maximum response to therapy usually occurs within 12-16 wk, but may be later (24 wk) in very young infants. Thus, HIV RNA levels should be measured at 4 wk and 3-4 mo after therapy initiation. Once an optimal response has occurred, the viral load should then be measured at least every 3-6 mo. If the response is unsatisfactory, another viral load should be determined as soon as possible to verify the results before a change in therapy is considered. Virologic failure is defined as a repeated plasma viral load ≥200 copies/mL after 6 mo of therapy. The CD4 cells respond more slowly to successful treatment particularly in patients with long standing infection and CD4 suppression. CD4 counts should be monitored every 3-4 mo and potentially can be done less frequently in adolescents and adults with documented virologic suppression. Potential toxicity should be monitored closely for the first 8-12 wk (including complete blood count, serum chemistries), and if no clinical or laboratory toxicity is documented, a follow-up visit every 3-4 mo is adequate. Monitoring for potential toxicity should be tailored to the drugs taken. These toxicities include but are not limited to hematologic complications (e.g., zidovudine); hypersensitivity rash (e.g., efavirenz); lipodystrophy (e.g., redistribution of body fat seen with NRTIs, protease inhibitors, which can take several years to emerge); hyperlipidemia (elevation of cholesterol and triglyceride concentrations); hyperglycemia, and insulin resistance (e.g., protease inhibitors); mitochondrial toxicity leading to severe lactic acidosis (e.g., stavudine, didanosine); electrocardiogram abnormalities (e.g., atazanavir, lopinavir); abnormal bone mineral metabolism (e.g., tenofovir disoproxil fumarate but not tenofovir alafenamide); and hepatic toxicity, including severe hepatomegaly with steatosis. After a patient is on a stable regimen, labs outside of CD4 count and viral load can be done every 6-12 mo. An important part of every visit is ongoing adherence counseling given the need for excellent adherence to cART to avoid the emergence of resistance. Detailed current guidelines for monitoring HIV-infected children during therapy can be found at http://aidsinfo.nih.gov .
Resistance to Antiretroviral Therapy
Young children usually are at greater risk than adults for developing resistance because they have higher viral loads than adults and are more limited by which ART options are available. The high mutation rate of HIV (mainly as a result of the absence of error-correcting mechanisms) results in the generation of viruses with multiple mutations everyday in the absence of cART. Failure to reduce the viral load to < 40 copies/mL on cART due to nonadherence resulting in subtherapeutic drug levels increases the risk for developing resistance by selecting those mutant viruses with a competitive advantage (i.e. drug resistance mutations). Even effectively treated patients do not completely suppress all viral replication, and persistence of HIV transcription and evolution of envelope sequences continues in the latent cellular reservoirs, though recent data show that this evolution does not appear to affect the emergence of resistance to cART in virologically suppressed patients. Accumulation of resistance mutations, particularly in nonadherent patients, progressively diminishes the potency of the cART and challenges the physician to find new regimens. For some drugs (e.g., nevirapine, lamivudine), a single mutation is associated with resistance, whereas for other drugs (e.g., zidovudine, lopinavir), several mutations are needed before significant resistance develops. Testing for drug resistance, especially when devising a new regimen, is the standard of care. Two types of tests are available; genotype is most commonly used but the phenotype may be helpful in select patients with complex viral resistance due to exposure to multiple cART regimens.
- 1. The phenotype measures the virus susceptibility in various concentrations of the drug. This allows calculation of the drug concentration that will inhibit the viral replication by 50% (IC50 ). The ratio of the IC50 and a reference virus IC50 is reported as the fold resistance change. Note this test is usually combined with a genotype when used but is largely reserved for patients with extremely complex mutations.
- 2. The genotype predicts the virus susceptibility from mutations identified in the HIV genome isolated from the patient and is the more commonly used test. Several online sites (e.g., http://hivdb.stanford.edu ) can assist in interpreting the test's results. Several studies show that the treatment success is higher in patients whose cART was guided by genotype or phenotype testing.
Neither method may detect drug resistance if the amount of the resistant virus is < 10% of the circulating population or if it is present only in the latent reservoir. Note that if a patient has not been taking cART for several weeks, the absence of selective drug pressure will make the dominant population of circulating viruses revert to the wild type, and resistance mutations can be missed.
It is recommended to test for drug resistance before initiating therapy and before changing treatment because of virologic failure. When changing therapy, the resistance test results should be considered in the context of previous resistance tests results, if done, and drugs used in previous regimens.
Supportive Care
Even before ART drugs were available, a significant impact on the quality of life and survival of HIV-infected children was achieved when supportive care was given. A multidisciplinary team approach is desirable for successful management. Following the initiation or change of cART, more frequent visits or contacts with the patient/caregivers for support and education will help in their acceptance and adjustment to the new regimen and will contribute to a better adherence. Close attention should be paid to the nutritional status, which is often delicately balanced and may require aggressive supplementation, especially in children with advanced disease. Painful oropharyngeal lesions and dental caries may interfere with eating, and thus routine dental evaluations and careful attention to oral hygiene should be encouraged. Paradoxically, an increasing number of adolescents with perinatally acquired or behavioral risk-acquired disease are obese. Some teens experience ART-related central lipoaccumulation (usually related to older agents), but others have poor dietary habits and inactivity as the cause of their obesity, just as others do who are obese in epidemic numbers in the United States. Their development should be evaluated regularly, with the provision of necessary physical, occupational, and/or speech therapy. Recognition of pain in the young child may be difficult, and effective nonpharmacologic and pharmacologic protocols for pain management should be instituted when indicated.
All HIV-exposed and HIV-infected children should receive standard pediatric immunizations. Live oral polio vaccine should not be given due to poor immunologic response in HIV+ children as well as concern for live vaccination in potentially immunocompromised children (Fig. 302.5 ). The risk and benefits of rotavirus vaccination should be considered in infants born to HIV-infected mothers. Because < 1% of these infants in resource-rich countries will develop HIV infection, the vaccine should be given. In other situations, the considerable attenuation of the vaccine's strains should be considered, and unless the infant has clinical symptoms of AIDS or a CD4 percentage of < 15%, vaccination is likely appropriate. Other live bacterial vaccines (e.g., bacillus Calmette-Guérin) should be avoided because of the high incidence of bacillus Calmette-Guérin–related disease in HIV-infected infants. Varicella and measles–mumps–rubella vaccines are recommended for children who are not severely immunosuppressed (i.e., CD4 cell percentage ≥ 15%, absolute CD4 count > 500 cells/µL for ages 1-5 yr), but these vaccines should not be given to severely immunocompromised children (i.e., CD4 cell percentage < 15%, absolute CD4 count < 500 cells/µL for age 1-5 yr). Of note, prior immunizations do not always provide protection, as evidenced by outbreaks of measles and pertussis in immunized HIV-infected children. The durability of vaccine-induced titers is often short, especially if vaccines are administered when the child's CD4 cell count is low, and reimmunization when the CD4 count has increased may be indicated. It is recommended that children with HIV receive quadrivalent meningococcal conjugate vaccine at a younger age than the routine schedule. Adolescent vaccines are also important, including the Tdap booster and HPV vaccine. The current recommended annotated vaccine schedule for HIV-infected children is found here: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html .
Prophylactic regimens are integral for the care of HIV-infected children. All infants between 4-6 wk and 1 yr of age who are proven to be HIV-infected should receive prophylaxis to prevent P. jiroveci pneumonia regardless of the CD4 count or percentage (Tables 302.5 and 302.6 ). Infants exposed to HIV-infected mothers should receive the same prophylaxis until they are proven to be noninfected; however, prophylaxis does not have to be initiated if there is strong presumptive evidence of noninfection (i.e., non–breastfed infant with two negative HIV PCR tests at older than 14 days and 4 wk of age, respectively). When the HIV-infected child is older than 1 yr of age, prophylaxis should be given according to the CD4 lymphocyte count (see Table 302.5 ). The best prophylactic regimen is 150 mg/m2 /day of TMP and 750 mg/m2 /day of SMX (maximum: 320/1,600 mg) given as 1-2 daily doses 3 days (consecutively or every other day) per wk. For severe adverse reactions to TMP-SMX, alternative therapies include dapsone, atovaquone, and aerosolized pentamidine.
Table 302.5
| AGE/HIV INFECTION STATUS | PJP PROPHYLAXIS | CD4 MONITORING |
| Birth to 4-6 wk, HIV-exposed | No prophylaxis | None |
| HIV infection reasonably excluded* | No prophylaxis | None |
| <1 yr, HIV-infected or HIV-indeterminate | Prophylaxis regardless of CD4 count or percentage | According to local practice for initiation or follow-up of cART |
| 1-5 yr, HIV-infected | Prophylaxis if CD4 < 500 cells/µL or < 15% † | According to local practice for initiation or follow-up of cART |
| >6 yr, HIV-infected | Prophylaxis if CD4 < 200 cells/µL or < 15% † ‡ | According to local practice for initiation or follow-up of cART |
* See text.
† More frequent monitoring (e.g., monthly) is recommended for children whose CD4 counts or percentages are approaching the threshold at which prophylaxis is recommended.
‡ Prophylaxis should be considered on a case-by-case basis for children who might otherwise be at risk for PJP, such as children with rapidly declining CD4 counts or percentages or children with category C conditions. Children who have had PJP should receive PJP prophylaxis until their CD4 count is ≥200 cells/mm3 for patients aged ≥6 yr, CD4 percentage is ≥15% or CD4 count is ≥500 cells/mm3 for patients aged 1 to <6 yr for >3 consecutive mo after receiving cART for ≥6 mo.
The National Perinatal HIV Hotline (1-888-448-8765) provides consultation on all aspects of perinatal HIV care.
cART, combined antiretroviral therapy; PJP, Pneumocystis jiroveci pneumonia.
Table 302.6
Prophylaxis to Prevent First Episode of Opportunistic Infections Among HIV-Exposed and HIV-Infected Infants and Children, United States*
| PATHOGEN | PREVENTIVE REGIMEN | ||
|---|---|---|---|
| INDICATION | FIRST CHOICE | ALTERNATIVE | |
| STRONGLY RECOMMENDED AS STANDARD OF CARE | |||
| Pneumocystis pneumonia † | HIV-infected or HIV-indeterminate infants aged 1-12 mo; HIV-infected children aged 1-5 yr with CD4 count of < 500 cells/µL or CD4 percentage of < 15%; HIV-infected children aged 6-12 yr with CD4 count of < 200 cells/µL or CD4 percentage of < 15%; >13 yr with CD4 count <200 or <15% |
TMP-SMX , 150/750 mg/m2 body surface area per day or 5-10 mg/kg/day (TMP)/25-50 mg/kg/day (SMX) (max: 320/1,600 mg) orally qd or bid 3 times weekly on consecutive days or qd or bid orally 3 times weekly on alternate days |
Dapsone: age ≥ 1 mo: 2 mg/kg (max: 100 mg) orally qd; or 4 mg/kg (max: 200 mg) orally once a week Atovaquone: age 1-3 mo and > 24 mo-12 yr: 30-40 mg/kg orally qd with food; age 4-24 mo: 45 mg/kg orally qd with food; ≥ 13 yr 1500 mg orally qd Aerosolized pentamidine: age ≥ 5 yr: 300 mg once a month by Respirgard II (Marquest, Englewood, CO) nebulizer |
| Malaria | Living or traveling to area in which malaria is endemic |
Same for HIV-infected and HIV-uninfected children. Refer to http://www.cdc.gov/malaria/ for the most recent recommendations. Mefloquine , 5 mg/kg orally 1 time weekly (max: 250 mg) Atovaquone/proguanil (Malarone) qd 11-20 kg: 62.5 mg/25 mg (1 pediatric tablet) 21-30 kg: 2 pediatric tablets 31-40 kg: 3 pediatric tablets >40 kg: 1 adult tablet (250 mg/100 mg) |
Doxycycline, 2.2 mg/kg body weight (maximum 100 mg) orally qd for children >8 yr Chloroquine, 5 mg/kg base (equal 7.5 mg/kg chloroquine phosphate) orally up to 300 mg weekly (only for regions where the parasite is sensitive) |
| Mycobacterium tuberculosis | |||
| Isoniazid-sensitive |
TST reaction ≥ 5 mm or Prior positive TST result without treatment or Close contact with any person who has contagious TB. TB disease must be excluded before start of treatment |
Isoniazid, 10-15 mg/kg body weight (max: 300 mg) qd for 9 mo or 20-30 mg/kg body weight (max: 900 mg) orally 2 times weekly for 9 mo; DOT highly recommended |
Rifampin, 10-20 mg/kg body weight (max: 600 mg) orally daily for 4-6 mo |
| Isoniazid-resistant | Same as previous pathogen; increased probability of exposure to isoniazid-resistant TB | Rifampin, 10-20 mg/kg body weight (max: 600 mg) orally daily for 4-6 mo | Consult TB expert |
| Multidrug-resistant (isoniazid and rifampin) | Same as previous pathogen; increased probability of exposure to multidrug-resistant TB | Choice of drugs requires consultation with public health authorities and depends on susceptibility of isolate from source patient | |
| Mycobacterium avium complex ‡ | For children age ≥ 6 yr with CD4 count of < 50 cells/µL; age 2-5 yr with CD4 count of < 75 cells/µL; age 1-2 yr with CD4 count of < 500 cells/µL; age < 1 yr with CD4 count of < 750 cells/µL |
Clarithromycin, 7.5 mg/kg (max: 500 mg) orally bid or Azithromycin, 20 mg/kg (max: 1,200 mg) orally once a week |
Azithromycin, 5 mg/kg body weight (max: 250 mg) orally qd or Children age ≥ 5 yr Rifabutin, 300 mg orally qd |
| Varicella-zoster virus § |
Exposure to varicella or shingles with no history of varicella or Zoster or seronegative status for VZV or Lack of evidence for age-appropriate vaccination |
Varicella-zoster immunoglobulin (VariZIG), 125 IU/10 kg (max: 625 IU) IM, administered ideally within 96 hr after exposure; potential benefit up to 10 days after exposure |
If VariZIG is not available and < 96 hr from exposure, acyclovir 20 mg/kg (max: 800 mg) 4 times a day for 5-7 days or IVIG, 400 mg/kg, administered once |
| Vaccine-preventable pathogens | Standard recommendations for HIV-exposed and HIV-infected children | Routine vaccinations (see Fig. 302.5 ) | |
| USUALLY RECOMMENDED | |||
| Toxoplasma gondii ¶ | Seropositive IgG to Toxoplasma and severe immunosuppression: age < 6 yr with CD4 percentage < 15%; age ≥ 6 yr with CD4 count < 100 cells/µL |
TMP-SMX, 150/750 mg/m2 orally qd or divided bid or Same dosage qd 3 times weekly on consecutive days or bid 3 times weekly on alternate days |
Dapsone, age ≥ 1 mo: 2 mg/kg or 15 mg/m2 (max: 25 mg) orally qd plus Pyrimethamine, 1 mg/kg (max: 25 mg) orally qd plus Leucovorin, 5 mg orally every 3d |
| Invasive bacterial infections | Hypogammaglobulinemia (i.e., IgG < 400 mg/dL) | IVIG 400 mg/kg body weight every 2-4 wk | |
| Cytomegalovirus |
CMV antibody positivity and severe immunosuppression (CD4 count < 50 cells/µL for >6 yr; CD4 percentage <5% for ≤6 yr) For children aged 4 mo–16 yr, valganciclovir oral solution 50 mg/mL at dose in milligrams = 7 × BSA × CrCl (up to maximum CrCl of 150 mL/min/1.73 m2 ) orally qd with food (maximum dose 900 mg/day) |
Valganciclovir, 900 mg orally qd with food for older children who can receive adult dosing | |
* Information in these guidelines might not represent FDA approval or FDA-approved labeling for products or indications. Specifically, the terms safe and effective might not be synonymous with the FDA-defined legal standards for product approval.
† Daily trimethoprim-sulfamethoxazole (TMP-SMX) reduces the frequency of certain bacterial infections. Compared with weekly dapsone, daily dapsone is associated with a lower incidence of PCP but higher hematologic toxicity and mortality rates. Patients receiving therapy for toxoplasmosis with sulfadiazine-pyrimethamine are protected against PCP and do not need TMP-SMX. TMP-SMX, dapsone-pyrimethamine, and possibly atovaquone (with or without pyrimethamine), protect against toxoplasmosis; however, data have not been prospectively collected.
‡ Substantial drug interactions can occur between rifamycins (i.e., rifampin and rifabutin) and protease inhibitors and nonnucleoside reverse transcriptase inhibitors. A specialist should be consulted.
§ Children routinely being administered intravenous immunoglobulin (IVIG) should receive VariZIG if the last dose of IVIG was administered more than 21 days before exposure.
¶ Protection against toxoplasmosis is provided by the preferred anti-Pneumocystis regimens and possibly by atovaquone.
CMV, cytomegalovirus; FDA, U.S. Food and Drug Administration; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IM, intramuscularly; IVIG, intravenous immunoglobulin; PCP, Pneumocystis pneumonia; TB, tuberculosis; TMP-SMX, trimethoprim-sulfamethoxazole; TST, tuberculin skin test; VZV, varicella-zoster virus.
From Centers for Disease Control and Prevention (CDC): Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children, MMWR Recomm Rep 58(RR-11):127-128, 2009, Table 1.
Prophylaxis against MAC should be offered to HIV-infected children with advanced immunosuppression (i.e., CD4 lymphocyte count < 750 cells/µL in children younger than 1 yr of age, < 500 cells/µL in children 1-2 yr of age, < 75 cells/µL in children 2-5 yr of age, and < 50 cells/µL in children > 6 yr of age) (see Table 302.6 ). The drugs of choice are azithromycin (20 mg/kg [maximum: 1,200 mg] once a week orally or 5 mg/kg [maximum: 250 mg] once daily orally) or clarithromycin (7.5 mg/kg bid orally). In rare situations, rifabutin 300 mg qd can be an alternative for children older than 6 yr of age though efficacy data in children is very limited.
Based on adult data, primary prophylaxis against most opportunistic infections may be discontinued if patients have experienced sustained (>6 mo duration) immune reconstitution with cART, even if they had previous opportunistic infections such as Pneumocystis pneumonia or disseminated MAC. HIV-infected children are at higher risk for TB and thus should have tuberculin skin testing (5 tuberculin units purified protein derivation) or interferon gamma release assay (IGRA) testing for TB at least once per year; an induration of 5 mm or more should be considered positive for the PPD. If the child is living in close contact with a person with TB, the child should be tested more frequently. Of note, the sensitivity of purified protein derivation and IGRA is reduced in severely immunocompromised patients. The Guidelines for Prevention and Treatment of Opportunistic Infections Among HIV-Exposed and HIV-Infected Children (http://aidsinfo.nih.gov ) should be consulted for these and other opportunistic infections that may occur in these populations. To reduce the incidence of opportunistic infections, parents should be counseled about (1) the importance of good hand washing, (2) avoiding raw or undercooked food (Salmonella) , (3) avoiding drinking or swimming in lake or river water or being in contact with young farm animals (Cryptosporidium) , and (4) the risk of playing with pets (Toxoplasma and Bartonella from cats, Salmonella from reptiles).
Prognosis
The improved understanding of the pathogenesis of HIV infection in children and the availability of more effective antiretroviral drugs has changed the prognosis considerably for children with HIV infection. The earlier cART is started, the better the prognosis. In settings with ready access to early diagnosis and antiretroviral therapy, progression of the disease to AIDS has significantly diminished. Since the advent of cART in the mid-1990s, mortality rates in perinatally infected children have declined more than 90% and many children survive to adolescence and adulthood. Even with only partial reduction of the viral load, children may have both significant immunologic and clinical benefits. In general, the best prognostic indicators are the sustained suppression of the plasma viral load and the restoration of a normal CD4+ lymphocyte count. If determinations of the viral load and CD4 lymphocytes are available, the results can be used to evaluate the prognosis. It is unusual to see rapid progression in an infant with a viral load < 100,000 copies/mL. In contrast, a high viral load (>100,000 copies/mL) over time is associated with a greater risk for disease progression and death. CD4 count is also another prognostic indicator with mortality rate significantly higher in profoundly immunosuppressed individuals. To define the prognosis more accurately, the use of changes in both markers (CD4 lymphocyte percentage and plasma viral load) is recommended.
Even in resource-limited countries where cART and molecular diagnostic tests are less available, the use of cART has had a substantial benefit on the survival of HIV-infected children and has reduced the likelihood of mortality by > 75%. Children with opportunistic infections (e.g., Pneumocystis pneumonia, MAC), encephalopathy and regressing developmental milestones, or wasting syndrome, which are all AIDS defining conditions, have the worst prognosis, with 75% dying before 3 yr of age. A higher risk of death was documented in children who did not receive TMP-SMX preventive therapy. Persistent fever and/or oral thrush, serious bacterial infections (meningitis, pneumonia, sepsis), hepatitis, persistent anemia (<8 g/dL), and/or thrombocytopenia (<100,000/µL) also suggest a poor outcome, with > 30% of such children dying before 3 yr of age. In contrast, lymphadenopathy, splenomegaly, hepatomegaly, lymphoid interstitial pneumonitis, and parotitis are associated with a slower progression of disease and a better prognosis. With sustained virologic suppression and maintained immunologic function, life expectancy is quite good. For adults and adolescents acquiring HIV, effective cART can restore life expectancy to near normal.
Prevention
Use of antiretroviral therapy for interruption of perinatal transmission from mother to child has been one of the greatest achievements of HIV research. Maternal cART is documented to decrease the rate of perinatal HIV-1 transmission to < 2%, and to < 1% if the mother's viral RNA level is < 1,000 copies/mL at delivery. Therefore, it is recommended that all pregnant women be tested for HIV, and if they are positive, should be treated with a cART regimen, irrespective of the viral load or CD4 count during pregnancy. All infants born to HIV-infected mothers should receive zidovudine prophylaxis for 6 wk; prophylaxis for 4 wk can be done in low-risk infants. Additional ARV therapy should be considered if the risk of acquiring HIV by the newborn is high. High-risk scenarios include infants born to mothers who received neither antepartum nor intrapartum ARV drugs or only intrapartum ARV drugs, infants born to mothers with a significant detectable viral load (>1,000 copies/mL) near delivery despite cART (particularly if it was a vaginal delivery), infants born to mothers of unknown HIV status who test positive at delivery or postpartum, or infants who have a positive HIV antibody test on screening after delivery. In these scenarios, three regimen options can be considered: (1) the addition of three doses of nevirapine (at birth, 48 hr, and 144 hr of life); (2) an empirical HIV therapy regimen of zidovudine, lamivudine, and nevirapine at treatment doses or (3) an empirical HIV therapy regimen of zidovudine, lamivudine, and raltegravir at treatment doses (note treatment doses of raltegravir for neonates are different than for older children with an escalating dose over the 6 wk of therapy due to evolving liver metabolism in neonates). Enthusiasm and support for treatment regimens (particularly option 2) have been driven by a case of an apparent functional cure in an infant in 2013 who went 2 yr without cART with virologic suppression before rebound of the infection occurred (the so-called Mississippi baby), as well as a large cohort of high-risk, exposed infants in Canada. The most experience and data exist for zidovudine, which can cause transient anemia or neutropenia in exposed infants. There is also a strong pool of data supporting the safety of lamivudine. For the remaining drugs for treatment of high risk infants, nevirapine has the the most experience of use but neither has robust data in premature infants. Dosing recommendations exist for nevirapine down to 32 wk but raltegravir can only be used in 37 wk and up. In high-risk infants, consultation with an experienced HIV specialist is highly recommended. The National Perinatal HIV Hotline (888-448-8765) provides 24-7 support from experienced HIV specialists to help in managing high-risk infants. Guidelines and current recommended doses for prophylaxis in newborns are updated at least yearly and can be accessed at http://www.aidsinfo.nih.gov . A complete blood count, differential leukocyte count, and platelet count should be performed at 4-8 wk of age to monitor zidovudine toxicity. This should be in conjunction with 4-6 wk of zidovudine prophylaxis for the infant. If the child is found to be HIV infected, baseline laboratory assessment (e.g., CD4 count, HIV RNA, complete blood count, chemistries, lipids, genotype) should be done and cART should be started as soon as possible. The viral load and CD4 lymphocyte counts should be determined at 1 and 3 mo of age and should be repeated every 3 mo. Cesarean section (C-section) as a prevention strategy was examined in a multinational meta-analysis, which showed that the combination of elective C-section and maternal zidovudine treatment reduced transmission by 87%. However, these data were obtained prior to the advent of cART, and the additional benefit of elective C-section to the cART-treated mother whose viral load is < 1,000 copies/mL is negligible. Thus, elective C-section at 38 wk of gestation should be considered only for women whose viral load is > 1,000 copies/mL in late gestation, to further reduce the risk of vertical transmission.
The WHO recommends that all pregnant women receive a cART regimen appropriate for their own health, which should be continued for the remainder of their lives. This approach has the potential to reduce transmission during breastfeeding and future pregnancies, lowers the transmission risk to sexual partners, improves maternal survival, and promotes simplified universal treatment regimens. It is not currently recommended that HIV+ women breastfeed in resource rich countries and there has been a least one case of mother-to-child-transmission via breastfeeding in a virologically suppressed mother.
Although the most effective way to prevent postpartum transmission of HIV is to eliminate breastfeeding altogether and substitute replacement feeding, there is evidence that early weaning may not be safe in resource-limited settings because of the high risk of malnutrition and diarrhea in formula-fed infants without a consistent source of clean water. Furthermore, exclusive breastfeeding (no additional solids or fluids other than water) results in less transmission than mixed feeding. Guidelines have evolved to recommend that HIV-infected mothers living in resource-limited settings should breastfeed their infants until at least 12 mo of age, with exclusive breastfeeding for the first 6 mo, and cART should continue to be provided to the mother. In settings where there are safe alternatives to breastfeeding, formula feeding is recommended. U.S. guidelines for prevention of mother-to-child transmission are regularly updated at http://aidsinfo.nih.gov/ and the international guidelines are regularly updated at the WHO website ( http://www.who.int/hiv/topics/mtct/en/ ).
Because perinatal transmission can be reduced dramatically by treating pregnant mothers, prenatal testing and identification of HIV-1 infection as early as possible in the mother is extremely important. The benefit of therapy both for the mother's health and to prevent transmission to the infant cannot be overemphasized. The recommended universal prenatal HIV-1 counseling and HIV-1 testing for all pregnant women has reduced the number of new infections dramatically in many areas of the United States and Europe. For women not tested during pregnancy, the use of rapid HIV antibody testing during labor or on the first day of the infant's life is a way to provide perinatal prophylaxis to an additional group of at-risk infants. Perinatal recommendations also now endorse the testing of pregnant women's partners to identify HIV+ partners who may transmit, leading to acute HIV infection which carries and extremely high risk of mother-to-child transmission.
Prevention of sexual transmission involves avoiding the exchange of bodily fluids. In sexually active adolescents, condoms should be an integral part of programs to reduce sexually transmitted diseases, including HIV-1. Unprotected sex with older partners or with multiple partners and the use of recreational drugs are often associated with acquisition of HIV-1 infection in adolescents and young adults. Educational efforts about avoidance of risk factors are essential for older school-age children and adolescents and should begin before the onset of sexual activity. In addition, promising research for sexually active adults may translate to increased prevention for adolescents. Three African trials demonstrated that male circumcision was associated with a 50–60% reduction in the risk of HIV acquisition in young men. For women, use of a 1% vaginal gel formulation of tenofovir during intercourse was found to reduce HIV acquisition by nearly 40% in one study, though subsequent trials have had variable efficacy; other topical microbicides are being investigated. A double-blind study of preexposure prophylaxis (PrEP) in MSM using once-daily dosing of coformulated tenofovir and emtricitabine resulted in a 44% reduction in the incidence of HIV. The incidence of HIV transmission was reduced by 73% when participants took the drug on 90% or more days. Studies of this regimen in other groups, including serodiscordant heterosexual couples, heterosexual individuals not in committed relationships, and intravenous drug users, showed excellent efficacy, as well (70–92%). All studies to date for PrEP have been in individuals 18 yr and up, however in adolescent patients with sufficiently high risk for acquisition, consideration should be given to using PrEP for HIV prevention. In addition, a large randomized multinational clinical trial of HIV serodiscordant adults demonstrated that effective ARV therapy in the HIV-infected partner reduced secondary transmission to an uninfected sexual partner by 96%. Further trials have confirmed that virologic suppression eliminates sexual transmission in heterosexual partners as well as men who have sex with men, spurning the catchphrase “U=U” or undetectable = untransmittable. The majority of these trials have been in adults, with limited participation by adolescents and young adults. Although much of the efficacy will likely be seen in young people, as well, further studies should be done on efficacy and acceptability in this age-group.
The course and prognosis of HIV infection has been radically improved by cART for all ages, particularly newer agents with less side effects. With good adherence, prolonged virologic suppression can be achieved and immune function can be preserved or reconstituted. However, lifelong adherence and side effects of medications are important challenges to recognize that can prevent patients from achieving good outcomes. Globally, great strides have been made in preventing mother-to-child transmission and increasing access to cART for children and adults, which is important for maintaining health as well as driving down sexual and vertical transmission with virologic suppression. However, there is still much work to be done in order to ensure the end of the global HIV epidemic, including continued advancement of our understanding of the immunology of HIV latency and reservoirs, HIV vaccines, and continued increasing of access to cART worldwide.