Human Papillomaviruses
Kristen A. Feemster
See also Chapter 687 .
Human papillomaviruses (HPVs) cause a variety of proliferative cutaneous and mucosal lesions, including common skin warts, benign and malignant anogenital tract lesions, oral pharyngeal cancers, and life-threatening respiratory papillomas. Most HPV-related infections in children and adolescents are benign (see also Chapter 687 ).
Etiology
The papillomaviruses are small (55 nm), DNA-containing viruses that are ubiquitous in nature, infecting most mammalian and many nonmammalian animal species. Strains are almost always species specific. Viral DNA is divided into an early region, which encodes proteins associated with viral replication and transcription, and a late region, which encodes capsid proteins necessary for virion assembly. These structural proteins are also the immunodominant antigens leading to type-specific immune responses. More than 100 different types of HPVs have been identified through the comparison of sequence homologies. The different HPV types typically cause disease in specific anatomic sites; more than 30 HPV types have been identified from genital tract specimens.
Epidemiology
HPV infections of the skin are common, and most individuals are probably infected with one or more HPV types at some times. There are no animal reservoirs for HPV; all transmission is presumably from person to person. There is little evidence to suggest that HPV is transmitted by fomites. Common warts, including palmar and plantar warts, are frequently seen in children and adolescents and typically infect the hands and feet, common areas of frequent minor trauma.
Human papillomavirus is also the most prevalent viral sexually transmitted infection in the United States. Up to 80% of sexually active women will acquire HPV through sexual transmission; most have their first infection within 3 yr of beginning sexual intercourse. Thus, HPV disproportionately affects youth, with 75% of new infections occurring in 15- to 24-yr-olds. The greatest risk for HPV in sexually active adolescents is exposure to new sexual partners, but HPV can still be acquired even with a history of one partner, underscoring the ease of transmission of this virus through sexual contact. It is estimated that after 11 acts of sexual intercourse, 100% of all HPV types infecting an individual will be transmitted to the other sexual partner. Couple studies show that there is high concordance in the genital area as well as between the hand and the genital area in the other partner. Whether the DNA detected in the hand is capable of transmitting infectious particles is unknown. Unlike other sexually transmitted infections, female-to-male transmission appears greater than male-to-female transmission. This may be because males in general have superficial transient infections or deposition. In turn, males do not develop an adequate immune response, so reinfections are quite common. The prevalence of HPV in women decreases with time, suggesting immune protection, whereas in men, the prevalence of HPV remains high across all ages.
As with many other genital pathogens, perinatal transmission to newborns can occur. Transmission from caregiver to child during the early childhood years has also been documented. However, both perinatal and early childhood infections appear transient. It remains unclear whether these HPV DNA detections are simply a deposition of caregiver DNA or a true infection. Detection of HPV DNA in older preadolescent children is rare. HPV DNA detection in nonsexually active adolescents has been reported, but a history of sexual activity in adolescents is not always disclosed and is therefore difficult to confirm. While caregivers can spread HPV to young children, if lesions are detected in a child older than 3 yr of age, the possibility of sexual transmission should be raised.
In adolescents, HPV DNA is most commonly detected without evidence of any lesion. Some of these detections are thought to be the result of partner deposition and hence do not represent a true infection. In older women, detection of HPV DNA is more commonly associated with a lesion. This is because the HPV DNA detected in older women reflects those HPV infections that became established persistent infections. Persistence is now the known necessary prerequisite for the development of significant precancerous lesions and cervical cancer.
Approximately 15–20% of sexually active adolescents have detectable HPV at any given time and have normal cytologic findings. The most common clinically detected lesion in adolescent women is the cervical lesion termed low-grade squamous intraepithelial lesion (LSIL) (Table 293.1 ). LSILs can be found in 25–30% of adolescents infected with HPV. External genital warts are much less common, occurring in < 1% of adolescents, but approximately 10% of individuals will develop genital warts in their lifetime. LSIL is a cytologic and histologic term to reflect the benign changes caused by an active viral infection and is likely present in most, if not all, women with HPV infection. The majority of women, however, have very minute or subtle lesions not easily detected by cytology. As with HPV DNA detection, most LSILs regress spontaneously in young women and do not require any intervention or therapy. Less commonly, HPV can induce more severe cellular changes, termed high-grade squamous intraepithelial lesions (HSILs) (see Chapter 568 ).
Table 293.1
Terminology for Reporting Cervical Cytology and Histology
| DESCRIPTIVE DIAGNOSIS OF EPITHELIAL CELL ABNORMALITIES | EQUIVALENT TERMINOLOGY |
|---|---|
| SQUAMOUS CELL | |
| Atypical squamous cells of undetermined significance (ASC-US) | Squamous atypia |
| Atypical squamous cells, cannot exclude HSIL (ASC-H) | |
| Low-grade squamous intraepithelial lesion (LSIL) | Mild dysplasia, condylomatous atypia, HPV-related changes, koilocytic atypia, cervical intraepithelial neoplasia (CIN) 1 |
| High-grade squamous intraepithelial lesion (HSIL) | Moderate dysplasia, CIN 2, severe dysplasia, CIN 3, carcinoma in situ |
| GLANDULAR CELL | |
|
Endometrial cells, cytologically benign, in a postmenopausal woman Atypical Endocervical cells, NOS Endometrial cells, NOS Glandular cells, NOS Endocervical cells, favor neoplastic Glandular cells, favor neoplastic |
|
|
Endocervical adenocarcinoma in situ Adenocarcinoma Endocervical Endometrial Extrauterine NOS |
|
NOS, not otherwise specified.
Although HSILs are considered precancerous lesions, they rarely progress to invasive cancer. HSILs occur in approximately 0.4–3% of sexually active women, whereas invasive cervical cancer occurs in 8 cases per 100,000 adult women. In true virginal populations, including children who are not sexually abused, rates of clinical disease are close to zero. In the United States, there are approximately 12,000 new cases and 3,700 deaths from cervical cancer each year. Worldwide, cervical cancer is the second most common cause of cancer deaths among women. HPV is also associated with a range of other anogenital cancers, including an estimated 4,600 cases of anal cancer and 11,100 cases of oropharyngeal cancers in men and women each year.
Some infants may acquire papillomaviruses during passage through an infected birth canal, leading to recurrent juvenile laryngeal papillomatosis (also referred to as respiratory papillomatosis ). Cases also have been reported after cesarean section. The incubation period for emergence of clinically apparent lesions (genital warts or laryngeal papillomas) after perinatally acquired infection is unknown but is estimated to be around 3-6 mo (see Chapter 417.2 ). It may be that infections can also occur during hygienic care from an infected parent.
Genital warts may represent a sexually transmitted infection even in some very young children. As such, genital warts appearing in childhood should raise suspicion for possible sexual abuse with HPV transmission during the abusive contact. A child with genital warts should therefore be provided with a complete evaluation for evidence of possible abuse (see Chapter 16.1 ), including the presence of other sexually transmitted infections (see Chapter 146 ). However, the presence of genital warts in a child does not confirm sexual abuse, because perinatally transmitted genital warts may go undetected until the child is older. Typing for specific genital HPV types in children is not helpful in diagnosis or to confirm sexual abuse status, because the same genital types occur in both perinatal transmission and abuse.
Pathogenesis
Initial HPV infection of the cervix or other anogenital surfaces is thought to begin by viral invasion of the basal cells of the epithelium, a process that is enhanced by disruption of the epithelium caused by trauma or inflammation. It is thought that the virus initially remains relatively dormant because virus is present without any evidence of clinical disease. The life cycle of HPV depends on the differentiation program of keratinocytes. The pattern of HPV transcription varies throughout the epithelial layer as well as through different stages of disease (LSIL, HSIL, invasive cancer). Understanding of HPV transcription enhances understanding of its ability to behave as an oncovirus. Early region proteins, E6 and E7, function as transactivating factors that regulate cellular transformation. Complex interactions between E6- and E7-transcribed proteins and host proteins result in the perturbation of normal processes that regulate cellular DNA synthesis. The perturbations caused by E6 and E7 are primarily disruption of the anti-oncoprotein p53 and retinoblastoma protein (Rb), respectively, contributing to the development of anogenital cancers. Disruption of these proteins results in continued cell proliferation, even under the circumstances of DNA damage, which leads to basal cell proliferation, chromosomal abnormalities, and aneuploidy, hallmarks of squamous intraepithelial lesion (SIL) development.
Evidence of productive viral infection occurs in benign lesions such as external genital warts and LSILs, with the abundant expression of viral capsid proteins in the superficial keratinocytes. The appearance of the HPV-associated koilocyte is a result of the expression of E4, a structural protein that causes collapse of the cytoskeleton. Low-level expression of E6 and E7 proteins results in cell proliferation seen in the basal cell layer of LSILs. LSILs are a manifestation of active viral replication and protein expression. In HSILs, expression of E6 and E7 predominates throughout the epithelium, with little expression of the structural proteins L1 and L2. This results in the chromosomal abnormalities and aneuploidy characteristic of the higher-grade lesions. The critical events that lead to cancer have not been verified; however, several mechanisms are thought to be critical, including viral integration into the host chromosome and activation of telomerase to lengthen chromosomes and avoid physiologic cell senescence. Over 150 HPV types have been documented and are classified by extent of their DNA homology into 5 genera, with the different types having different life-cycle and disease characteristics. The predominant group is α HPV types, which are associated with cutaneous and mucosal anogenital infections and cancers. β, γ, µ, and ν cause predominantly benign cutaneous lesions but can be difficult to manage in severely immunocompromised individuals. Β types are commonly detected on the skin without any apparent lesions but are associated with the development of skin cancers in those with epidermodysplasia verruciformis or other forms of immunodeficiencies. Genital lesions caused by the α HPV types may be broadly grouped into those with little to no malignant potential (low risk) and those with greater malignant potential (high risk). Low-risk HPV types 6 and 11 are most commonly found in genital warts and are rarely found isolated in malignant lesions. High-risk HPV types are those types that are associated with anogenital cancers, specifically cervical cancer. HPV 16 and 18 are thought to be more oncogenic than other HPV types because they comprise 70% of cervical cancers, whereas each of the other 12 high-risk types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73) contributes less than 1–9%. HPV 16 appears to be even more important in anal and HPV-associated oropharyngeal cancers, comprising close to 90% of these cancers. HPV 16 is also commonly found in women without lesions or in those with LSILs, making the connection with cancer confusing. Genital warts and SIL are commonly associated with the detection of multiple HPV types, including a combination of low- and high-risk HPV types. Data show that it is likely that a single lesion arises from a single HPV type. Detection of multiple HPV types reflects the presence of cervical and anal coexisting lesions. Almost all (95%) incident low-risk and high-risk HPV DNA detections, with or without detectable SIL, will spontaneously resolve within 1-3 yr. Although HPV 16 has a slower rate of regression than some of the other high-risk types, the majority of incident HPV 16 detections also will resolve. Data suggest that clearance of an HPV type results in natural immune protection against reinfection with that same type. Redetections of the same type are not common and when found are often associated with a history of a new sexual partner, suggesting that these are not reactivated infections but are due to new exposures. These redetections rarely result in high-grade disease. Persistent high-risk–type infections are associated with increased risk for development of HSILs and invasive cancer. Progression of HSIL to invasive cancer is still rare, with only 5–15% showing progression. Approximately 50% of HPV 16–associated HSILs and 80% of non–HPV 16 HSILs will spontaneously regress in young women. Genital and common warts in general also resolve without therapy but may take years to do so. Genital warts in only extremely rare conditions can become malignant.
Most infants with recognized genital warts are infected with the low-risk types. In contrast, children with a history of sexual abuse have a clinical picture more like that of adult genital warts, consisting of mixed low- and high-risk types. There are rare reports of HPV-associated genital malignancies occurring in preadolescent children and adolescents. On the other hand, precancerous HSILs do occur in sexually active adolescents. There is a concern that younger age of sexual debut has contributed to the increase in invasive cervical cancers seen in women younger than 50 yr of age in the United States, specifically cervical adenocarcinomas. Persistent HPV infections are considered necessary but not sufficient for the development of invasive cancers. Other risk factors for which there is relatively strong suggestive evidence of association include smoking cigarettes, prolonged oral contraceptive use, greater parity, and Chlamydia trachomatis and herpes simplex virus infections.
Clinical Manifestations
The clinical findings in HPV infection depend on the site of epithelial infection.
Skin Lesions
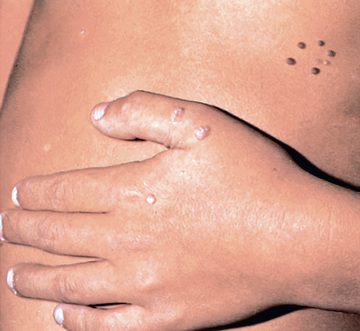
The typical HPV-induced lesions of the skin are proliferative, papular, and hyperkeratotic. Common warts are raised circinate lesions with a keratinized surface (Fig. 293.1 ). Plantar and palmar warts are practically flat. Multiple warts are common and may create a mosaic pattern. Flat warts appear as small (1- to 5-mm), flat, flesh-colored papules.
Genital Warts
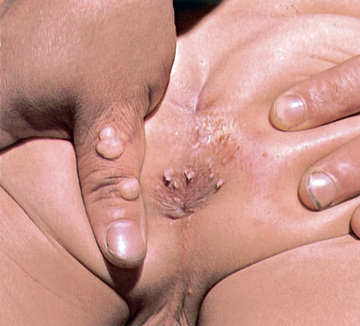
Genital warts may be found throughout the perineum around the anus, vagina, and urethra, as well as in the cervical, intravaginal, and intraanal areas (Fig. 293.2 ). Intraanal warts occur predominantly in patients who have had receptive anal intercourse, in contrast with perianal warts, which may occur in men and women without a history of anal sex. Although rare, lesions caused by genital genotypes can also be found on other mucosal surfaces, such as the conjunctivae, tongue, gingivae, and nasal mucosa. They may be single or multiple lesions and are frequently found in multiple anatomic sites, including the cervix. External genital warts can be flat, dome shaped, keratotic, pedunculated, and cauliflower shaped and may occur singly, in clusters, or as plaques. On mucosal epithelium, the lesions are softer. Depending on the size and anatomic location, lesions may be pruritic and painful, may cause burning with urination, may be friable and bleed, or may become superinfected. Adolescents are frequently disturbed by the development of genital lesions. Other rarer lesions caused by HPV of the external genital area include Bowen disease, bowenoid papulosis, squamous cell carcinomas, Buschke-Löwenstein tumors, and vulvar intraepithelial neoplasias.
Squamous Intraepithelial Lesions and Cancers
Squamous intraepithelial lesions detected with cytology are usually invisible to the naked eye and require the aid of colposcopic magnification and acetic acid. With aid, the lesions appear white and show evidence of neovascularity. SILs can occur on the cervix, vagina, vulva, penis, and intraanus. HPV-associated squamous cell lesions can also be found in the oropharynx. Invasive cancers tend to be more exophytic, with aberrant-appearing vasculature. These lesions are rarely found in non–sexually active individuals.
Laryngeal Papillomatosis
The median age at diagnosis of recurrent laryngeal papillomatosis is 3 yr. Children present with hoarseness, an altered cry, and sometimes stridor. Rapid growth of respiratory papillomas can occlude the upper airway, causing respiratory compromise. These lesions may recur within weeks of removal, requiring frequent surgery. The lesions do not become malignant unless treated with irradiation.
Diagnosis
The diagnosis of external genital warts and common warts may be reliably determined by visual inspection of a lesion by an experienced observer and does not require additional tests for confirmation. A biopsy should be considered if the diagnosis is uncertain, the lesions do not respond to therapy, or the lesions worsen during therapy.
Screening for cervical cancer in young women begins with cytology, which is either performed by Papanicolaou smear or liquid-based cytology. Screening guidelines, which were updated in 2012 by the American Cancer Society and the U.S. Preventive Services Task Force, recommend starting screening at age 21 yr. Screening earlier is more likely to result in unnecessary referrals for colposcopy, because most lesions, including both LSILs and HSILs in this age-group, are likely to regress. Guidelines recommend screening with cytology every 3 yr. At 30 yr of age, screening can also include co-testing with HPV DNA at an interval of every 5 yr. This is not recommended earlier, because HPV infections are extremely common in young women, resulting in a very low positive-predictive value in this age-group.
The recommended terminology used for cytologic evaluation is based on the Bethesda system (see Table 293.1 ). Recent updates to the terminology used for histology uses similar terms. Many clinicians still prefer the World Health Organization terminology using cervical intraepithelial neoplasia (CIN) 1, 2, and 3 (see Table 293.1 ). Although the purpose of screening is to identify CIN 3+ lesions, the majority of CIN lesions are found in women who were referred for atypical squamous cells of undetermined significance (ASC-US) or LSILs on cytology. On the other hand, few CIN 3 or cancers exist in women younger than 24 yr of age. Thus, for women 21-24 yr of age, ASC-US and LSILs are treated the same. The current preferred recommendation for young women with ASC-US or LSILs is to repeat cytology every 12 mo for up to 24 mo. For persistent ASC-US or LSILs at 2 yr of follow-up, referral for colposcopy is recommended. Women 21-24 yr of age with HSIL at any visit should be referred for colposcopy and biopsy. In adult women, HSIL can be treated without histologic confirmation. However, this approach should be avoided in those 21-24 yr of age, because HSIL is often misdiagnosed in this group or will resolve spontaneously.
In women older than 21 yr of age, high-risk HPV testing is acceptable to assist in ASC-US triage. This recommendation is based on the observations that adult women with ASC-US and a positive HPV test result for high-risk types are more likely to have CIN 2/3 than women with a negative HPV test result. However, in women with ASC-US and a positive HPV test for high-risk types, repeat cytology is recommended for confirmation. In women 21-24 yr of age referred for colposcopy and found to have no lesion or biopsy-confirmed LSIL after ASC-US or LSIL cytology, repeat cytology is recommended at 12 mo intervals. If ASC-US or LSIL has persisted after 2 yr or if HSIL is present at any time, referral for colposcopy is recommended. In women with biopsy-confirmed LSIL after atypical squamous cells of high grade (ASC-H) or HSIL, observation with cytology and colposcopy is recommended at 6 mo intervals for up to 2 yr. For persistent ASC-H or HSIL at 2 yr or progression at any time, treatment is recommended. Any young woman with histology-confirmed HSIL can be followed by colposcopy and cytology at 6 mo intervals if the patient is compliant. If HSIL continues to persist after 2 yr of follow-up, treatment is recommended. When CIN 3 is specified, treatment is recommended. These guidelines and updates can be found at http://www.asccp.org .
Very sensitive tests for the presence of HPV DNA, RNA, and proteins are becoming generally available, although they are not required for the diagnosis of external genital warts or related conditions. There are no indications for HPV DNA testing in women younger than 21 yr of age or children. HPV DNA testing is not recommended in women 21-30 yr of age but is acceptable for ASC-US triage.
Diagnosis of juvenile laryngeal papillomatosis (JRP) is made based on laryngeal examination.
There are no routine screening recommendations for noncervical or oropharyngeal lesions.
Differential Diagnosis
A number of other conditions should be considered in the differential diagnosis of genital warts, including condyloma latum, seborrheic keratoses, dysplastic and benign nevi, molluscum contagiosum, pearly penile papules, neoplasms, Bowen disease, bowenoid papulosis, Buschke-Löwenstein tumors, and vulvar intraepithelial neoplasias.
Condyloma latum is caused by secondary syphilis and can be diagnosed with darkfield microscopy and standard serologic tests for syphilis. Seborrheic keratoses are common, localized, hyperpigmented lesions that are rarely associated with malignancy. Molluscum contagiosum is caused by a poxvirus, is highly infectious, and is often umbilicated. Pearly penile papules occur at the penile corona and are normal variants that require no treatment.
Treatment
Most common (plantar, palmar, skin) warts eventually resolve spontaneously (see Chapter 687 ). Symptomatic lesions should be removed. Removal includes a variety of self-applied therapies, including salicylic acid preparations and provider-applied therapies (cryotherapy, laser therapy, electrosurgery). Genital warts are benign and usually remit, but only over an extended period. It is recommended that genital lesions be treated if the patient or the parent requests therapy. Treatments for genital warts are categorized into self-applied and provider-applied. No one therapy has been shown to be more efficacious than any other. Recommended patient-applied treatment regimens for external genital warts include topical podofilox, imiquimod, and sinecatechins. Podofilox 0.5% solution (using a cotton swab) or gel (using a finger) is applied to visible warts in a cycle of applications twice a day for 3 days followed by 4 days of no therapy, repeated for up to a total of 4 cycles. Imiquimod 5% cream is applied at bedtime, 3 times a week, every other day, for up to 16 wk; the treated area should be washed with mild soap and water 6-10 hr after treatment. Sinecatechins (15% ointment) is a topical product from green tea extract used for external genital wart treatment that can be used 3 times daily for up to 16 wk. Provider-applied therapies include surgical treatments (electrosurgery, surgical excision, laser surgery) and office-based treatment (cryotherapy with liquid nitrogen or a cryoprobe, podophyllin resin 10–25%, and bichloroacetic or trichloroacetic acid). Office-based treatments are usually applied once a week for 3-6 wk. Podophyllin resins have lost favor to other methods because of the variability in preparations. Intralesional interferon is associated with significant adverse effects and is reserved for treatment of recalcitrant cases.
Many therapies are painful, and children should not undergo painful genital treatments unless adequate pain control is provided. Parents and patients should not be expected to apply painful therapies themselves. None of the patient-applied therapies are approved for use during pregnancy, and podophyllin resin is contraindicated in pregnancy. For any of the nonsurgical treatments, prescription is contraindicated in a patient with any history of hypersensitivity to any product constituents.
If HPV exposure as a result of sexual abuse is suspected or known, the clinician should ensure that the child's safety has been achieved and is maintained.
When indicated, the most common treatments for CIN 2/3 are ablative and excisional treatments, including cryotherapy, laser, and loop electrosurgical excisional procedures. Once confirmed by histology with CIN 1, LSILs can be observed indefinitely. The decision to treat a persistent CIN 1 rests between the provider and patient. Risks of treatment, including premature delivery in a future pregnancy, should be discussed prior to any treatment decision. Treatment in pregnancy is not recommended unless invasive cancer is present.
JRP is commonly treated with surgical removal of lesions, but laser and microdebriders are also used. There are also several reports describing the use of adjunctive treatments, including antivirals and the quadrivalent human papillomavirus vaccine. However, the effectiveness of adjunctive therapy is not consistent.
Complications
The presence of HPV lesions in the genital area may be a cause of profound embarrassment to a child or parent. Complications of therapy are uncommon; chronic pain (vulvodynia) or hypoesthesia may occur at the treatment site. Lesions may heal with hypopigmentation or hyperpigmentation and less commonly with depressed or hypertrophic scars. Surgical therapies can lead to infection and scarring. Premature delivery and low birthweight in future pregnancies are complications of excisional therapy for CIN.
It is estimated that 5–15% of untreated CIN 3 lesions will progress to cervical cancer. Most cancer is prevented by early detection and treatment of these lesions. Despite screening, cervical cancer develops rapidly in a few adolescents and young women. The reason for the rapid development of cancer in these rare cases remains unknown, but host genetic defects are likely underlying causes. Juvenile laryngeal papillomas rarely become malignant, unless they have been treated with irradiation. Vulvar condylomas rarely become cancerous. HPV-associated cancers of the vagina, vulva, anus, penis, and oral cavity are much rarer than cervical tumors, and therefore screening for them is not currently recommended. However, anal, vaginal, and vulvar cancers are more common in women with cervical cancer; hence, it is recommended to screen women with cervical cancer for other anogenital or oropharyngeal tumors with visual and/or digital inspection.
Prognosis
With all forms of therapy, genital warts commonly recur, and approximately half of children and adolescents require a second or third treatment. Recurrence is also evident in patients with juvenile laryngeal papillomatosis. Patients and parents should be warned of this likelihood. Combination therapy for genital warts (imiquimod and podofilox) does not improve response and may increase complications. Prognosis of cervical disease is better, with 85–90% cure rates after a single treatment with the loop electrosurgical excision procedure. Cryotherapy has a slightly lower cure rate. Recalcitrant disease should prompt an evaluation and is common in immunocompromised individuals, specifically men and women infected with HIV.
Prevention
The only means of preventing HPV infection is to avoid direct contact with lesions. Condoms may reduce the risk for HPV transmission; condoms also prevent other sexually transmitted infections, which are risk factors associated with SIL development. In addition, condoms appear to hasten the regression of LSILs in women. Avoiding smoking cigarettes is important in preventing cervical cancer. Prolonged oral contraceptive use and parity have been shown to be risks for cervical cancer. However, the mechanisms associated with these factors have not been identified, and consequently no change in counseling is recommended.
HPV vaccines show efficacy against type-specific persistence and development of type-specific disease, including the cervix, vagina, vulva, and anus. A quadrivalent HPV vaccine containing types 6, 11, 16, and 18 was licensed in the United States in 2006, and a bivalent HPV vaccine containing types 16 and 18 was licensed in the United States in 2009. A 9-valent vaccine containing types 6, 11, 16, 18, 31, 33, 45, 52, and 58 was approved in 2014. The types targeted by the nonavalent vaccine account for up to 85% of cervical cancer cases. The efficacy of these vaccines is mediated by the development of neutralizing antibodies. Prelicensure studies demonstrate 90–100% efficacy in the prevention of persistent HPV infection, CIN 2/3, adenocarcinoma in situ, anogenital warts, and precancerous vaginal and vulvar lesions. Since vaccine introduction, data from Sweden and Australia show a decrease in national rates of genital warts within 4 yr of implementing vaccination programs. Recent data from the United States show significant reductions in the prevalence of the HPV types contained in the quadrivalent vaccine among adolescent and young adult females in the years 2009-2012 (postvaccine) compared with 2003-2006 (prevaccine). Additionally, the HPV vaccine–type prevalence was 2.1% in vaccinated compared with 16.9% in unvaccinated 14- to 24-yr-old sexually active females. A systematic review of 20 studies conducted in nine high-income countries showed reductions of at least 68% in the prevalence of HPV 16 and 18 among 13- to 19-yr-olds in countries with HPV vaccination rates > 50%. Available effectiveness data suggest that HPV vaccination confers herd immunity in addition to individual protection.
Vaccination in the United States is recommended routinely for all adolescents at 11-12 yr of age and is administered intramuscularly in the deltoid region in a two-dose series at 0 and 6 -12 mo. A two-dose series was approved and recommended in 2016 for younger adolescents who initiate the HPV vaccine series prior to age 15 yr based upon immunogenicity data showing a comparable immune response among younger adolescents who receive a two-dose series compared with older adolescents who receive a three-dose series. Vaccination is also recommended for adults through age 45 yr if they have not been previously vaccinated.
It is important that vaccination take place in children before they become sexually active, because the rate of HPV acquisition is high shortly after the onset of sexual activity. Vaccine can be given to adolescents as young as 9 yr of age, and a catch-up vaccination is recommended in girls 13-26 yr and in boys 13-21 yr. For males who are gay, bisexual, or have sex with males, who are immunocompromised (including HIV infection), or who are transgender, catch-up vaccination can continue through age 45. For any adolescent who receives his or her first HPV vaccine dose at age 15 or older, a three-dose series at 0, 1-2, and 6 mo is recommended. The three-dose series is also recommended for adolescents and young adults 9-26 yr old who have an immunocompromising condition. Individuals who are already infected with one or more vaccine-related HPV types prior to vaccination are protected from clinical disease caused by the remaining vaccine HPV types. Therefore, a history of prior HPV infection is not a contraindication to vaccine receipt. However, HPV vaccines are not therapeutic.
Postlicensure vaccine safety surveillance has not identified any serious adverse events attributable to HPV vaccine receipt. Three large observational studies and safety monitoring through active and passive surveillance networks among more than 1 million individuals have not identified any association between HPV vaccination and outcomes such as autoimmune disorders, stroke, or venous thrombotic emboli. Vaccination can cause fever in approximately 1 in 60 and discomfort at the injection site for 1 in 30 vaccine recipients. Syncope has also been found to be correlated with vaccine administration in 0.1% of vaccine recipients. Therefore, it is advised that adolescents remain seated for 15 min following vaccination.
Despite an excellent safety and efficacy profile, HPV vaccine uptake has been slow. Immunization rates consistently lag behind rates for the other vaccines included in the adolescent immunization platform. In 2015, only 56.1% of 13- to 17-yr-olds received at least one HPV vaccine dose compared with 81.6% who received at least one dose of the quadrivalent meningococcal vaccine and 86.4% who received Tdap. Reasons for the slow uptake include inconsistent provider recommendation, lack of knowledge about HPV, parental belief that vaccination is not necessary for younger adolescents, and misconceptions about vaccine safety, among others. There is a growing body of literature evaluating interventions to improve HPV vaccine uptake. One important strategy is a strong, consistent recommendation in which HPV vaccines are presented in the same way as Tdap and meningococcal vaccines.